Abstract
The effect of ranged short-term (24 h) hypoxia on morphological and functional parameters of hemocytes in the Pacific oyster (Crassostrea gigas) were investigated using flow cytometry and light microscopy. The control group was kept at 100% oxygen saturation, experimental groups were exposed to moderate (30% oxygen saturation) and severe hypoxia (3% oxygen saturation). Hypoxia had no effect on morphometric parameters of hemocytes, but induced considerable changes in their functional characteristics, leading to shifts in the cellular composition of hemolymph. In the oysters exposed to moderate oxygen deficiency, a compensatory response consisted in an increase in the granulocyte count (by 20%) and an increase in the spontaneous production of reactive oxygen species in agranulocytes (by 40%) and granulocytes (by 90%). Severe short-term hypoxia inhibited the ability of hemocytes to generate an oxidative burst and induced a decrease in the granulocyte count, indicating the inability of oysters to maintain normal functional state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Oxygen deficiency is one of the most significant environmental factors that influence the vital activity of aquatic organisms [1]. In the littoral and sublittoral zones, hypoxia can ensue due to natural cycles of fluctuations in dissolved oxygen levels or eutrophication [2]. The duration of hypoxic exposure under natural conditions can vary from several hours to several months. Persistent hypoxia is considered to be the main reason for the decline in the biodiversity of aquatic habitats [3]. Short-term hypoxia, in turn, can negatively affect the functional state of aquatic organisms. Benthic and sedentary aquatic species, including bivalve mollusks, are particularly susceptible to hypoxia [4]. It should be noted that many bivalvian species are intensively cultivated all over the world, gaining not only biological, but also economic significance [5]. The Pacific oyster (Crassostrea gigas, Thunberg 1973) is considered one of the main worldwide objects of aquaculture because of its high growth rate, euryhalinity, high adaptive potential toward oxygen deficiency and temperature fluctuations [6]. The optimal oxygen regime for aquatic organisms, specifically for bivalve mollusks, is the dissolved oxygen concentration in water at a level of 7.5–9 mg O2/L. However, C. gigas often inhabits shallow coastal areas characterized by eutrophication and poor water mixing, due to which such areas may become hypoxic [7, 8, 9]. It was noted that with a decrease in the oxygen concentration below 3 mg O2/L, in the oyster organism, oysters reveal various physiological disorders, low resistance to bacterial pathogens, as well as reduced growth rate and survival rate of individual mollusks [10, 11, 12, 13, 14].
In the Black Sea, the Pacific oyster has been cultivated for more than 40 years [15]. For farmed oyster species, a deeper insight into the mechanisms and consequences of the impact of natural environmental factors on the immune system is of great importance. The latter arouses a high interest in studying the influence of environmental stress factors on the ability of the immune system to resist infectious agents [16]. Hemocytes circulating in the mollusk hemolymph are considered to be the main cell type that reflects the physiological status of an organism due to their extensive functional role, specifically the involvement in shell regeneration, digestion, transport of nutrients, and protective immune responses [16, 17, 18, 19].
The cellular immune response in mollusks includes phagocytosis, encapsulation, enzymatic destruction of pathogens, and generation of reactive oxygen species (ROS) [20]. The humoral component of the immune system in bivalve mollusks is based on the production of C-type lectins, antimicrobial and peptidoglycan-recognizing proteins, and some other compounds [21, 22, 23]. Many works have been devoted to the impact of hypoxia on the functional state of hemocytes and their ability to generate an immune response. For example, the incubation of Perna viridis under conditions of oxygen deficiency elicits a decrease in ROS production [23, 24]. On the other hand, in Mytilus galloprovincialis, Mytilus coruscus and Chlamys farreri, short-term hypoxia leads to an increase in this parameter [25, 26, 27]. It is well known that oxygen deficiency leads to a decrease in the total hemocyte count [24, 25, 28, 29] and a change in their ratio in hemolymph [25, 26]. The ratio of hemocyte types is considered as an indicator of the effectiveness of the cellular immune status of an organism, since granular hemocytes are more capable of eliciting an immune response compared to their agranular counterparts [30]. At the same time, the proportion of granular hemocytes in M. coruscus decreased after incubation at 2 mg O2/L [25], while in M. galloprovincialis, this parameter increased after a daily incubation at 0.3 mg O2/L [26]. Based on the above data, it can be assumed that the hypoxic effect, depending on the degree and duration, can exert both a stimulatory and an inhibitory effect on the cellular immune component in mollusks. The boundaries of the adaptive potential to oxygen deficiency is especially important to assess for mass aquaculture objects, since during the production cycle, e.g., transportation, sizing and redistribution across oyster banks, mollusks often face short-term oxygen deficiency, which may last up to 24 h. At the same time, a short-term but deep hypoxic exposure can transcend the adaptive potential of cultivated objects.
