Abstract
Hibernation can be considered as a circannual cycle of feeding and fasting. During winter, the absence of dietary substrates in fat-storing hibernators leads to a reduction in the mass of fats and digestive organs. We examined the seasonal changes in body mass and activity of digestive enzymes in the pancreas and small intestine of the torpid Eptesicus nilssonii (Keyserling & Blasius, 1839). Adult females and males were captured in autumn (November, early hibernation), winter (February, mid-hibernation) and spring (March–April, late hibernation) in northwestern Russia (Republic of Karelia). Our findings indicated that male and female E. nilssonii reduced body mass during hibernation, but females exhibited a slower decline in body mass during hibernation period than males. No significant differences in the activity of digestive enzymes were found between males and females. Pancreatic protease activity was the highest in autumn, decreased in the mid-hibernation season, and remained low during hibernation of E. nilssonii. There was no difference in the activity of amylase and lipase in the pancreas among all periods of hibernation. However, the activity of protease, amylase and lipase in small intestine was higher before the emergence from hibernation in spring than during autumn. This study provides new information of the maintenance of intestinal hydrolytic enzyme activity after months of fasting. E. nilssonii, a fat-storing hibernator, appears to maintain a digestive function during the hibernation season, apparently to allow efficient absorption of nutrients that are ingested after terminal arousal in the spring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Many mammals living in northern latitudes encounter foraging problems associated with periods of adverse environmental conditions [1–3]. The ability to hibernate is a strategy enabling animals to survive seasons of restricted food supplies and extreme cold temperatures [4]. Hibernation is characterized by torpor bouts that may last from a few days to weeks. During torpor, animals reduce substantially their metabolic rate, body temperature and other physiological functions. Torpor bouts alternate with energy-expensive rewarming episodes where animals achieve normothermia [1, 2]. The majority of hibernating species accumulate fat stores during the active season (late summer–autumn). Lipids are the dominant source of energy during the metabolic depression characteristic of the hibernating state [2, 4]. In fat-storing hibernators (Spermophilus tridecemlineatus Mitchill, 1821 and Marmota marmota L., 1758), there is reduction in the body mass and the tissue mass of all digestive organs (stomach, intestine, caecum and colon) that occurs during the extended fast periods (6–7 months) [5, 6]. In addition, hibernation is accompanied by changes in the morphology of the small intestine (a decrease in the length and density of the villi of the jejunum) and the activity of digestive enzymes [4, 7, 8]. The expression of genes encoding sucrase, isomaltase and nutrient transporters are preserved in torpid S. tridecemlineatus despite the dramatic reduction in mass of the gastrointestinal tract [4, 9]. Previously, it was shown that the pancreatic amylase activity and expression fell only 40–50% in S. tridecemlineatus during the torpid state, and it is remarkable that they remain at the same high level until the end of hibernation [7]. Thus, the digestive organs must retain basal function (partially or fully) at the low metabolic rate values during torpor, apparently to allow efficient immediate absorption of nutrients that are ingested in spring [2]. The digestive system during hibernation and metabolic changes are relatively well studied in large hibernating mammals, especially rodents [2] but data from smaller, free-living bats are sparse [8, 10].
According to the observations in northwestern Russia, five resident vespertilionid bat species (Vespertilionidae) hibernate in natural and man-made roosts: Eptesicus nilssonii (Keyserling & Blasius, 1839), Plecotus auritus (L., 1758), Myotis brandtii (Eversmann, 1845), M. daubentonii (Kuhl, 1817) and М. mystacinus (Kuhl, 1817) [11]. Eptesicus nilssonii, a typical cave-roosting species in winter, is one of the most known bat species in the underground roosts (caves and mines) of northwestern Russia [11–13]. The hibernation period of E. nilssonii can last more than seven months [13]. Studies on P. auritus and M. daubentonii indicate that maximum and average duration of torpor bouts can be significantly shorter than recorded for E. nilssonii [14]. The Myotis species and E. nilssonii do not feed during arousals from torpor, since there are almost no night aerial insects available in winter [15]. Eptesicus nilssonii may provide a natural model system to study mechanisms that increase the tolerance of digestive organs to atrophy and dysfunction after a long-term fast. We estimated changes in body mass and the activity of digestive enzymes in tissues (pancreas and small intestine) of E. nilssonii during early (November) and mid-hibernation (February), and before emergence from hibernation in the spring (March–April).
