Abstract
In three Far Eastern male forest cats (Prionailurus bengalensis euptilura) and four male domestic cats (Felis catus) with preimplanted (under general anesthesia) loggers, deep subcutaneous back temperature and motor activity were recorded in captive conditions for two months in the fall–winter period. It was found that in fall, at positive daytime temperatures, the circadian rhythm of body temperature is absent in Far Eastern forest cats and weakly expressed in domestic cats. However, it reappears during the period of winter cold in the form of fluctuations, synchronous in all animals, with an amplitude of 3–4°C and acrophase in the middle of the daylight period and a minimum in the middle of the night. For comparison, the recording of the “core” body temperature and activity in two female domestic cats kept in the same season in the laboratory revealed no comparable rhythms. It is concluded that the rest-activity and deep subcutaneous temperature circadian biorhythms are not constant characteristics of the body of Far Eastern forest and domestic cats, but can appear, disappear and change radically with changes in ambient temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The Far Eastern (Amur) forest cat (Prionailurus bengalensis euptilura) is a northern subspecies of the Bengal cat. This little-studied wild species has a number of interesting ecological and physiological adaptations that allow it to endure a long and frosty winter, although this animal does not have the ability to move on loose snow and cannot hunt during this season [1–4]. Here, for the first time, we made an attempt to record, using implanted loggers, rest-activity and body temperature circadian rhythms of male Far Eastern forest cats in captive conditions in the fall–winter period. For comparison, similar records were made in parallel in male domestic cats (Felis catus) under the same conditions.
Although cats of both sexes have been one of the most common laboratory animals throughout the 20th century and the main object of somnological experiments, ecophysiological studies have never been carried out on them. It is unknown how the feline organism behaves not only in the Far Eastern forest cats, but also in domestic cats with a constant stay in natural or semi-natural conditions. There are only a few fairly old works that compared the rest-activity and body temperature circadian rhythms in cats under laboratory conditions, the results of which are very contradictory and not very convincing [5–7]. Some authors believe that regular circadian fluctuations in the sleep-wake cycle and body temperature are completely absent in domestic cats [8]. To verify the presence (or absence) of circadian rhythms in these animals under domestic conditions, the results were compared with data obtained from the two female laboratory cats.
MATERIALS AND METHODS
Several Far Eastern forest and domestic cats were constantly kept from the moment of birth in spacious individual enclosures in a forest area at the Center for Collective Use “Live Collection of Wild Species of Mammals” on the territory of the scientific/experimental base (SEB) Chernogolovka (Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences; SIEE RAS). Each enclosure had a small shelter house with hay bedding. The enclosures were in natural light. There were no additional illumination either in the enclosures themselves or on the adjacent territory. Animals received a rationed feed (200 g of minced chicken) once a day at about 6 PM, 6 days a week. Three adult male Far Eastern forest cats (age 2–3 years, weight 5–6 kg) and four adult male domestic cats (age 2–3 years, weight 4 kg) were generally anesthetized (for Far Eastern forest cats, 0.1 mg Zoletil + 0.7/0.8 mg Rometar per animal; for domestic cats, 0.08 mg Zoletil + 0.6/0.7 mg Rometar per animal; i.m.) and implanted through a small incision at the withers with ecologgers (miniature autonomous temperature sensors equipped with accelerometers, manufactured by Embi Research LLC, Novosibirsk [9]) between the muscles of the interscapular region of the back, to a depth of 2–3 cm from the body surface. The incision was sutured; the animals were given an antibiotic and placed back in their enclosures.
Deep subcutaneous body temperature was measured in centigrade (°C), and motor activity was measured in conventional units (fractions of G, where dG is the resulting acceleration in three dimensions). The temperature and motion sensors were taking measurements every minute, averaged them over a period set by the user, and stored them in internal memory. In this study, the averaged period was 10 minutes. In this mode, the ecologger battery held its charge for 9–10 weeks. Recording lasted 65 days in the fall–winter period, from 10.28.2021 to 01.05.2022. At the end of the experiment, the animals were reanesthetized in the same way to remove the loggers and suture the incision; finally, the animals were given an antibiotic and returned to their enclosures.
