Abstract
Hydrogen sulfide is involved in the functional regulation of various organs, both in physiological conditions and in pathology, and, specifically, is a major participant of the inflammatory process. In this study, we studied the role and mechanisms of action of hydrogen sulfide in lipopolysaccharide (LPS)-induced relaxation of the capsule of bovine mesenteric lymph nodes (LNs). Isolated strips of the LN capsule were incubated for 6 h in F-10 Ham medium supplemented with LPS from Escherichia coli O55:B5. At the end of incubation, LN capsule preparations were examined on a myograph setup. Contractile properties were evaluated using phenylephrine, relaxation properties—with papaverine. The involvement of H2S and NO in LPS-induced relaxation was determined through the use of specific inhibitors. Incubation of LN capsular strips in F-10 Ham + LPS led to a sustained inhibition of phasic contractions and a decrease in the level of tonic tension of LN capsule smooth muscles due to the effect of H2S synthesized by the enzymes cystathionine-γ-lyase, cystathionine-β-synthase and 3-mercaptosulfurtransferase, as well as NO produced by inducible NO-synthase. Our data show that H2S is one of the major compounds that are quickly produced in the LNs during inflammation. We believe that H2S is a key molecule that, along with NO, triggers the inflammatory remodeling of LNs, thus promoting a rapid increase in their size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The lymphatic vasculature, which includes lymph capillaries, lymph vessels and lymph nodes (LN), is one of the main participants of inflammation and plays an important role in both the development and resolution of the inflammatory process [1, 2]. Historically, it so happened that for a long time, when studying LN functions, the main focus was on hematopoietic cells involved in antigen recognition (dendritic cells) and immune responses (B and T lymphocytes) [3, 4]. The followed the works which demonstrated the important role of LN-resident lymphatic endothelial cells (LECs) in the implementation of LN-mediated immune functions [5, 6]. It was found that the LN contains at least three distinct groups of LECs: subcapsular sinus, cortical and medullary. It was also shown that, apart from different location, different types of LECs express a variety of chemokines that regulate the migration of immune cells within the LN [7]. Moreover, it has recently been shown that LN-resident LECs are potent antigen-presenting cells involved in maintaining peripheral T cell tolerance [8]. In recent years, there has been obtained evidence that LN non-hematopoietic stromal cells perform not only support and trophic functions, but are also involved in the initiation and maintenance of immunity [9]. It is believed that various stromal cells play an active role in the immune response due to their interaction with dendritic cells and lymphocytes [10]. In order to adapt to the influx of a large number of neutrophils, macrophages, dendritic cells (DCs) and lymphocytes involved in the immune response, stromal cells are able to rapidly divide to remodel the LN [11].
A unique feature of LNs is their ability to have their size changed significantly: during inflammation, their volume can increase 10–20-fold compared to the normal size within a few days, and this growth is accompanied by proliferative expansion and remodeling of the LN vascular-stromal compartment while retaining its basic structure [12]. In this process, it is generally accepted to distinguish several phases: initiation, expansion, and recovery phases [13]. The published data from several research groups show that, among the five subsets of LN-resident stromal cells, only fibroblastic reticular cells have evident contractile abilities, expressing a-actin and smooth muscle myosin light chain [14, 15]. It is assumed that it is these cells that play the main role in LN remodeling during inflammation, although the mechanism through which they contribute to a rapid increase in the LN size remains unclear [16].
LNs, which are the main organs in the adaptive immunity system, are under intensive investigation in different laboratories [11, 17]. The Pubmed search with the key words “endothelial cells in a lymph node” yields more than 1600 publications, and with the key words “stromal cells in a lymph node” more than 1100 works over the past 15 years. At the same time, against the background of an array of publications on the role of stromal cells in the process of inflammatory LN remodeling, there are surprisingly few data on the involvement of LN capsule cells in this process. Meanwhile, as shown in our previous studies, it is the LN capsule containing a significant amount of connective tissue fibers and smooth muscle cells (SMCs) that is the most rigid component of the LN, which performs the function of its framework and determines its size and shape [18, 19].
In our later works, it was established that the contractile function of the capsule of bovine mesenteric LNs changes considerably when modeling inflammation: fast phasic contractions discontinue followed by quite a rapid tonic tension decline [20]. We have also shown that under physiological conditions, H2S is involved in the regulation of phasic and tonic contractions of the bovine mesenteric LN capsule [21]. The role of H2S in inflammation and sepsis has been a subject of debate for a long time. To date, it is commonly accepted that H2S is an important regulatory factor of organ functions in various types of pathology, including inflammation [22]. In the present work, the goal was to study the role of endogenous H2S in the regulation of the contractile function of the SMC capsule of bovine mesenteric LNs and their remodeling in LPS-induced inflammation on a model described previously [20]. Since the “active” components responsible for the biological effects of H2S are not fully identified by now [23], in this article, the term H2S will unite all the forms of H2S (including polysulfides) that will be present in solution at physiological values of pH and temperature.