MATERIALS AND METHODS
Ethical procedures
The investigation was performed in accordance with the US National Institute of Health ‘Guide for the Care and Use of Laboratory Animals’ as published in the NIH publication No. 85–23, 1996. The Ethics Committee of the Institute of Biology, Karelian Research Centre approved all animal care procedures prior to initiation of the experiment (permit number: 2015-02-12; 2016-02-01; 2017-01-18). The research was carried out using the equipment of the Core Facility of the Karelian Research Centre of the Russian Academy of Sciences.
Study species
Eptesicus nilssonii belongs to the global genus of serotine bats, Eptesicus (family Vespertilionidae, subfamily Vespertilioninae). This species has a wide trans-continental distribution across the Eurasian continent. The northern bat utilizes a variety of different overwintering sites such as man-made mines, bunkers, cellars and caves. This study was conducted in the underground roosts (Fig. 1) of the Republic of Karelia (61–63° N, 30–36° E) under the terms of permits (the Game Management Directorate of the Republic of Karelia, nos. 00009-2015, 00011-2016 and 00013-2017). The males and females of E. nilssonii were captured and weighed (10♂♂ / 19♀♀).
We collected bats in November (early hibernation, 3 ♂♂, 3 ♀♀), February (mid-hibernation, 4 ♂♂, 6 ♀♀) and March–April (late hibernation, 3 ♂♂, 10 ♀♀). The bats were induced to a hibernating state in a cold room (4–7°C) and transported to the laboratory. All the bats were torpid during collection, transportation and stayed at the laboratory until sacrifice. Samples of the pancreas and the small intestine of torpid bats were obtained after decapitation of animals the day after transportation. To determine the digestive enzyme activities, we isolated the pancreas and the entire length of the small intestine by separating the tissues from the mesentery. Fresh specimens of the small intestine were cut into segments, opened longitudinally, rinsed and placed in Eppendorf tubes, and stored at –80°C until the analyses were conducted.
Determination of digestive enzyme activities
For determination of digestive enzyme activity, tissue samples were homogenized in 2.0 mL ice-cold buffer solution (pH 6.9, composed of 20 mM Na2HPO4 and 6.7 mM NaCl). Intestinal and pancreatic homogenates were centrifuged to eliminate solid tissue residuals (at 6000 × g for 15 min, 4°C).
The digestive enzyme activity was determined in the supernatant fraction of freshly prepared homogenate. Amylase activity was assayed spectrophotometrically using starch as the substrate. The principle of the assay was that in the presence of amylase, a sample of starch will be hydrolyzed to shorter polysaccharides and iodine forms a blue to black complex with amylose in starch (starch–iodine complex). The reaction mixture containing 0.5 mL of 0.08% starch solution, 0.4 mL of 0.1 M phosphate buffer (pH 7.2), and 0.1 mL of 3% NaCl solution added to control and test tubes was incubated at 37°C for 10 min. Then, 0.01 mL of pancreatic supernatant / small intestine supernatant were added to the test tubes and incubated at 37°C for 10 min. The hydrolytic reaction was stopped by acidification, using 1 mL of 0.1N HCl which was also added to control tubes followed by addition of 0.01 mL of pancreatic supernatant / small intestine supernatant samples. The assay mixture (for a color reaction) was prepared by mixing 0.05 mL of 0.1 N HCl, 0.01 mL of iodine reagent (0.1 N solution of iodine in 0.1 N solution of KI in distilled water) and 1 mL of distilled water. Then 1 mL of the assay mixture and 3 mL of distilled water were added to the test and control tubes. The absorbance of the formed product (starch–iodine complex) was assayed spectrophotometrically at 600 nm (spectrophotometer Thermo Spectronic Genesys 20, Thermo Fisher Waltham, USA). All samples were run in duplicate, and the results are expressed as the units of enzyme that hydrolyse 1 mg of starch per minute per 1 g of analyzed tissue.