The goal of this study was to explore the effect of ranged short-term hypoxia on morphological and functional characteristics of hemolymph cell components in a mass aquaculture object, the bivalve Pacific oyster Crassostrea gigas (Thunberg, 1793).
MATERIALS AND METHODS
Pacific oysters C. gigas (n = 30, weight 8.6 ± 0.4 g, shell length 25.4 ± 1.4 mm) were obtained from an oyster-mussel farm (Sevastopol Bay, Sevastopol). To adapt to laboratory conditions and relieve transportation-induced stress, oysters were kept for a week in containers with running seawater (oxygen content 7.9 mg/L, i.e. 100% oxygen saturation of water; temperature 22°C, salinity 19.5 ‰, pH 8.1 ± 0.01). During acclimation, the mollusks were fed with a mixture of microalgae (5–10 mL/50 L of aquarium water; cell concentration 2–3 × 106/mL). The control group of mollusks (n = 10) was kept at an oxygen concentration of 7.9 mg/L. Hypoxia was created in vivo by blowing gaseous nitrogen through seawater for 1.5–2 h until a dissolved oxygen concentration of 30% (2.4 mg/L; n = 10) and 3% (0.2 mg/L; n = 10) versus the reference level. Upon reaching a desired level of hypoxic exposure, the mollusks were kept in oxygen-deficient water for 1 day. The dissolved oxygen concentration in experimental and control aquaria was monitored using a Starter 300D portable dissolved oxygen meter (Ohaus, USA) with a temperature sensor. The constancy of the dissolved oxygen concentration in experimental aquaria was achieved by a periodic water aeration. Salinity and pH were monitored using an ST20S salt meter (Ohaus, USA) and ST2100-F pH meter (Ohaus, USA). The pH of seawater, salinity, and temperature were identical in both control and experimental groups and corresponded to the acclimation period.
At the end of 24-h hypoxic exposure, hemolymph was collected. Hemolymph samples (0.5–1 mL) were taken from the heart via a sterile syringe and washed thrice in sterile filtered seawater (300 g, 5 min). Samples obtained from the same mollusk were analyzed individually. The morphometric characteristics of hemocytes sedimented by centrifugation were assessed on smears stained using the Pappenheim method [31]. At least 1000 cells were analyzed per smear. In each hemocyte, the morphological parameters were assessed, and the largest cell and nucleus diameters were measured (disregarding the pseudopodia). The nucleus-to-cytoplasmic ratio was calculated as the ratio of the nucleus diameter to the cell diameter [32, 33]. The functional characteristics of hemocytes were analyzed using flow cytometry (FC500 cytometer, Beckman Coulter) and Flowing Software 5.2. To assess the DNA content and proliferative cell activity, hemocytes were stained with SYBR Green I Dye (Sigma Aldrich) as described previously [34]. The types of hemocytes were identified and their percentage versus the total cell count in the suspension were carried out on two-parameter cytograms based on the distribution of SYBR Green-positive particles by their relative size (forward scatter, FS) and granularity (lateral scatter, SS). The hemocyte mortality rate was determined using propidium iodide (PI, Sigma Aldrich), a membrane-impermeable fluorescent DNA stain [32]. The ability of hemocytes to spontaneously produce ROS was assessed by the fluorescence of 2-7-dichlorofluorescein diacetate (DCF-DA) dye (Merck, Germany) according to the standard staining protocol [32].