MATERIALS AND METHODS
The material for this study was taken at the slaughter house of the Prinevskoye Breeding Plant CJSC. Fifteen min after exsanguination, sentinel LNs were cut out from the mesentery of the bulls (Bos taurus taurus) aged 16–18 months and washed in cold physiological saline solution (PSS). The capsule strips with a small amount of the cortical matter were excised from LNs. The capsule strips (n = 54 from 23 animals) were delivered to the laboratory in chilled (2–4°C) PSS. In the laboratory, they were thoroughly cleared from the surrounding connective tissue and pericapsular fat, and preparations were prepared for subsequent examination (strips 10 mm long and 2 mm wide). In a part of strips, the subcapsular sinus (SS) was removed. The quality control of SS endothelial removal was performed by assessing the relaxation response of the capsule strips to acetylcholine (no response indicated a high-quality deendothelialization).
The prepared LN capsule strips were divided into several groups (Table 1). The preparations were incubated in the F-10 Ham medium (DIA-M, Russia) added with benzylpenicillin (100 U/mL) and streptomycin (10 mg/mL) at 37°C for 6 h. In all groups, the F-10 Ham medium was additionally added with indomethacin (10 µM) to suppress the possible formation of vasoactive prostanoids. For the incubation of strips of groups 3 and 4, the F-10 Ham medium was added with lipopolysaccharide (LPS) from Escherichia coli O55:B5 (20 µg/mL), for strips of groups 5 and 6—with LPS + actinomycin D (RNA synthesis inhibitor), for strips of groups 7 and 8—with LPS + cycloheximide (protein synthesis inhibitor).
Upon termination of incubation, the preparations were washed twice with PSS and placed into a myograph chamber with a FORT-10 force transducer (WPI, USA). The information from the latter, after being converted in a LabMaster ADC, was recorded on the computer hard disk. The experiments were carried out at a continuous flow of PSS of the following composition (mM): NaCl 120.4; KCl 5.9; CaCl2 2.5; MgCl2 1.2; NaH2PO4 1.2; NaHCO3 15.5; glucose 11.0; bovine serum albumin 10 g/L. PSS was saturated with a gas mixture containing 95% O2 and 5% CO2. The temperature of the incubation solution in the chamber was maintained at 38.0 ± 0.1°C. The preparations were subjected to an initial tension of 16 mN. This tension value was chosen because after a similar tension and a 30-min stabilization, the level of tonic tension of the intact LN capsule strips (group 1) was set at 10.3 ± 1.2 mN, which corresponded to a transmural pressure of about 4 cm H2O. To check contractile strip responses, a hyperpotassium solution was used (with 55 mM NaCl in PSS replaced by KCl). After assessing the contractility of the preparations, the latter were washed twice with PSS.
The following reagents were used in the present study: LPS from Escherichia coli O55:B5 (100 µg/mL; Sigma-Aldrich, USA), benzylpenicillin sodium salt (100 U/mL; Kraspharma, Russia), streptomycin (10 mg/mL; Pharmasyntez, Russia). Other reagents were purchased from Sigma-Aldrich (USA) and used at the following concentrations: actinomycin D 100 nM; cycloheximide 10 µM; 1400W (N-([3-(aminomethyl)phenyl] methyl)ethanimidamide dihydrochloride) 20 µM; DL-propargylglycine (PPG) 10 µM; aminooxyacetate (aminooxyacetic acid hemihydrochloride, AOAA) 100 µM; L-aspartic acid (L-Asp) 10 µM; phenylephrine, ((R)-(–)-phenylephrine hydrochloride, PE) 100 µM; papaverine hydrochloride (PP) 40 µM; acetylcholine chloridea 1 µM; indomethacin 3 µM.
All reagents were dissolved in PSS immediately before an experiment. Actinomycin D and indomethacin were preliminarily dissolved in dimethyl sulfoxide (DMSO), cycloheximide—in ethanol, and the required volume of the concentrate/PSS was added before an experiment. DMSO diluted 1:1000 and ethanol diluted 1:2000 did not cause statistically significant changes in contractile activity parameters of the LN capsule strips. Details of experiments with the above chemicals are described in the Results section.
All experimental procedures in this study complied with the ethical standards approved by the legal acts of the Russian Federation, principles of the Basel Declaration, and recommendations of the Bioethics Committee at the Pavlov Institute of Physiology.