Lipase activity was determined spectrophotometrically, using glycerol tributyrate as a substrate. For this, two mL of the substrate (pH 8.2, 0.4 mL of tributyrin was dissolved in 100 mL of 0.1 M Tris-HCI buffer) was added to all tubes (test and control) and incubated at 37°C for 15 min (incubation 1). After that, 0.5 mL of supernatant fraction of homogenate was added to the test tubes and the mixture was incubated at 37°C for 20 min (incubation 2). The reaction was stopped by adding 1 mL of 5% trichloroacetic acid (TCA) to the tubes. The supernatant fraction of homogenate was added to the control tube after incubation 2 following addition of TCA. After the formation of a white precipitate, the tubes were centrifuged at 2500 × g for 20 min and 2 mL of the supernatant fraction was mixed with 0.1 mL of 10 N H2SO4, 0.5 mL of 0.1 M periodic acid solution (HIO4) and 0.5 mL of 10% sodium metabisulfite solution (Na2S2O5) (reaction mixture). Next, 2.5 mL of a solution of the disodium salt of chromotropic acid was added to tubes with 0.5 mL of the reaction mixture and incubated at 100°C for 30 min (incubation 3). Lipase hydrolyzes glycerol tributyrate to free fatty acid and glycerol. The amount of the product of the lipase activity in the form of glycerol was determined spectrophotometrically at 530 nm (spectrophotometer Thermo Spectronic Genesys 20, Thermo Fisher Waltham, USA). The concentrations of glycerol in the test tubes were determined by using the standard curve of known concentrations of this substance. Standard tubes were treated similarly to the test and control tubes, but without incubation 1 and incubation 2. The results were expressed as µmoles of substrate hydrolysed per minute per 1 g of analyzed tissue.
Total proteolytic activity (TPA) of pancreas and small intestine homogenates were evaluated using the conventional proteinase substrate—hemoglobin. The reaction mixture containing 2.5 mL of 0.1 M Tris-HCl buffer (pH 7.8) and 1 mL of 1% hemoglobin solution (in the same buffer) added to control and test tubes was incubated at 37°C for 15 min. Then, 0.5 mL of pancreatic supernatant / small intestine supernatant were added to the test tubes and incubated at 37°C for 20 minutes. The control tubes were incubated at 37°C for 20 minutes without homogenate. The reaction was stopped by adding 2 mL of 5% TCA. The tubes were centrifuged at 1500 × g for 20 min and the optical density of the supernatant was measured, at 280 nm (spectrophotometer SF-2000, Russia). The proteolytic activity is determined by the difference in optical density between the test and control samples. One unit of enzyme activity is defined as the amount of protease, producing an absorption corresponding to 1 µmole of tyrosine in 1 min using a calibration curve prepared in the micromolar range with the 1 mM tyrosine solution.
Statistical analysis
Statistical analysis was performed using MS Excel and Statgraphics software. Since some data were not normally distributed, we used median values (Me), percentiles (25%, 75%), and min/max indicators for their descriptions. In view of the small number of samples in each group, the non-parametric Mann–Whitney U-test was used to compare body mass as well as enzyme activity between groups of bats caught in different seasons. Statistical significance was assumed for values of p < 0.05. Data were analysed using a three-way MANOVA, with the effects of season and sex as factors.
Locations of underground shelters of bats in Karelia: 1—Ruskeala (61°57' N, 30°35' E), 2—Sona (4 caves; 61°43' N, 32°14' E), 3—Pertnavolok (62°10' N, 33°57' E), 4—Gizhozero (62°28' N, 35°05' E), 5—Medvezhyegorsk (3 underground concrete buildings; 62°54' N, 34°26' E), 6—Shcheleiki (61°07' N, 35°40' E).
RESULTS
Changes in body mass of adult E. nilssonii captured in the Republic of Karelia are summarized in Fig. 2. Bats showed decreases in body mass from mid-November to late April. In this period, the average decrease in body mass was 2.7 g for males (р < 0.05) and 0.4 g for females. The body mass of females was significantly higher than males at the end of hibernation (Mann–Whitney U-test, р < 0.05) (Fig. 2).