Statistical data processing was carried out using the RStudio version 4.0.5 software. The Kolmogorov–Smirnov test showed that the distribution of hemocyte dimensional characteristics disobeys a normal distribution. To analyze the significance of the hypothesis on the presence of differences between the samples of microscopic results, the Mann–Whitney U-test was used. The results of cytometric studies were analyzed using one-way ANOVA and the Tukey’s test. The critical significance level was taken as 0.05. The results were presented as S x ± SE (mean and the error of the mean).
RESULTS
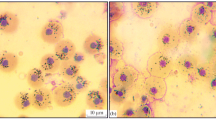
Using light microscopy, three type of hemocytes were identified in the oyster hemolymph: agranulocytes, hyalinocytes, and granulocytes (Figs. 1a–1c). Agranulocytes are the smallest cells with a diameter of 8.9 ± 0.4 µm characterized by a predominantly round shape, high nucleus-to-cytoplasmic ratio (0.6 ± 0.03 a.u.), and the absence of cytoplasmic granular inclusions and pseudopodia.
The cells with a largest diameter, granulocytes, had a low nucleus-to-cytoplasmic ratio (0.4 ± 0.04 a.u.); their cytoplasm contained basophilic and eosinophilic granular inclusions, and the nuclei were mainly located excentrically. Besides, granular cells formed pseudopodia. The diameter of hyalinocytes was 10.2 ± 0.8 µm, the diameter of their nuclei was 5.7 ± 0.4 µm. Hyalinocytes, like granulocytes, had pseudopodia, but their cytoplasm lacked granular inclusions, while the nucleus was mainly centrally located. The fluorescence peak of a DNA-specific dye SYBR Green I in control and experimental groups corresponded to a diploid set of chromosomes without signs of division (Fig. 2a). Using flow cytometry and based on the distribution of particles by forward (FS) and side (SS) scattering, 3 subpopulations of cells with different relative size and granularity level were identified (Fig. 2b). Agranular cells, agranulocytes (37.5 ± 14.2%) and hyalinocytes (58.8 ± 15.7%), were predominant in the hemolymph of oysters. The relative granulocyte count in the control sample was 8.8 ± 4.6%. The classification of hemocytes is described in more detail in our previous work [35].
24-h hypoxia had no effect on the morphometric characteristics of oyster hemocytes, however, it significantly affected the ratio of hemocyte types in hemolymph (Fig. 3). Incubation under conditions of 30% oxygen saturation of water led to a significant increase in the relative granulocyte count (by 20%, p < 0.05) (Fig. 3c), while the percentage of agranulocytes and hyalinocytes remained intact. After one-day incubation under conditions of 3% oxygen level of that under normoxia, the relative agranulocyte count increased by 58% (Fig. 3a), while the hyalinocyte count decreased by 50% (Fig. 3b). The hemocyte mortality rate did not exceed 2% in both control and experimental groups. No hypoxia-induced changes in the proliferative activity of hemolymph cells were observed.
Incubation of oysters under conditions of 30% oxygen saturation of water led to an increase in the spontaneous ROS production: in agranulocytes, on average, by 40% (Fig. 4a) and in granulocytes by more than 90%. At the same time, moderate hypoxic load had no significant effect on ROS production by hyalinocytes. Incubation in 3% oxygen-saturated water inhibited ROS production in all cell types (Fig. 4).
Morphological characteristics of hemocytes in the Pacific oyster Crassostrea gigas under normoxic and hypoxic conditions. (a) Normoxia. (b) 30% oxygen saturation of water. (c) 3% oxygen saturation of water. 1—agranulocytes, 2—hyalinocytes, 3—granulocytes. Smears stained using a combined Pappenheim method were viewed under a light microscope (Biomed PR-2 Lum) equipped with a camera (Levenhuk C NG Series). Scale bar, 10 µm.