For comparison, in two female laboratory cats with chronically implanted (for the purpose of another experiment) Pavlovian intragastric fistulas, a similar recording was carried out by placing ecologgers inside the fistula. In one cat (no. 1), recording was carried out in the summer–fall (08.22.2019–10.01.2019), while in the other (no. 2)—in the fall–winter (10.22.2021–12.28.2021) periods. All this time, the cats were kept in the laboratory at room temperature and under mainly natural light with the overhead light off, water and food were available ad libitum.
The data from the ecologgers’ information storages were read by a special device and processed in Excel with the determination of the mean values and the standard error of the mean (M ± SEM).
All experimental procedures performed in this study complied with the ethical standards approved by the legal acts of the Russian Federation and the principles of the Basel Declaration, and also were approved by the Bioethics Committee of the SIEE RAS.
RESULTS
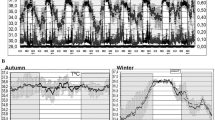
As seen from the fragments of records of the Far Eastern forest cats (Fig. 1), the dynamics of the recorded parameters in the fall (October 31–November 11) and winter (December 26–January 3) periods differed significantly. Daily data averaging (Fig. 2, left side) confirmed this impression. During the fall period, when mean air temperature was +6°C in the daytime and +4°C at night, no clear circadian rhythm of subcutaneous body temperature was observed. The deep subcutaneous temperature of the back showed random fluctuations around the “set point” of 36.1°C. On the contrary, locomotor activity underwent a regular circadian shift with lower values during daylight hours, which is natural for nocturnal predators. However, with the onset of cold weather (–9°C in the daytime and –13°C at night), the dynamics of deep subcutaneous back temperature changed significantly (Fig. 2, right side). At night, the subcutaneous back temperature of the Far Eastern forest cats dropped to 33.2°C. Approximately since midnight, deep subcutaneous back temperature began to increase rapidly, reaching a maximum (36.5°C) at dawn and remaining as a plateau for 5 h. Then, its gradual decline began, ending between midnight and 1AM. The mean value of the deep subcutaneous back temperature in Far Eastern forest cats in winter was 35.1°C, i.e. 1°C lower than in fall. These fluctuations in body temperature occurred synchronously and in phase in all three animals. At the same time, the motor activity rhythm of the animals smoothed out, and the difference between daytime and nighttime activity levels disappeared.
In domestic cats (Figs. 3, 4), deep subcutaneous back temperature fluctuated around the “set point” of 36.9°C during fall; at the same time, it dropped slightly at night, reaching a minimum (36.2°C) by 5AM, and rapidly rose afterward, reaching 37.5°C by dawn (6:30–7AM). After dawn, body temperature dropped sharply to 36.4°C and rose again to 38°C by 1PM (noon local solar time). Then the subcutaneous body temperature of domestic cats underwent a gradual undulating decrease. Motor activity did not change significantly; however, it is noticeable that both rises in body temperature (at 6AM and 1PM) coincided with a slight increase in the dG value.
In winter, subcutaneous temperature at night dropped to 34.4°C. From about 3AM, it began to rise, reaching the acrophase (38.3°C) by 2PM, after which there was a sharp decline, ending at about 7PM. From 7PM to 3AM, the subcutaneous back temperature of domestic cats remained at a low level of 34.4–35°C. The mean value of deep subcutaneous back temperature in domestic cats in winter was 35.8°C, i.e. 1.1°C lower than in fall. As for motor activity, its pronounced peak was noted, with the maximum coinciding with the acrophase of body temperature. All these fluctuations occurred synchronously and in phase in all four cats.
In laboratory cats kept at room temperature, there were no significant and regular fluctuations in the temperature of the loggers located inside the gastric fistula, with the exception of a slight (by 0.3°C) drop in body temperature at sunrise (Figs. 5, 6). In general, the temperature in the stomach cavity was much higher than between the back muscles (38.5–38.6 versus 36.9°C on average) and more stable, obviously reflecting the thermoregulation of the body’s “core”. The motor activity in cat No. 1 showed slight aperiodic rises, but no consistent relationship with the light–dark cycle was seen.