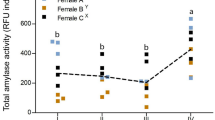
We demonstrated the presence of TPA, amylase and lipase activities in the pancreas and small intestine of E. nilssonii during the whole hibernation period (Fig. 3). No significant differences in the activity of digestive enzymes were found between males and females (MANOVA, p > 0.05), so the data were combined. Pancreatic protease activity was higher in early hibernation than in either mid-hibernation or late hibernation (Mann–Whitney U-test, р < 0.05). The amylase and lipase activities in the pancreas of the torpid bats were nearly the same among the three seasons (p > 0.05). The TPA in small intestine increased 15.3-fold from early to late hibernation (р < 0.05). The small intestine amylase and lipase activities in late hibernation were higher than in early hibernation (р < 0.05) (Fig. 3).
DISCUSSION
Mammalian hibernators display strong circannual rhythms in metabolism and body mass. The vespertilionid bats deposit additional fat in a short period just before hibernation with a concomitant loss of this fat during winter [2, 16]. However, male and female bats may spend their energy reserves differently throughout the hibernation season [16]. Sexual dimorphism in lipid metabolism is the result of hormone action and of other modulators [17]. Our results indicate that male E. nilssonii decline in body mass at a faster rate than females within the hibernation season (Fig. 2). Similarly, females of some species such as the big brown bat E. fuscus (Beauvois, 1796) and the tricolored bat Perimyotis subflavus (F. Cuvier, 1832) also exhibit a slower decline in body mass during winter than males [18, 19]. In accordance with previous results [16], female little brown bats (M. lucifugus Le Conte, 1831) emerge from hibernation with greater energy reserves in comparison with male. These observations are consistent with the “the thrifty female hypothesis” that adult females should minimize their energy expenditure and rely more heavily on the deep torpid state in winter [20]. Kunz et al., [21] suggested that females must retain sufficient fat upon awakening from hibernation to mediate the hormonal changes necessary for reproductive function. Our findings may reflect an evolutionary strategy that female E. nilssonii with larger remaining fat stores may be able to emerge from the torpid state earlier in the spring. During periods of inclement weather and when the number of insects is low, these energy reserves will allow them to survive [22].
In addition to dietary change, other unfavorable environmental factors of the hibernation could potentially alter body mass. The interbout arousal is accompanied by intense metabolic activity with simultaneous depletion of fat stores. Observation from several laboratories on behavioral changes in bats during the hibernation period led to the hypothesis that the circadian rhythm of torpor may be compromised by fungal infection (White-nose syndrome, WNS), resulting in an increase in the number of euthermic arousals in northeastern United States and Canada [23–25]. There are significant decreases in body fat in a short period of hibernation in WNS-infected animals, likely contributing to subsequent mortality [23]. The large reserves of fat can contribute to the survival of infected animals during hibernation.
Seasonal fluctuations in the resource availability cause drastic changes in the morphology and functions of hibernator digestive organs [2, 4]. Bauman et al. [26] have documented that the protein contents were lower (by 50%) in the pancreas of torpid M. lucifugus in comparison with active bats. The pancreatic mass is ∼40–50% higher during the summer season in S. tridecemlineatus than in hibernating animals [27]. Our results indicate that pancreatic protease activity in torpid E. nilssonii is reduced during hibernation (Fig. 3). This study is the first to report a seasonal change in digestive enzyme activity in the organs of E. nilssonii during hibernation. The protein biosynthesis and denaturation are extremely costly in energy during the hibernation period [28]. The structural changes were demonstrated in pancreatic acinar cells of the hazel dormouse Muscardinus avellanarius (L., 1758) during torpor [29]. A marked decrease in protein synthesis occurs during hibernation in pancreatic acinar cells of M. avellanarius, with a reduction in the Golgi apparatus and size of the zymogen granules, as well as a flattening of the cisternae of the rough endoplasmic reticulum [29].