The results were statistically processed using StatSoft STATISTICA 6.1.478. The Shapiro–Wilk test was used to check normal distribution of data. Since the data matched the Gaussian distribution, they were presented as the arithmetic mean and standard error of the mean (M ± SEM). The Student’s t-test was used for data comparison. Differences were considered statistically significant at p < 0.05.
RESULTS
LN capsule strips (SS+) and (SS–) incubated for 6 h in F-10 Ham medium, after washing and keeping for 30 min in PSS in the myograph chamber, showed a stable tone level, against the background of which rhythmic phasic contractions were recorded. The tone value and phasic contraction parameters of the preparations are presented in Table 2. The LN capsule strips without the SS had a higher tone and a slightly higher frequency of phasic contractions compared to intact preparations.
Since there are very scarce data on the effects of LPS on the contractile function of LN capsule SMCs, we evaluated contractile activity parameters of intact and deendothelialized LN capsule strips after the addition of LPS to the incubation medium (after 1 and 6 h). A study of the preparations incubated in F-10 Ham + LPS during 1 h failed to reveal significant changes in the parameters of their contractile function compared to the preparations of groups 1 and 2 (not shown). LN capsule strips treated with LPS for 6 h (groups 3 and 4) had a lower tone level; none of the preparations of these groups showed spontaneous phasic contractions (Table 2).
To study the mechanisms of LPS action on the capsule of bovine mesenteric LNs, the medium containing LPS was added with an RNA synthesis inhibitor actinomycin D (groups 5 and 6) and a protein synthesis inhibitor cycloheximide (groups 7 and 8). After a 6-h incubation, LN capsule strips were examined according to the standard protocol. It was found that contractile activity parameters of the LN capsule strips in groups 5–8 were little different from those in groups 1 and 2. The frequency of phasic contractions of the strips in groups 5–8 were somewhat lower than in groups 1 and 2, but this decrease was insignificant and in various experiments amounted to 3–7% of the values recorded in groups 1 and 2.
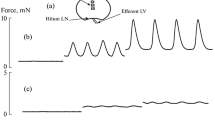
To study the relaxation properties of LN capsule strips after incubation in the F-10 Ham medium, the strips were washed twice with PSS and placed into the myograph chamber. All preparations were subjected to an initial tension of 16 mN. After a 30-min stabilization in the chamber, a stable level of strip tonic tension was established (Table 2 and Fig. 1). Then, a papaverine solution (40 µM) was injected into the myograph chamber, causing a maximum relaxation. Figure 1 shows the fragments of the two experiment records (Fig. 1a—tension changes in the LN capsule strip incubated in F-10 Ham + LPS, Fig. 1b—tension changes in the LN capsule strip incubated in F-10 Ham without LPS).
Papaverine-induced relaxation of LN capsule strips in different groups was different. The relaxation amplitude of the preparations incubated in F-10 Ham medium without LPS was significantly higher compared to that of the strips incubated with LPS. However, detailed analysis showed that both groups of preparations, under the effect of papaverine, reached approximately the same level of tension. These data are extremely interesting as they suggest that SMCs in LN capsule strips incubated with LPS were already significantly relaxed prior to papaverine exposure.
In the following experiments, in order to explore the signaling pathways that were affected (activated or inhibited) by LPS in LN capsule SMCs, leading to the dilatation of the intact (SS+) LN capsule strips, the bath solution (PSS) was added with the following enzyme inhibitors: an iNOS inhibitor 1400W, a cystathionine-γ-lyase (CSE) inhibitor DL-propargylglycine (PPG), a cystathionine-β-synthase (CBS) inhibitor aminooxyacetate (AOAA), and a 3-mercaptopurivate sulfurtransferase (3-MST) inhibitor L-Aspartic acid (L-Asp). In the first series of experiments, after the end of the incubation of LN capsule strips in F-10 Ham alone and F-10 Ham + LPS, the preparations were placed into a myograph chamber, set a standard tension, and kept for 30 min to attain stabilization. After that, phenylephrine (PE) was added to the bath solution, and after 5 min, the tonic tension value developed by the strips under the effect of PE was measured. In response to PE application, the strips reacted with a pronounced increase in tone. The contractile reactions of the strips incubated in F-10 Ham showed a greater amplitude compared to those of the strips incubated in F-10 Ham + LPS. Next, the bath solution with PE was replaced with pure PSS; after 10 min, 1400W was added to PSS, and 20 min later, PE was re-injected, and the tonic tension value was measured again and compared with the previous measurement.