Subpopulations of hemocytes in the Pacific oyster as identified by flow cytometry. (a) DNA content. Hemocytes were suspended in sterile filtered seawater (cell concentration 1–2 × 106/mL–1), incubated with SYBR Green I for 40 min in the dark (final dye concentration in the smple 10 µM). CV—Coefficient of variation. (b) Hemocyte distribution based on relative size (forward scatter, FS) against granularity (side scatter, SS) values to reveal three hemocyte subpopulations: 1—agranulocytes, 2—hyalinocytes, 3—granulocytes. The plots are shown for the control group of mollusks.
Effect of short-term hypoxia on the ratio of hemocyte types in the Pacific oyster Crassostrea gigas. (a) Changes in the relative agranulocyte count. (b) Changes in the relative hyalinocyte count. (c) Changes in the relative granulocyte count. Mollusks were divided into three groups, which were held in water with different oxygen concentrations: c—control group, 100% oxygen saturation (n = 10); 30 h—experimental group, 30% oxygen saturation (n = 10); 3 h—experimental group, 3% oxygen saturation (n = 10). Hemocyte suspension was stained with a DNA dye SYBR Green I (final concentration in a sample, 10 µmol/L; incubation time, 40 min in the dark) to identify cell types in a Beckman Coulter FC500 flow cytometer. The ratio of cell types was assessed on the forward scatter vs. side scatter (FS/SS) histogram. *—Significant differences between control and experimental groups (p < 0.05).
Effect of oxygen deficiency on the ability of Pacific oyster Crassostrea gigas hemocytes to spontaneously generate reactive oxygen species (ROS). (a) Agranulocytes. (b) Hyalinocytes. (c) Granulocytes. The mollusks were kept for 24 h under conditions of oxygen deficiency: 30 h—experimental group, 30% oxygen saturation (n = 10); 3 h—experimental group, 3% oxygen saturation (n = 10). Spontaneous ROS production was by the fluorescence intensity of hemocytes stained with DCF-DA (final concentration in the sample, 10 µmol/L). Dye fluorescence was analyzed in the flow cytometer fluorescence channel 1 (FL1; 530/30 nm band pass). DCF-DA fluorescence intensity level is presented on the graph as % of the control level. *—Significant differences between control and experimental groups (p < 0.05).
DISCUSSION
It is well known that, in bivalve mollusks, oxygen deficiency often causes a decline in the total count of circulating hemocytes and changes in the ratio of their types [23, 25, 28]. In our study, a deeper hypoxic load (3% of the normoxic level) led to a reduction in the percentage of granulocytes and hyalinocytes, as well as an increase in the proportion of agranulocytes in oyster hemolymph. The opposite effect, an increase in the percentage of granulocytes, was detected in the group of oysters after incubation at 30% oxygen saturation of water.
Among the possible reasons underlying changes in the cellular composition of the oyster hemolymph, the following are most significant:
1. Proliferative activity of hemocytes and their precursors [25];
2. Death of a certain type of hemocytes [23];
3. Functional changeovers of one type of hemocytes into another (granulation vs. degranulation) [36, 37];
4. Migration of hemocytes into tissues [38].
Presumably, agranular cells represent immature hemocytes, the proliferation of which can occur directly in the hemolymph [39, 40, 41, 42]. Within a short-term experiment, it is unlikely that a change in the ratio of hemocyte types can be elicited by the proliferative activity in hematopoietic tissue. This is supported by the data on the absence of dividing hemocytes in oyster hemolymph samples in both experimental groups. Similarly, there were no changes in the proportion of dead cells in hemolymph during hypoxia. At the same time, granulocytes are characterized by degranulation and, as a consequence, an increase in the relative proportion of agranular cells in hemolymph [43]. The possible migration of granulocytes into tissues should also not be ruled out, since this type of cells is known to be able to actively relocate from the hemolymph vessels to the gills, mantle, and other tissues and organs of mollusks [44]. Thus, short-term changes in the cellular composition of hemolymph during hypoxia are implemented via rapid adaptive rearrangements, among which the most probable are degranulation of granulocytes and/or their migration into tissues under deep hypoxia, as well as the functional transition of agranulocytes into granulocytes at a moderate lack of oxygen. It is noteworthy that an increase in the proportion of granular cells at 30% oxygen saturation of water and its decrease after one-day exposure to deep hypoxia (3% of oxygen saturation of water of the normoxic level) may reflect the development of a compensatory adaptive response in C. gigas under conditions of moderate hypoxic load. The latter assumption is further confirmed by an increase in the level of DCF-DA fluorescence in agranulocytes and granulocytes after incubation of oysters at 30% oxygen saturation and its reduction in all cell types after 24-h hypoxia at 3% oxygen saturation of water. The changes we revealed in the level of spontaneous ROS production are generally consistent with the literature data. For example, in M. coruscus and C. farreri, oxygen deficiency induced an increase in ROS production [25], while the opposite effect was observed in P. viridis [23, 24]. The mechanisms underlying the effect of oxygen deficiency on the ability of hemocytes to generate an oxidative burst remain a matter of debate. The decrease in ROS production is thought to be due to a metabolic adjustment with the involvement of the HIF factor in response to hypoxic exposure [45]. It is also likely that the increase we detected in the ROS concentration in oyster hemocytes is a consequence of the reorganization of the mitochondrial respiratory chain, since mitochondria represent the main source of ROS in C. gigas hemocytes, and the lack of oxygen can induce changes in the mitochondrial electron transport chain [46, 47]. This assumption is indirectly confirmed by the data that the intracellular level of ROS in oyster hemolymph cells correlates with changes in the mitochondrial membrane potential under hypoxia [14].
Thus, the results of this work indicate that the pattern of changes in oyster hemolymph parameters is determined not only by the duration of hypoxic exposure, but also by the concentration of dissolved oxygen. The increase in the relative granulocyte count and the ability to generate an oxidative burst probably indicate the development of a compensatory response in the Pacific oyster due to exposure to a moderate lack of oxygen. Apparently, short-term incubation under conditions of 30% dissolve oxygen saturation occurs within the range of C. gigas tolerance to hypoxia. Deep hypoxia, in turn, induced a decrease in the percentage of granulocytes in hemolymph and a suppression of ROS production in hemocytes, exerting a suppressive effect on the cellular immune response in oysters.
REFERENCES
Paulmier A, Ruiz-Pino D (2009) Oxygen minimum zones (OMZs) in the modern ocean. Prog Oceanogr 80(3–4): 113–128. https://doi.org/10.1016/j.pocean.2008.08.001
Howarth R, Chan F, Conley DJ, Garnier J, Doney SC, Marino R, Billen G (2011) Coupled biogeochemical cycles: eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front Ecol Environ 9(1): 18–26. https://doi.org/10.1890/100008
Sirakov I, Slavcheva-Sirakova D (2015) The influence of climate changes on the hydrobionts: a review. JBES 6(3): 315–329.
Weinstock JB, Collin R (2021) Hypoxia and warming are associated with reductions in larval bivalve abundance in a tropical lagoon. Mar Ecol Prog Ser 662: 85–95. https://doi.org/10.3354/meps13630
Wijsman JWM, Troost K, Fang J, Roncarati A (2019) Global production of marine bivalves. Trends and challenges. In: Goods and services of marine bivalves. Springer, Cham, pp 7–26.
Harris J (2008) Pacific oyster, Crassostrea gigas (Thunberg, 1793). In: Aquatic Invasive Species Profile Aquat Invasions. pp 1–12.