In contrast, the amylase and lipase activities in the pancreas are preserved despite depression in metabolism of torpid E. nilssonii. Previously it was shown that the pancreatic amylase activity and expression decreased by 40–50% in S. tridecemlineatus during the torpid state, it is remarkable that they remain at the same high level until the end of hibernation [7]. Maintaining a basal amylase activity in the pancreas during the torpor may allow them to survive seasons of restricted food supplies in the spring after terminal arousal (a time when food is of lower quality) [7]. The changes in pancreatic amylase activity that have been observed may be the consequence of altered insulin metabolism during the annual cycle. Insulin, the anabolic hormone, plays a key role in the transition away from catabolism upon refeeding. Previously it was shown that the insulin concentration progressively increased in M. lucifugus during the deep torpid state (winter–early spring) [26]. In rodent hibernators (S. tridecemlineatus and M. flaviventris), the plasma insulin levels significantly increase before hibernation [30]. After reaching maximum values in the autumn, plasma insulin concentrations then decline to summer levels during the torpid state [30].
In hibernating rodent species, there is maintenance of the expression of sucrase, isomaltase and transporters involved in glucose uptake despite a decrease in mucosal jejunal villi length and density [9]. Our results demonstrated that the activity of protease, amylase and lipase in small intestine of E. nilssonii were higher in the period of late hibernation than in either early hibernation or mid-hibernation (Fig. 3). The molecular mechanism for these changes in the digestive function during torpor is unknown. Recently, it has been reported that the activity of amylase, chitobiase, endochitinase and glucosaminidase are detected in fresh faeces of the Mediterranean horseshoe bat Rhinolophus euryale (Blasius, 1853) throughout the whole winter [8]. However, R. euryale arouse from hibernation frequently and feed on insects (Lepidoptera) [8]. It is improbable that the Northern bats in the North are able to feed during the winter, since there are practically no nocturnal insects available [15]. Previously, it was shown that the bats have smaller small intestines and significantly greater villous amplification of small intestine surface area than similarly sized non-flying mammals [31, 32]. The ability to hydrolyze nutrients in the intestinal lumen directly after feeding restarts, without prior synthesis of new digestive enzyme protein, may allow the body protein supplies to be used for other functions, such as intestinal growth and membrane transport [7].
In conclusion, we have shown that a prolonged fast (5–7 months) and hypothermia during hibernation have effects on the body mass and activity of digestive enzymes in the pancreas and small intestine of E. nilssonii. Our results suggest that the male and female reduce body mass from mid-October to late April, but females use fat reserves more conservatively during hibernation to ensure energy availability for spring reproduction. The pancreatic protease activity was higher in early hibernation than in either mid-hibernation or late hibernation. However, the activity of amylase and lipase in the pancreas does not change seasonally. Winter fasting leads to increases in the activity of hydrolytic enzymes (protease, amylase and lipase) in the small intestine of E. nilssonii. We conclude that the digestive enzyme activity increased in the organs of the torpid Northern bats despite the hibernation-related fasting, which excludes the presence of insects in the underground roosts in the winter months. The physiological significance of these changes has yet to be determined. Apparently, they might facilitate the rapid resumption of digestive function after arousal in the spring. Confirmation of this idea requires further research to understand the molecular genetic mechanisms of the adaptations of the digestive system during the torpid state.
