Abstract
The sympathetic nervous system (SNS) plays an important role in regulating the metabolic and secretory functions of white adipose tissue. In this study, it was aimed to investigate the long term effects of bilateral retroperitoneal adipose tissue denervation and high fat diet (HFD) on general metabolic status and serum levels of some adipokines in rats. For this purpose, we fed denervated and control rats with control and high fat diet for 70 days. At the end of the feeding program, denervation caused an increase in serum triglyceride (p < 0.01), leptin (p < 0.05) and adiponectin levels (p < 0.01), as well as body weight (p < 0.05) in the group with the control diet (CD). We also observed that the serum adiponectin levels (p < 0.05), of the animals in the denervated HFD group was higher than the non-denervated group whereas the body weight and leptin/adiponectin ratio were reduced. Our data indicate that bilateral denervation of a pad of white adipose tissue in rats causes different changes in metabolic parameters and circulating adipokine levels at different energy states, and in the long term, while denervation mimicked obesity in the control diet group, it to some extent suppressed the effects of a high fat diet in the HFD group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
White adipose tissue (WAT) is a loose connective tissue formed by mature fat cells and the stromal vascular fraction, consisting of macrophages, fibroblasts, blood cells, endothelial cells and adipose precursor cells [1]. There are two main metabolic pathways in WAT. The first is the conversion and storage of excess energy into triglyceride (TG) molecules in the presence of a positive energy balance, such as high-fat dietary nutrition. The second, if necessary, is the breakdown of triglycerides into free fatty acids and glycerol [2]. In addition, as an endocrine organ, WAT synthesizes adipokines, such as leptin and adiponectin, which are involved in many physiological and pathological events. These metabolic and secretory properties of adipose tissue are tightly controlled by the endocrine and autonomic nervous system [3].
Factors of neural origin play an important role in the control of energy homeostasis. The central and autonomic nervous system controls energy metabolism by regulating metabolic functions such as food intake, energy consumption and storage. In fact, the metabolic and secretory functions of various tissues or organs are under the control of the autonomic nervous system and this applies to the liver, pancreas, and adrenal glands, as well as to the muscles. In addition, the metabolic, secretory and plasticity properties of adipose tissue are tightly controlled by the autonomic nervous system [4]. Adrenergic receptors of WAT also play an important role in regulating lipolysis in the fat cells. In addition, adipose tissue secretes factors such as leptin, BDNF, VEGF, TNF, affecting sensory neural networks and transmitting signals from adipose tissue and cells found therein to the brain [5]. Although the effects of sympathetic nervous system (SNS) on adipose tissue have been demonstrated, innervation and effects of the parasympathetic nervous system on adipose tissue have remained controversial [3, 6]. To examine the effects of the nervous system on adipose tissue, denervation experiments, in which nerve fibres are disabled, have been used. Following WAT denervation, the growth in adipose tissue due to cell proliferation was detected without measurable changes in fat cell metabolism. SNS controls both lipolysis and growth of adipose tissue. In addition, many studies have shown that SNS suppresses the secretion of leptin and adiponectin from the adipose tissue [5, 7]. Most adipose tissue denervation studies involve short-term (days or 1–2 weeks) and unilateral denervation. Long-term studies in which a pair of white fat pads are completely disabled and metabolic evaluations are made in different energy balance levels due to the diet are very limited.
Recently, it has been suggested that via sensory nerves acting on the central nervous system, white adipose tissue can regulate the energy metabolism of the whole body by affecting the organs that have important roles in energy metabolism such as brain, liver, and brown fat tissue [5]. In this study, it was aimed to investigate the effect of positive energy balance, created by a long-term (70-days) high-fat diet (HFD), and bilateral retroperitoneal adipose tissue denervation, on general metabolic parameters and adipokine levels in adult rats.
MATERIALS and METHODS
Animals and study design
In our study, 32 male Sprague–Dawley rats, 3–5 weeks old weighing 100–150 g, obtained from KTU Medical Faculty Surgical Research Center were used. All experiments were performed with the approval of the Animal Experiments Local Ethics Committee of Karadeniz Technical University (protocol number 2013/29). All rats were fed control food (D12450J, Reseach Diet, USA) for 48 hours for adaptation. Then, the rats were randomly divided into 4 groups with 8 rats in each group and body weights were measured at the beginning of the diet protocol.