Gray JS, Wu RSS, Or YY (2002) Effects of hypoxia and organic enrichment on the coastal marine environment. Mar Ecol Prog Ser 238:249–279. https://doi.org/10.3354/meps238249
Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160(8): 1875–1888. https://doi.org/10.1007/s00227-012-1954-1
Wu RS (2002) Hypoxia: from molecular responses to ecosystem responses. Mar Pollut Bull 45(1–12): 35–45. https://doi.org/10.1016/s0025-326x(02)00061-9
Baker SM, Mann R (1992) Effects of hypoxia and anoxia on larval settlement, juvenile growth, and juvenile survival of the oyster Crassostrea virginica. Biol 182(2): 265–269. https://doi.org/10.1128/AEM.00317-08
Macey B M, Achilihu IO, Burnett KG, Burnett LE (2008) Effects of hypercapnic hypoxia on inactivation and elimination of Vibrio campbellii in the Eastern oyster, Crassostrea virginica. Appl Environ Microbio 74(19): 6077–6084. https://doi.org/10.1016/j.jprot.2018.12.009
Khan B, Ringwood AH (2016) Cellular biomarker responses to hypoxia in eastern oysters and Atlantic ribbed marsh mussels. Mar Ecol Prog Ser 546: 123–133. https://doi.org/10.3354/meps11622
Sokolov EP, Markert S, Hinzke T, Hirschfeld C, Becher D, Ponsuksili S, Sokolova IM (2019) Effects of hypoxia-reoxygenation stress on mitochondrial proteome and bioenergetics of the hypoxia-tolerant marine bivalve Crassostrea gigas. J Proteom 194: 99–111. https://doi.org/10.1016/j.jprot.2018.12.009
Andreyeva AY, Gostyukhina OL, Kladchenko ES, Vodiasova EA, Chelebieva ES (2021) Acute hypoxic exposure: effect on hemocyte functional parameters and antioxidant potential in gills of the Pacific oyster, Crassostrea gigas. Mar Environ Res 169: 105389. https://doi.org/10.1016/j.marenvres.2021.105389
Zolotarev V (1996) The Black Sea ecosystem changes related to the introduction of new mollusc species. Marine Ecology 17: 227–236. https://doi.org/10.1111/j.1439-0485.1996.tb00504.x
Allam B, Raftos D (2015) Immune responses to infectious diseases in bivalves. J Invertebr Pathol 131: 121–136. https://doi.org/10.1016/j.jip.2015.05.005
Fisher WS (1988) Environmental influence on bivalve hemocyte function. Am Fish Soc Symp 18: 225–237.
Auguste M, Balbi T, Ciacci C, Canonico B, Papa S, Borello A, Canesi L (2020) Shift in immune parameters after repeated exposure to nanoplastics in the marine bivalve Mytilus. Front Immunol 11: 426. https://doi.org/10.3389/fimmu.2020.00426
Loker ES, Adema CM, Zhang SM, Kepler TB(2004) Invertebrate immune systems–not homogeneous, not simple, not well understood. Immunol Rev 198(1): 10–24. https://doi.org/10.1111/j.0105-2896.2004.0117.x
Allam B, Espinosa EP (2016) Bivalve immunity and response to infections: are we looking at the right place? Fish Shellfish Immunol 53: 4–12. https://doi.org/10.1016/j.fsi.2016.03.037
Wootton EC, Dyrynda EA, Ratcliffe NA (2003) Bivalve immunity: comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua). Fish Shellfish Immunol. 15(3): 195–210. https://doi.org/10.1016/S1050-4648(02)00161-4
Rodrigues J, Brayner FA, Alves LC, Dixit R, Barillas-Mury C (2010) Hemocyte differentiation mediates innate immune memory in Anopheles gambiae mosquitoes. Science 329(5997): 1353–1355. https://doi.org/ 10.1126/science.1190689
Wang Y, Hu M, Shin PK, Cheung SG (2011) Immune responses to combined effect of hypoxia and high temperature in the green-lipped mussel Perna viridis. Mar Pollut Bull 63: 201–208. https://doi.org/10.1016/j.marpolbul.2011.05.035
Wang Y, Hu M, Cheung SG, Shin PKS, Lu W, Li J (2012) Immune parameter changes of hemocytes in green-lipped mussel Perna viridis exposure to hypoxia and hyposalinity. Aquaculture 356: 22–29. https://doi.org/10.1016/j.aquaculture.2012.06.001
Sui Y, Kong H, Shang Y, Huang X, Wu F, Hu M, Wang Y (2016) Effects of short-term hypoxia and seawater acidification on hemocyte responses of the mussel Mytilus coruscus. Mar Pollut Bull 108: 46–52. https://doi.org/10.1016/j.marpolbul.2016.05.001
Andreyeva AY, Efremova ES, Kukhareva TA (2019) Morphological and functional characterization of hemocytes in cultivated mussel (Mytilus galloprovincialis) and effect of hypoxia on hemocyte parameters. Fish Shellfish Immunol 89: 361–367.