REFERENCES
Mohr SM, Bagriantsev SN, Gracheva EO (2020) Cellular, molecular, and physiological adaptations of hibernation: the solution to environmental challenges. Annual Review of Cell and Developmental Biology 36: 315–338. https://doi.org/10.1146/annurev-cellbio-012820-095945
Kurtz CC, Otis JP, Regan MD, Carey HV (2021) How the gut and liver hibernate. Comparative Biochemistry and Physiology—Part A: Molecular and Integrative Physiology 253: 110875. https://doi.org/10.1016/j.cbpa.2020.110875
Giroud S, Habold C, Nespolo RF, Mejías C, Terrien J, Logan SM, Henning RH, Storey KB (2021) The Torpid state: recent advances in metabolic adaptations and protective mechanisms. Frontiers in Physiology 11: 623665. https://doi.org/10.3389/fphys.2020.623665
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological Reviews 83(4): 1153–1181. https://doi.org/10.1152/physrev.00008.2003
Carey HV (1990) Seasonal changes in mucosal structure and function in ground squirrel intestine. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 259: 385–392. https://doi.org/10.1152/ajpregu.1990.259.2.R385
Hume D, Beiglböck C, Ruf T, Frey-Roos F, Bruns U, Arnold W (2002) Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). Journal of Comparative Physiology B 172(3): 197–207. https://doi.org/10.1007/s00360-001-0240-1
Balslev-Clausen A, McCarthy JM, Carey HV (2003) Hibernation reduces pancreatic amylase levels in ground squirrels. Comparative Biochemistry and Physiology – Part A 134: 573–578. https://doi.org/10.1016/s1095-6433(02)00363-x
Maxinová E, Sustr V, Uhrin M (2017) Digestive enzymes in Rhinolophus euryale (Rhinolophidae, Chiroptera) are active also during hibernation. European Journal of Ecology 3(1): 91–96. https://doi.org/10.1515/eje-2017-0010
Carey HV, Martin SL(1996) Preservation of intestinal gene expression during hibernation. American Journal of Physiology Gastrointestinal and Liver Physiology 271: G805–G813. https://doi.org/10.1152/ajpgi.1996.271.5.G805
Buchholz R, Wells P, Conaway C (1958) Digestive enzymes of mole, bat and rat. Journal of Mammalogy 39(3): 452–454. https://doi.org/10.2307/1376175
Belkin VV, Fyodorov FV, Ilyukha VA, Yakimova AE (2021) Characteristics of the bat (Chiroptera) population in protected areas in the northern and middle taiga subzones of European Russia. Nature Conservation Research 6: 17–31. https://doi.org/10.24189/ncr.2021.002
Siivonen Y, Wermundsen T (2008) Characteristics of winter roosts of bat species in southern Finland. Mammalia 72: 50–56. https://doi.org/10.1515/MAMM.2008.003
Belkin VV, Panchenko DV, Tirronen KF, Yakimova AE, Fedorov FV (2015) Ecological status of bats (Chiroptera) in winter roosts in Eastern Fennoscandia. Russian Journal of Ecology 46: 463–469. https://doi.org/10.1134/S1067413615050045
Anufriev AI, Revin YV (2006) Bioenergetics of hibernating bats (Chiroptera, Vespertilionidae) in Yakutia. Plecotus et al 9: 8–17.
Blomberg AS, Vasko V, Meierhofer MB, Johnson JS, Eeva T, Lilley TM (2021) Winter activity of boreal bats. Mammalian Biology 101: 609–618. https://doi.org/10.1007/s42991-021-00111-8
Jonasson KA, Willis CK (2011) Changes in body condition of hibernating bats support the thrifty female hypothesis and predict consequences for populations with white-nose syndrome. PloS one 6(6): e21061. https://doi.org/10.1371/journal.pone.0021061
Wang X, Magkos F, Mittendorfer B (2011) Sex differences in lipid and lipoprotein metabolism: it’s not just about sex hormones. The Journal of Clinical Endocrinology & Metabolism 96: 885–893. https://doi.org/10.1210/jc.2010-2061
Ploskey GR, Sealander JA (1979) Lipid deposition and withdrawal before and during hibernation in Pipistrellus subflavus (Chiroptera: Vespertilionidae). The Southwestern Naturalist 24: 71–78. https://doi.org/10.2307/3670626
Beer JR, Richards AG (1956) Hibernation of the big brown bat. Journal of Mammalogy 37: 31–41.