Male Far Eastern forest cats. Fragments of a 2-month recording. Top: body temperature (T, upper curves) and motor activity (dG, lower curves) during the fall period (October 31–November 10, 2021). M ± SEM (n = 3). The abscissa shows the time of day in h. On the left ordinate—deep subcutaneous temperature of the interscapular region of the back (10°C/scale). On the right ordinate—accelerometer readings (0.8 dG/scale). Shaded areas are night periods. Below: the same in winter (December 26, 2021–January 04, 2022).
Far Eastern forest cats. Intraday dynamics of body temperature (top) and locomotor activity (bottom), averaging over 3 animals × 10 days (M ± SEM) in fall (left, mean temperature +6°C during the day and +4°C at night) and winter (right, mean temperature –9°C during the day and –13°C at night). The abscissa shows the time of day in h. On the ordinate axes, at the top—deep subcutaneous temperature of the back (6.4°C/scale), at the bottom—accelerometer readings (0.2 dG/scale). Shaded areas—night periods.
Male domestic cats. Mean data for 4 animals (M ± SEM). Designations as in Fig. 1.
Domestic cats. Mean data for 4 animals × 10 days (M ± SEM). Designations as in Fig. 2.
Left: laboratory female domestic cat no. 1, summer–fall period. Right: laboratory female domestic cat no. 2, fall–winter period, 1°C/scale and 0.17 dG/scale for upper and lower ordinates, respectively. Other designations as in Fig. 2.
DISCUSSION
Circadian rhythms of activity and body temperature are influenced by such external factors as ambient temperature and light. However, seasonal studies of resting activity and body temperature were only carried out in just a few mammalian species, such as the sheep [10], horse [11], giant African rat [12], wolverine [13], and oryx [14]. In cats, similar studies have not been reported in the literature. The present study shows that the rest-activity and subcutaneous body temperature circadian biorhythms are not constant characteristics of the organism of the Far Eastern forest cats and domestic cats, but can appear, disappear, and radically change while the ambient temperature changes. At positive values of the ambient air temperature, the circadian rhythm of the subcutaneous back temperature in Far Eastern and domestic cats is weakly expressed or completely absent. Small carnivores, sables and ferrets, living under similar conditions at the SEB Chernogolovka, also demonstrate weakly pronounced rest-activity and abdominal temperature circadian rhythms in fall, which is not clearly associated with changes in illumination [15]. However, at negative ambient temperatures, cats demonstrate rhythmic fluctuations in subcutaneous temperature. At the same time, the synchronous and in-phase nature of the emerging biorhythms in all our animals indicates that they are subject to some external pacemakers. Judging from our data, the circadian fluctuations in external temperature and the alternation of day and night can be such pacemakers.
Obviously, with the onset of frosty weather, the organism of an animal living in enclosures has to adapt, maintaining a relatively constant temperature of the body “core”. To do this, the animal strongly reduces heat loss by decreasing the peripheral blood flow, as reflected in the time course of deep subcutaneous temperature. These cyclic fluctuations, in turn, reflect the ambient air temperature (maximum in the afternoon and minimum in the early hours of the night/morning). Interestingly, the similar dynamics of subcutaneous temperature, reflecting the ambient temperature, is also observed in the oryx during adaptation to high temperatures [14].
As for physical activity, the Far Eastern forest cats, which accumulate a thick subcutaneous fat layer by winter, demonstrate no need for the additional muscle warm-up. In winter, their motor activity somewhat decreases at night and increases in the daytime, so that in general it is not higher in winter than in fall. Domestic cats, which have no such a powerful “thermal insulation”, have to move around their enclosures for additional warming up. At the same time, an increase in daytime motor activity in fall and, especially, in winter was unexpected in “nocturnal” predators, domestic cats.