Chen MY, Yang HS, Delaporte M, Zhao SJ, Xing K (2007) Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J Exp Mar Biol Ecol 345(1): 52–60. https://doi.org/10.1016/j.jembe.2007.01.007
Nogueira L, Mello DF, Trevisan R, Garcia D, da Silva Acosta D, Dafre AL, de Almeida EA (2017) Hypoxia effects on oxidative stress and immunocompetence biomarkers in the mussel Perna perna (Mytilidae, Bivalvia). Mar Environ Res 126: 109–115. https://doi.org/10.1016/j.marenvres.2017.02.009
Matozzo V, Monari M, Foschi J, Papi T, Cattani O, Marin MG (2005) Exposure to anoxia of the clam Chamelea gallina: I. Effects on immune responses. J Exp Mar Biol 325(2): 163–174. https://doi.org/10.1016/j.jembe.2005.04.030
Wang W, Li M, Wang L, Chen H, Liu Z, Jia Z, Song L (2017) The granulocytes are the main immunocompetent hemocytes in Crassostrea gigas. Dev Comp Immunol 67: 221–228. https://doi.org/10.1016/j.dci.2016.09.017
Piaton E, Fabre M, Goubin Versini I, Bretz Grenier MF, Courtade Saïdi M, Vincent S, Cochand Priollet B (2016) Guidelines for May Grünwald–Giemsa staining in haematology and non gynaecological cytopathology: recommendations of the French Society of Clinical Cytology (SFCC) and of the French Association for Quality Assurance in Anatomic and Cytologic Pathology (AFAQAP). Cytopathology 27(5): 359–368. https://doi.org/10.1111/cyt.12323
Kladchenko ES, Andreyeva AY, Kukhareva TA, Soldatov AA (2020). Morphologic, cytometric and functional characterisation of Anadara kagoshimensis hemocytes. Fish Shellfish Immunol 98:1030–1032.
Carballal MJ, Lopez MC, Azevedo C, Villalba A (1997) Hemolymph cell types of the mussel Mytilus galloprovincialis. Diseases of aquatic organisms 29(2):127–135.
Andreyeva AY, Kladchenko ES, Kukhareva TA, Sakhon EG (2019) Analysis of Cell Cycle and Morphological and Functional Abnormalities of Mytilus galloprovincialis Lam., 1819 (Bivalvia) Hemocytes from Coastal Ecosystems near Sevastopol, Crimea. Inland Water Biol 12(2): 96–103.
Andreyeva AY, Kladchenko ES, Vyalova OY, Kukhareva TA (2021) Functional Characterization of the Pacific Oyster, Crassostrea gigas (Bivalvia: Ostreidae), Hemocytes Under Normoxia and Short-Term Hypoxia. Turkish J Fish Aquat Sci 21(3):125–133.