Denervation of the fat tissue was performed as explained in previous studies [8]. Animals were anaesthesized under ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anaesthesia. Sympathetic and sensory nerves innervating the retroperitoneal adipose tissue are very close to each other [8, 3]. In this study, both sympathetic and sensory nerve fibers were cut during the denervation procedure. The following experimental groups were formed:
– Control group (CD)—Only the abdomens were opened and sutured. Fed with control diet. (D12450J, Research Diet, USA);
– Denervated control group (dCD)—Retroperitoneal fat tissue is bilaterally denervated. Fed with control diet (D12450J, Research Diet, USA;
– High fat diet group, (HFD)—Only the abdomens were opened and sutured. Fed with high fat diet (D12492, Research Diet, USA);
– Denervated high fat diet group (dHFD)—Retroperitoneal fat tissue is bilaterally denervated. Fed with high fat diet (D12492, Research Diet, USA).
Food and water were given ad libitum to all animals throughout the adaptation and following diet protocols.
Body weights were measured at 15-days intervals. At the end of the total 70-days diet period, the rats were weighed and after a 12-hour feed-free period, they were sacrificed by decapitation at 09:00–10:00 AM. Blood and tissue samples were collected.
Analysis of serum glucose and triglyceride levels
Fasting glucose and triglyceride (TG) levels in the serum samples were measured using a Roche Hitachi Cobas 8000 auto analyzer in Farabi Hospital routine medical biochemistry laboratory of Karadeniz Technical University.
Determination of serum adiponectin, leptin, ınsulin, resistin and plasminogen activator inhibitor-1 (PAI-1) levels
Rat adiponectin, leptin, insulin, resistin and PAI-1 were measured by ELISA kits (Adiponectin, catalog number 201-11-0759, SunRed, China; Leptin, catalog number RD291001200R, Biovendor, Czech Republic; Insulin, catalog number A05105, SPI Bio, China; Resistin, catalog number RD391016200R, Biovendor, Czech Republic; PAI-1, catalog number 201-11-0637, SunRed, China) according to the manufacturers’ instructions.
Norepinephrine measurement in the adipose tissue
Denervation was verified by measuring retroperitoneal fat tissue norepinephrine (NE) content using ELISA (IBL, Germany). Approximately 150 mg of fat tissue was placed in 500 µL of cold homogenization solution (PBS 10 mM, pH 7.4) in an Eppendorf tube. Samples were frozen in liquid nitrogen (–196°C) for approximately 45 s, then thawed at 2–8°C. 500 µL of PBS was added again and then tissues were homogenized for 30 seconds at 6000 rpm with a hand homogenizer. It was then centrifuged at 3000 rpm for 20 minutes, supernatants were separated and used for measurements. Results were expressed as ng/g wet tissue.
Statistical Analysis
The data from the present study were analyzed using SPSS software version 22 (IBM Corp., Armonk, NY, USA). The Kruskal–Wallis test was used to compare multiple variables in independent groups not exhibiting normal distribution and the Mann–Whitney U test was used to compare two-way variables. Results were given as mean and (±) standard error of mean (SEM). p < 0.05 was considered statistically significant.
RESULTS
Weight gain and final body weights in the groups
The results of the 70-day diet period, the weight changes in the study groups were as given in Table 1 and Fig. 1. The animals for the HFD group had the highest body weight at the end of the experiment. It was observed that denervation led to significantly higher final body weight (CD: 384.3 ± 10.6 g, dCD: 424.8 ± 9 g, p = 0.02) in rats consuming the control diet. However, the final body weights of the dHFD group were lower compared to non-denervated rats (HFD: 460 ± 9.9 g, dHFD: 427.1 ± 14.9 g, p = 0.03).