Our experiments with recording the “core” body temperature in a pair of domestic female cats confirm the point of view of Jouvet and other authors on the absence of rest-activity and body temperature circadian rhythms in these animals during constant stay at room temperature [5, 8]. These rhythms obviously form under the influence of external factors, the main of which is the low ambient temperature.
Noteworthy is a clear temperature drop that occurs at dawn in female laboratory cats. According to the averaged data, this decline turned out to be very similar to that in domestic male cats living in enclosures at much lower fall temperatures, being however 5 times less in its amplitude (cf. Fig. 6 and Fig. 4). This decline was noted when averaging data of all six domestic animals involved in the present study, regardless of their sex, environmental conditions and logger location, differing only in amplitude. This allows such a fluctuation to be considered as a real marker of the circadian rhythmicity of the body temperature in domestic cats.
A comparison of these results with our previous [15] and literature data [16–19] shows that mammals have at least three different strategies for adapting to cold.
(1) Hibernation and torpor [16–19].
(2) An increase in behavioral activity aimed at additional warming up during the 12-h active period of the day, with the maximum body (abdominal) temperature reached in the middle of the subjective night, which allows the animal (facultative hibernators, Mongolian hamsters) to maintain internal heat during the 12-h period of behavioral rest (subjectively, in the daytime). It is reflected in an increase in the amplitude of synchronous circadian rhythms of body temperature (threefold, from 0.5 to 1.6°C) and motor activity (twofold) [15].
(3) Shivering and non-shivering thermogenesis [18] without pronounced behavioral activation in the night and morning hours and with the maximum subcutaneous temperature achieved in the middle of the day (Far Eastern forest and domestic cats). It is reflected in the generation of a pronounced circadian rhythm of body temperature (with an amplitude of deep subcutaneous temperature fluctuations of 3–4°C) without significant changes (at least in Far Eastern forest cats) in the parameters of motor activity.
Thus, the present study represents the first attempt to record such fundamental characteristics as circadian rhythms of body temperature and motor activity level at different ambient temperatures in Far Eastern forest cats as compared with their domestic relatives.
REFERENCES
Pavlova EV, Naidenko SV (2008) Noninvasive monitoring of glucocorticoids in feces of the Bengal cat (Prionailurus bengalensis euptilurus). Zool J 87(11): 1375–1381. (In Russ).
Antonevich AL, Alekseeva GS, Vasilieva NA, Pavlova EV, Loshchagina JA, Duplyakina SYu, Naidenko SV (2019) Social play changes reflect differences in biology and development of three felids. Rus J Theriol 18(2): 80–90. https://doi.org/10.15298/rusjtheriol.18.2.02
Seryodkin IV, Burkovskiy OA (2019) Food habit analysis of the Amur leopard cat Prionailurus bengalensis euptilurus in the Russian Far East. Biol Bull 46(6): 648–653. https://doi.org/10.1134/S1062359019660038
Naidenko S, Chistopolova M, Hernandez-Blanco JA, Erofeeva M, Rozhnov V (2021) The effect of highway on spatial distribution and daily activity of mammals. Transp Res Part D 94: 102808. https://doi.org/10.1016/j.trd.2021.102808
Hawking F, Lobban MC, Gammage K, Worms MJ (1971) Circadian rhythms (activity, temperature, urine and microfilariae) in dog, cat, hen, duck, thamnomys and gerbillus. J Interdisсipl Cycle Res 2(4): 455–473. https://doi.org/10.1080/09291017109359289
Kuwabara N, Seki K, Aoki K (1986) Circadian, sleep and brain temperature rhythms in cats under sustained daily light-dark cycles and constant darkness. Physiol Behav 38(2): 283–289.
Randall W, Cunningham JT, Randall S, Liittschwager J, Johnson RF (1987) A two-peak circadian system in body temperature and activity in the domestic cat, Felis catus. J Therm Biol 12(1): 27–37.
Jouvet M (2016) Le sommeil, la conscience et l’éveil. Paris, Odile Jacob.