Foley DA, Cheng TC (1977) Degranulation and other changes of molluscan granulocytes associated with phagocytosis. J Invertebr Pathol 29(3): 321–325. https://doi.org/10.1016/S0022-2011(77)80037-2
Rebelo MDF, Figueiredo EDS, Mariante RM, Nóbrega A, de Barros CM, Allodi S (2013) New insights from the oyster Crassostrea rhizophorae on bivalve circulating hemocytes. PLoS One 8(2): e57384. https://doi.org/10.1371/journal.pone.0057384
Lau YT, Gambino L, Santos B, Espinosa EP, Allam B (2018) Transepithelial migration of mucosal hemocytes in Crassostrea virginica and potential role in Perkinsus marinus pathogenesis. J Invertebr Pathol 153: 122–129. https://doi.org/10.1016/j.jip.2018.03.004
Ottaviani E, Franchini A, Barbieri D, Kletsas D (1998) Comparative and morphofunctional studies on Mytilus galloprovincialis hemocytes: Presence of two aging-related hemocyte stages. Ital J Zool 65(4):349–354. https://doi.org/10.1080/11250009809386772
Delaporte M, Synard S, Pariseau J, McKenna P, Tremblay R, Davidson J, Berthe FC (2008) Assessment of haemic neoplasia in different soft shell clam Mya arenaria populations from eastern Canada by flow cytometry. J Invertebr Pathol 98(2):190–197. https://doi.org/10.1016/j.jip.2007.12.005
Aladaileh S, Nair SV, Birch D, Raftos DA (2007) Sydney rock oyster (Saccostrea glomerata) hemocytes: morphology and function. J Invertebr Pathol 96(1):48–63. https://doi.org/10.1016/j.jip.2007.02.011
Cima F, Matozzo V (2018) Proliferation and differentiation of circulating haemocytes of Ruditapes philippinarum as a response to bacterial challenge. Fish Shellfish Immunol 81:73–82. https://doi.org/10.1016/j.fsi.2018.07.010
de Freitas Rebelo M, de Souza Figueiredo E, Mariante RM, Nóbrega A, de Barros CM, Allodi S (2013) New insights from the oyster Crassostrea rhizophorae on bivalve circulating hemocytes. PLoS One 8(2):e57384. https://doi.org/10.1371/journal.pone.0057384
Huang J, Li S, Liu Y, Liu C, Xie L, Zhang R (2018) Hemocytes in the extrapallial space of Pinctada fucata are involved in immunity and biomineralization. Sci Rep 8(1): 1–11. https://doi.org/10.1038/s41598-018-22961-y
Michiels C, Minet E, Mottet D, Raes E (2002) Regulation of gene expression by oxygen: NF-kappaB and HIF-1, two extremes. Free Radic Biol Med 33:1231–1242. https://doi.org/10.1016/S0891-5849(02)01045-6
Donaghy L, Kraffe E, Le Goïc N, Lambert C, Volety AK, Soudant P (2012) Reactive oxygen species in unstimulated hemocytes of the Pacific oyster Crassostrea gigas: a mitochondrial involvement. PloS one 7(10): e46594. https://doi.org/10.1371/journal.pone.0046594
Donaghy L, Artigaud S, Sussarellu R, Lambert C, Le Goïc N, Hégaret H, Soudant P (2013) Tolerance of bivalve mollusc hemocytes to variable oxygen availability: a mitochondrial origin? Aquat Living Resour 26(3): 257–261. https://doi.org/10.1051/alr/2013054
Funding
The effect of hypoxia on the morphological parameters of hemocytes was studied within the framework of the state assignment No. 21102500161-4 “Organization of the immune system of cultivated aquatic organisms and the influence of environmental factors on the functioning of organism defense systems”. The study of the effect of oxygen deficiency on the functional parameters of hemocytes was supported by a grant of the President of the Russian Federation for the state support of young Russian PhD researchers (project No. MK609.2020.4).
Author information
Authors and Affiliations
Contributions
Conducting experiments and data analysis (E.S.K., A.Yu.A., T.A.K.); graphic data representation and statistical data processing (E.S.K., T.A.K.); preparing and correcting a manuscript (A.Yu.K.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no evident or potential conflict of interest in relation with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 1, pp. 43–50https://doi.org/10.31857/S004445292201003X.
Rights and permissions
About this article
Cite this article
Kladchenko, E.S., Andreyeva, A.Y. & Kukhareva, T.A. Effect of Ranged Short-Term Hypoxia on Functional and Morphological Parameters of Hemocytes in the Pacific Oyster Сrassostrea gigas (Thunberg, 1793). J Evol Biochem Phys 58, 45–53 (2022). https://doi.org/10.1134/S0022093022010045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022010045