Serum glucose, TG and insulin levels
Glucose, TG and insulin values of the groups are given in Table 1. Serum glucose levels showed no difference between the groups (p > 0.05). It was observed that serum TG value of the HFD group was higher in the non-denervated groups (CD: 69.2 ± 7.2 mg/dL, HFD: 98.6 ± 8.6 mg/dL, p = 0.02). In rats consuming control diet, bilateral retroperitoneal adipose tissue denervated resulted in higher serum TG values (CD: 69.2 ± 7.2 mg/dL, dCD: 120 ± 14.2 mg/dL, p < 0.01). However, there was a slight but insignificant decrease in serum TG values due to denervation in rats fed with HFD (HFD: 98.6 ± 8.6 mg/dL, dHFD: 80.6 ± 14.3 mg/dL, p = 0.16). In denervated groups, the serum TG value was lower in the HFD rats (dCD: 120 ± 14.2 mg/dL, dHFD: 80.6 ± 14.3, p = 0.06). Serum insulin values did not show statistically significant changes due to denervation in both CD and HFD rats (p > 0.05) but insulin levels of the HFD group were higher compared to control group rats (CD: 0.18 ± 0.04 ng/mL, HFD: 0.58 ± 0.16 ng/mL, p = 0.03).
Retroperitoneal fat tissue weights and NE levels
The total (right and left) retroperitoneal fat tissue weight due to denervation was found to be higher in rats consuming the control diet (CD: 6.0 ± 0.7 g, dCD: 9.2 ± 1.1 g, p = 0.02). The use of HFD also caused an increase in retroperitoneal fat tissue (CD: 6.0 ± 0.7 g, HFD: 9.8 ± 1.2 g, p = 0.03). However, denervation had no effect on retroperitoneal fat tissue weight in rats using HFD (HFD: 9.8 ± 1.2 g, dHFD: 9.5 ± 0.8 g, p = 0.72) (Table 1).
Following denervation, the NE levels were reduced in retroperitoneal fat tissues (Table 2). The reduction in NE verified the success of adipose tissue denervation. It was observed that the NE values decreased in the denervated control group (CD: 188.8 ± 9 ng/g wet tissue, dCD: 126 ± 11.2 ng/g wet tissue, p < 0.01).
Serum leptin, adiponectin, resistin and PAI-1 levels
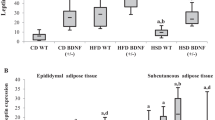
Serum leptin levels (Table 2) were higher in the HFD group (CD: 2.2 ± 0.5 ng/mL, HFD: 5.5 ± 0.9 ng/mL, p = 0.01). An increase in leptin level due to denervation was observed in the CD group (CD: 2.2 ± 0.5 ng/mL, dCD: 4.9 ± 0.9 ng/mL, p = 0.03). In rats consuming HFD, denervation was observed to cause a decrease in serum leptin levels but the reduction did not reach a statistically significant level (HFD: 5.5 ± 0.9 ng/mL, dHFD: 2.9 ± 0.3 ng/mL, p = 0.05).
The type of diet did not affect serum adiponectin levels in non-denervated groups (CD: 8.3 ± 0.4 mg/L, HFD: 8.5 ± 0.3 mg/L, p = 0.95) (Table 2). Similarly, no difference was observed between the denervated groups (p > 0.05). A significant increase in serum adiponectin level due to denervation was observed in the CD group (CD: 8.3 ± 0.4 mg/L, dCD: 9.7 ± 0.2 mg/L, p < 0.01). Similar increase was observed in the denervated HFD group (HFD: 8.5 ± 0.3 mg/L, dHFD: 9.9 ± 0.4 mg/L, p = 0.02).
Resistin levels were found to be higher in the HFD group than in the CD group (CD: 21.1 ± 1.9 ng/mL, HFD: 31.3 ± 3.6 ng/mL, p = 0.05; dCD: 22.4 ± 1.8 ng/mL, dHFD: 36.7 ± 5.8 ng/mL, p = 0.02). Denervation did not change serum resistin levels either in animals fed with control diet (CD: 21.1 ± 1.9 ng/mL, dCD: 22.4 ± 1.8 ng/mL, p = 0.79 or in the HFD group (HFD: 31.3 ± 3.6 ng/mL, dHFD: 36.7 ± 5.8 ng/mL, p = 0.87).
Diet and denervation did not lead to any significant differences in serum PAI-1 values (p > 0.05) (Table 2).