Petrovskii DV, Romashchenko AV, Troitskii SYu, Moshkin MP (2015) Between-strain differences in hypothermic response in mice after intranasal administration of Pto nanoparticles. Vavilovsk Zhurn Genetiki Selektsii—Vavilov J Gen Breed 19(4): 439–444. https://doi.org/10.18699/VJ15.058
Fuchs B, Sørheim KM, Chincarini M, Brunberg E, Stubsjøen SM, Bratbergsengen K, Hvasshovd SO, Zimmermann B, Lande US, Grøva L (2019) Heart rate sensor validation and seasonal and diurnal variation of body temperature and heart rate in domestic sheep. Vet Anim Sci 8: 100075. https://doi.org/10.1016/j.vas.2019.100075
Giannetto C, Aragona F, Arfuso F, Piccione G, De Caro S, Fazio F (2022) Diurnal variation in rectal and cutaneous temperatures of horses housed under different management conditions. Int J Biometeor 66(8): 1601–1611. https://doi.org/10.1007/s00484-022-02304-3
Dzenda T, Ayo JO, Lakpini CAM, Adelaiye AB (2011) Diurnal, seasonal and sex variations in rectal temperature of African giant rats (Cricetomys gambianus, Waterhouse). J Therm Biol 36: 255–263. https://doi.org/10.1016/j.jtherbio.2011.03.010
Thiel A, Evans AL, Fuchs B, Arnemo JM, Aronsson M, Persson J (2019) Effects of reproduction and environmental factors on body temperature and activity patterns of wolverines. Front Zool 16: 21. https://doi.org/10.1186/s12983-019-0319-8
Davimes JG, Alagaili AN, Bhagwandin A, Bertelsen MF, Mohammed OB, Bennett NC, Manger PR, Gravett N (2018) Seasonal variations in sleep of free-ranging Arabian oryx (Oryx leucoryx) under natural hyperarid conditions. Sleep 41: 5. https://doi.org/10.1093/sleep/zsy038
Kovalzon VM, Averina OA, Minkov VA, Petrin AA, Vysokikh MYu (2020) Unusual correlation between rest–activity and body temperature rhythms in the naked mole rat (Heterocephalus glaber) as compared to five other mammalian species. J Evol Biochem Physiol 56(5): 451–458. https://doi.org/10.1134/S0022093020050087
Pastukhov YuF, Maksimov AL, Haskin VV (2003) Adaptation to cold and subarctic conditions: the problems of thermophysiology, V. 1. ISRC “Arktika”, Magadan. (In Russ).
Mohr SM, Bagriantsev SN, Gracheva EO (2020) Cellular, molecular, and physiological adaptations of hibernation: The solution to environmental challenges. Annu Rev Cell Dev Biol 36: 13.1–13.24. https://doi.org/10.1146/annurev-cellbio-012820-09594
Frare C, Williams CT, Drew KL (2021) Thermoregulation in hibernating mammals: The role of the “thyroid hormones system”. Mol Cell Endocrinol 519: 111054. https://doi.org/10.1016/j.mce.2020.111054
Junkins MS, Bagriantsev SN, Gracheva EO (2022) Towards understanding the neural origins of hibernation. J Exp Biol 225(1): 229542. https://doi.org/10.1242/jeb.229542
ACKNOWLEDGMENTS
The authors are grateful to G.N. Fesenko for participating in this study, as well as the anonymous reviewer of the JEBP for a careful reading of the text and valuable comments that helped improve it.
Author information
Authors and Affiliations
Contributions
Conceptualization and experimental design (V.M.K., S.V.N.); data collection (A.D.K., G.S.A., M.N.E); data processing (V.M.K.); writing and editing the manuscript (V.M.K., S.V.N.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 5, pp. 449–456https://doi.org/10.31857/S0044452922050059.
Rights and permissions
About this article
Cite this article
Kovalzon, V.M., Komarova, A.D., Alekseeva, G.S. et al. Motor Activity Dynamics and Body Temperature in Far Eastern Forest and Domestic Cats in the Fall–Winter Period. J Evol Biochem Phys 58, 1381–1388 (2022). https://doi.org/10.1134/S002209302205009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002209302205009X