Humphries MM, Thomas DW, Kramer DL (2003) The role of energy availability in Mammalian hibernation: a cost-benefit approach. Physiological and Biochemical Zoology 76(2): 165–179. https://doi.org/10.1086/367950
Kunz TH, Wrazen JA, Burnett CD (1998) Changes in body mass and fat reserves in pre-hibernating little brown bats (Myotis lucifugus). Ecoscience 5: 8–17. https://doi.org/10.1080/11956860.1998.11682443
Racey PA, Swift SM (1981) Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. Journal of Reproduction and Fertility 61(1): 123–9. https://doi.org/10.1530/jrf.0.0610123
Reeder DM, Frank CL, Turner GG, Meteyer CU, Kurta A, Britzke ER, Vodzak ME, Darling SR, Stihler CW, Hicks AC, Jacob R, Grieneisen LE, Brownlee SA, Muller LK, Blehert DS (2012) Frequent arousal from hibernation linked to severity of infection and mortality in bats with white-nose syndrome. PLoS One 7(6): e38920. https://doi.org/10.1371/journal.pone.0038920
Turner JM, Warnecke L, Wilcox A, Baloun D, Bollinger TK, Misra V, Willis CK (2015) Conspecific disturbance contributes to altered hibernation patterns in bats with white-nose syndrome. Physiology & Behavior 140: 71–78. https://doi.org/10.1016/j.physbeh.2014.12.013
Mayberry HW, McGuire LP, Willis CKR (2018) Body temperatures of hibernating little brown bats reveal pronounced behavioural activity during deep torpor and suggest a fever response during white-nose syndrome. Journal of Comparative Physiology B 188(2): 333–343. https://doi.org/10.1007/s00360-017-1119-0
Bauman WA (1990) Seasonal changes in pancreatic insulin and glucagon in the little brown bat (Myotis lucifugus). Pancreas 5: 342–346. https://doi.org/10.1097/00006676-199005000-00015
Bauman WA, Meryn S, Florant GL (1987) Pancreatic hormones in the nonhibernating and hibernating golden mantled ground squirrel. Comparative Biochemistry and Physiology—Part A 86: 241–244. https://doi.org/10.1016/0300-9629(87)90324-0
Macrae JC, Lobley GE, Lobley GE (2003) Protein turnover—what does it mean for animal production? Canadian Journal of Animal Science 83: 327–340. https://doi.org/10.4141/A03-019
Malatesta M, Zancanaro C, Marcheggiani F, Cardinali A, Rocchi MBL, Capizzi D, Vogel P, Fakan S, Gazzanelli G (1998) Ultrastructural, morphometrical and immunocytochemical analyses of the exocrine pancreas in a hibernating dormouse. Cell and Tissue Research 292: 531–541. https://doi.org/10.1046/j.0021-8782.2002.00103.x
Buck MJ, Squire TL, Andrews MT (2002) Coordinate expression of the PDK4 gene: a means of regulating fuel selection in a hibernating mammal. Physiological Genomics 8: 5–13. https://doi.org/10.1152/physiolgenomics.00076.2001
Price ER, Brun A, Caviedes-Vidal E, Karasov WH (2015) Digestive adaptations of aerial lifestyles. Physiology (Bethesda) 30(1): 69–78. https://doi.org/10.1152/physiol.00020.2014
Karasov WH, Caviedes-Vidal E (2021) Adaptation of intestinal epithelial hydrolysis and absorption of dietary carbohydrate and protein in mammals and birds. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 253: 110860. https://doi.org/10.1016/j.cbpa.2020.110860
ACKNOWLEDGMENTS
The authors express their gratitude to the associates of the Laboratory of ecological physiology of animals (IB FRC KRC), especially to A.V. Morozov, for his assistance in conducting the experiment.
Funding
The research was supported by the state orders (project no. FMEN-2022-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All applicable international, national and institutional principles of handling and using experimental animals for scientific purposes were observed. This study did not involve human subjects as research objects. This study protocol was approved by the Research and Ethical Committee of the Institute of Biology, Karelian Research Centre, RAS.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Antonova, E.P., Belkin, V.V., Ilyukha, V.A. et al. Seasonal Changes in Body Mass and Activity of Digestive Enzymes in Eptesicus nilssonii (Mammalia: Chiroptera: Vespertilionidae) during Hibernation. J Evol Biochem Phys 58, 1055–1064 (2022). https://doi.org/10.1134/S002209302204010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002209302204010X