DISCUSSION
The neural feedback loop between the adipose tissue and the brain plays a crucial role in numerous physiological processes, particularly the regulation of energy homeostasis and body fat mass [3]. This mechanism can be better understood by studying the effects of intervention in nutrition and neural functioning as the factors affecting it.
The results of this study showed that denervation of white adipose tissue produces different responses in animals with different energy states caused by their diet. Significant weight gain was observed following denervation in rats fed with the control diet (Table 1). These data correlate with the findings that in denervated adipose tissue, adipocyte number and adipocyte size increase, leading to the obesity-like status [3, 9]. According to the literature, regional sympathectomy of white adipose tissue also impairs adipose thermogenesis and leaves mice susceptible to obesity [5]. In our study, the weight gain in bilaterally denervated adipose tissues only partially contributed to the increase in the body weight in the dCD group but the significant increase in triglyceride levels in this group indicates that metabolic changes may be effective in weight gain as well.
In the non-denervated groups HFD caused an increase of about 75 g in body weight compared to the CD group. However, in the denervated groups this increase was found to be only 3 g on average (Table 1). Previously, Nishi and colleagues reported a reduction in body weight due to denervation in rats fed HFD for 8 weeks [11]. Other authors also observed no difference in weights of mice with denervated white adipose tissue fed HFD for 4 weeks, which was attributed to the sympathetic tone changes due to developing obesity [12].
In addition, the reduction of weight gain in the case of positive energy balance of denervation may be dependent on a change in nutritional behavior induced by denervation
Considering the potential of the adipose tissue to regulate the whole body metabolism by using the brain-adipose tissue cross-talk via secreted adipokines, the changes in the adipokine profile with HFD may have reduced the effect of denervation in the group fed with HFD.
We have also found that denervation led to an increase in the retroperitoneal fat tissue weight in the CD group (Table 1, p = 0.02). Fat mass is controlled by the number of fat cells and the regulation of their size [3]. As was reported before, in the denervated adipose tissue, no difference was found in the lipoprotein lipase activity, which ensures the uptake of exogenous lipids into the adipose tissue and the GLUT 4 protein level, which mediates glucose transport necessary for de novo lipid synthesis [10]. However, increases in tissue weights have been attributed to decreased lipolysis due to decreased amount of NE. While leptin increases glucose utilization in tissues with its central effect, it suppresses glucose utilization as well as lipogenesis in the white adipose tissue [13]. The removal of this inhibitory effect of leptin due to denervation may contribute to the increase in the tissue weight. However, in our study no difference was found in retroperitoneal fat tissue weights between the groups consuming high-fat feed (p = 0.72). While an average increase of 4 g was observed in retroperitoneal adipose tissue in non-denervated rats due to high-fat feed consumption, an increase of 0.3 g was observed in denervated rats. The mechanisms mentioned above may be responsible for this difference.
Besides other factors, synthesis and secretion of adipokines in the white adipose tissue is controlled by the sympathetic nervous system, through catecholamines. The control of leptin synthesis and secretion by NE is well documented and many studies have shown that β-adrenoreceptor stimulation reduces leptin secretion [14, 15]. Also, leptin secretion was reduced when 3T3L1 fat cells were co-cultured in the presence of primary sympathetic neurons [14, 16]. Consistent with these results, in our study the amount of leptin was increased due to denervation in the CD group (p = 0.03). The amount of leptin in the blood is directly proportional to the fat mass in the body [17] and increased serum leptin levels in the HFD group also correlated with increased body weight in our study. However, in the dHFD group the amount of leptin was insignificantly lower than in the HFD group (Table 2, p = 0.05). Similar data demonstrating that denervation caused a decrease in the amount of leptin in rats fed with HFD for 8 weeks was reported by Nishi and colleagues [11]. The changes in leptin levels that we observed may have occurred as a result of body weight and action of the other factors, which determine serum leptin levels (such as the amount of subcutaneous adipose tissue, insulin, TNF-α), that reacted/responded to denervation. The serum leptin/adiponectin ratio is often used as an indicator of systemic inflammation in metabolic diseases [18, 19] and it has been reported that denervation increases inflammation in adipose tissue [5]. Although in our study this rate was found to be higher in the dCD group than in the CD group, it was not significant. However, denervation-related leptin/adiponectin ratio was decreased in the HFD group (p = 0.01, Table 2). This effect of denervation might be regulated by such factors as calcitonin gene-related peptide and substance P, which are secreted from the sensory nerves innervating the adipose tissue and play an important role in local inflammation [5].
Adiponectin synthesis is suppressed by the sympathetic nervous system [3]. In our study, we found that serum adiponectin levels were higher in the denervated groups due to the removal of inhibition, regardless of the diet type (Table 2). There are data reporting that chronic treatment of diabetic (db/db) mice with β-adrenoreceptor agonists resulted in increased plasma adiponectin levels and decreased insulin levels [20]. Adiponectin is an adipokine that increases insulin sensitivity. Increased adiponectin levels increases fatty acid transfer, oxidation and energy loss in the muscles [23]. Besides the direct effect of β-adrenergic activation on lipolysis and insulin signaling molecules, it also indirectly enhances insulin resistance by decreasing the amount of adiponectin that increases insulin sensitivity [21]. However, in our study, denervation did not change the concentration of resistin, which has an important role in insulin resistance (Table 2). There were also no differences between the PAI-1 values in our study (Table 2).
Taken together, our data demonstrate that while denervation mimicked obesity in the control diet group, denervation to some extent suppressed the effects of a high fat diet in the HFD group. Although regional fat tissues are innervated by different nerve fibers, the fat tissues are communicating via the central nervous system. It has been shown that sympathetic denervation of some visceral adipose tissues is perceived by other non-denervated adipose tissues resulting in changes in tissue sizes and synthesis of some proteins [3, 5, 7, 22]. As we have shown here, in the rats consuming a control diet, denervation led to body weight gain, leptin increase, increased inflammation, hyperglycemia and hyperlipidemia. Such drastic changes probably emerged as a common metabolic result of mutual communication of all fat tissues, rather than independent reaction of a single denervated fat pad. Besides, the adipose tissue has an influence on other organs that regulate metabolism over the brain [5]. Likewise, suppression of obesity-related changes in the HFD group must have also emerged as a result of common metabolic communication. Our data confirm the suggestions that the metabolic and secretory heterogeneity in white adipose tissues is primarily due to their innervation [3, 5] and that bilateral denervation of retroperitoneal adipose tissue may cause different metabolic responses depending on the duration of denervation and energy balance.
CONCLUSION
In conclusion, it has been shown that bilateral denervation of retroperitoneal fat tissue can cause different changes in metabolic parameters and circulating adipokine levels depending on energy balance in rats. However, more comprehensive studies are needed to elucidate the nature and underlying mechanism of such different response.
REFERENCES
Frühbeck G, Yang K (2008) Adipose Tissue Protocols, Second ed. Humana Press, Totowa.
Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA (2008) Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab 95: 117–126. https://doi.org/10.1016/j.ymgme.2008.06.012
Bastard JP, Fève B (2013) Physiology and Physiopathology of Adipose Tissue. Springer, Paris.
Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM (2002) Selective parasympathetic innervation of subcutaneous and intra-abdominal fat functional implications. J Clin Invest 110: 1243–1250. https://doi.org/10.1172/JCI15736
Guilherme A, Henriques F, Bedard AH, Czech MP (2019) Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nat Rev Endocrinol 15:207–225. https://doi.org/10.1038/s41574-019-0165-y
Bartness TJ, Liu Y, Shrestha YB, Ryu V (2014) Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol 35:473–493. https://doi.org/10.1016/j.yfrne.2014.04.001
Harris RB (2012) Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 20:1355–1364. https://doi.org/10.1038/oby.2012.95
Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ (2014) Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 537:199–225. https://doi.org/10.1016/B978-0-12-411619-1.00011-2
Cousin B, Bascands-Viguerie N, Kassis N, Nibbelink M, Ambid L, Casteilla L, Pénicaud L (1996) Cellular changes during cold acclimatation in adipose tissues. J Cell Physiol 167:285–289. https://doi.org/10.1002/(SICI)1097-4652(199605)167:2<285::AID-JCP12>3.0.CO;2-7
Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF, Penicaud L (2013). Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 133: 2255–2262. https://doi.org/10.1210/endo.133.5.8404678
Nishi EE, Ferreira GR, Garcia ML, Campos RR, Bergamaschi CMT (2020). Effects of white adipose tissue denervation on cardiovascular and renal parameters in wistar rats treated with high-fat diet. FASEB Journal 34: 1–1. https://doi.org/10.1096/fasebj.2020.34.s1.08969
Zhu Q, Shen M, Liu X, Shi H (2016) Effects of adipose tissue denervation on fat cell metabolism. Endocr Rev 37:i1–i1699 https://endo.confex.com/endo/2016endo/webprogram/Paper27034.html
Bonzón-Kulichenko E, Fernández-Agulló T, Moltó E, Serrano R, Fernández A, Ros M, Carrascosa JM, Arribas C, Martínez C, Andrés A, Gallardo N (2011) Regulation of insulin-stimulated glucose uptake in rat white adipose tissue upon chronic central leptin infusion: effects on adiposity. Endocrinology 152:1366–1377. https://doi.org/10.1210/en.2010-0858
Cammisotto PG, Bukowiecki LJ (2002) Mechanisms of leptin secretion from white adipocytes. Am J Physiol Cell Physiol 283:C244–C250. https://doi.org/10.1152/ajpcell.00033.2002
Ricci MR, Lee MJ, Russell CD, Wang Y, Sullivan S, Schneide SH, Brolin RE, Fried SK (2005) Isoproterenol decreases leptin release from rat and human adipose tissue through posttranscriptional mechanisms. Am J Physiol Endocrinol Metab 288:E798–E804. https://doi.org/10.1152/ajpendo.00446.2004
Turtzo LC, Marx R, Lane MD (2001) Cross-talk between sympathetic neurons and adipocytes in coculture. Proc Natl Acad Sci USA 98:12385–12390. https://doi.org/10.1073/pnas.231478898
Vázquez-Vela ME, Torres N, Tovar AR (2008) White adipose tissue as endocrine organ and its role in obesity. Arch Med Res 39:715–728. https://doi.org/10.1016/j.arcmed.2008.09.005
Jialal I, Adams-Huet B, Duong F, Smith G (2014) Relationship between retinol-binding protein-4/adiponectin and leptin/adiponectin ratios with insulin resistance and inflammation. Metab Syndr Relat Disord 12:227–230. https://doi.org/10.1089/met.2014.0013
López-Jaramillo P, Gómez-Arbeláez D, López-López J, López-Lópe C, Martínez-Ortega J, Gómez-Rodríguez A, Triana-Cubillos S (2014) The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm Mol Biol Clin Investig 18:37–45. https://doi.org/10.1515/hmbci-2013-0053
Fu L, Isobe K, Zeng Q, Suzukawa K, Takekoshi K, Kawakami Y (2007) β-adrenoceptor agonists downregulate adiponectin, but upregulate adiponectin receptor 2 and tumor necrosis factor-alpha expression in adipocytes. Eur J Pharmacol 569:155–162. https://doi.org/10.1016/j.ejphar.2007.05.005
Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R (2001) Adiponectin gene expression is inhibited by β-adrenergic stimulation via protein kinase A in 3T3-L1 adipocytes. FEBS Lett 507:142–146. https://doi.org/10.1016/s0014-5793(01)02960-x
Foster MT, Bartness TJ (2006) Sympathetic but not sensory denervation stimulates white adipocyte proliferation. Am J Physiol Regul Integr Comp Physiol 291:R1630–R1637. https://doi.org/10.1152/ajpregu.00197.2006
Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T (2001) The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7(8): 941–946. https://doi.org/10.1038/90984
Funding
This study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (114S147).
Author information
Authors and Affiliations
Contributions
The basic idea and study design: C.K., animal experiments A.A., data collection and analysis: T.A.R., N.S., İ.İ.A., and manuscript writing and edit: A.A, İ.A.
Corresponding author
Ethics declarations
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
Rights and permissions
About this article
Cite this article
Kahraman, C., Rendi, T.A., Sağlam, N. et al. Reciprocal Effects of Adipose Tissue Denervation and High Fat Diet on Serum Metabolic Parameters and Adipokine Levels in Rats: A Long Term Study. J Evol Biochem Phys 58, 410–417 (2022). https://doi.org/10.1134/S0022093022020090
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022020090