On other LN capsule strips, studies were conducted along to the same protocol, but with inhibitors of the enzymes synthesizing H2S (PPG + AOAA + L-Asp) added to the bath solution. With these inhibitors of H2S-producing enzymes, the amplitude of the PE-induced contractile reaction of LN capsule strips incubated in F-10 Ham + LPS increased. In another series of experiments, to determine the role of the endothelium in LPS-induced dilatation of the LN capsule, studies were carried out on LN capsule strips with the SS removed. The experimental protocol, as well as the concentrations of the reagents, were the same. The results of these two series of experiments are shown in Fig. 2.
Since LPS had a relaxing effect on the LN capsule, whereas Fig. 2 demonstrates contractile responses of LN capsule strips, it is rather difficult to analyze these data. Therefore, here we present the results of the same experiments in an alternative way. We proceeded from the assumption that the difference in the amplitudes of PE-induced contractions of LN capsule strips incubated in F-10 Ham and F-10 Ham + LPS represents essentially a value of LPS-induced relaxation. For this reason, Fig. 3 shows the values of LPS-induced relaxation of LN capsule strips (intact and deendothelialized) recorded in pure PSS and PSS added with inhibitors of NO- and H2S-producing enzymes. The application of 1400W was accompanied by a significant decrease in the relaxation amplitude of the LN capsule. In turn, a complex of H2S synthesis inhibitors also led to a significant attenuation of LPS-induced relaxation of the LN capsule.
Since the enzymatic way of H2S production in most biological tissues is considered to be the basic [24], in the following studies, when determining specific enzymes that catalyze H2S production in the LN capsule during inflammation, we used a single inhibitor per experiment. LN capsule strips with the preserved SS were exposed to PE, and the contraction amplitude was assessed afterwards. Then, successively, PE was washed out, one of the inhibitors of H2S-producing enzymes was added to the bath solution, and (after 20 min) PE was added to the solution again. The amplitude of the contractile response was measured and compared to that of PE-induced contraction in a bath solution without an inhibitor. The difference in the amplitudes of PE-induced contractions of LN capsule strips in pure PSS and PSS added with an inhibitor was considered a relaxation induced by H2S that was produced by a corresponding inhibited enzyme. The data from this series of experiments are shown in Fig. 4. The application of each of the three inhibitors of the H2S-synthesizing enzymes was accompanied by a significant increase in the contraction amplitude, i.e. a decrease in the relaxing effect of LPS (Fig. 4a). For comparison, Fig. 4b shows the results of similar experiments on LN capsule strips incubated in F-10 Ham without LPS. Interestingly, the tendency toward increasing the amplitude of PE-induced contractions (although not statistically significant) in these preparations was only observed with the inhibition of cystathionine-γ-lyase.
Reactions of LN capsule strips to papaverine. (a) Reaction of a strip incubated in F-10 Ham + LPS, (b) reaction of a strip incubated in F-10 Ham medium without LPS. The left part of the curve shows strip’s tone 30 min after placement into a myograph chamber and creating an initial tension of 16 mN. ↓PP—time point of papaverine addition to PSS. The right part of the curve demonstrates the papaverine-induced dynamics of strip’s tone. The vertical arrow shows the amplitude of strip relaxation. Bottom—timeline (min).
Amplitude of phenylephrine-induced contraction of intact strips of the bovine mesenteric LN capsule (SS+) and analogous strips with subcapsular sinus removed (SS–) after incubation in F-10 Ham (LPS–) and F-10 Ham (LPS+) in the presence of an inducible NO synthase inhibitor 1400W and a mixture of inhibitors of H2S-producing enzymes: DL-propargylglycine (PPG) + aminooxyacetate (AOAA) + L-Aspartic acid (L-Asp). PSS—physiological saline solution. The amplitude of the PE-induced contraction of intact strips in PSS was taken as 100%.
Relaxation amplitude of intact strips of the bovine mesenteric LN capsule (SS+) and strips with the subcapsular sinus removed (SS–) after incubation in F-10 Ham + LPS in the presence of an inducible NO synthase inhibitor 1400W and a mixture of inhibitors of H2S-producing enzymes: DL-propargylglycine (PPG) + aminooxyacetate (AOAA) + L-Aspartic acid (L-Asp). PSS—physiological saline solution. * Differences are significant, p < 0.01.
Amplitude of phenylephrine-induced contraction of LN capsule strips with preserved subcapsular sinus, incubated in F-10 Ham + LPS (a) and F-10 Ham without LPS (b) in physiological saline solution (PSS) and in the presence of inhibitors of H2S-producing enzymes: cystathionine-γ-lyase (PPG), cystathionine-β-synthase (AOAA) and 3-mercaptopurivate sulfurtransferase (L-Asp). The contraction amplitude of the preparations is shown as % of the contraction amplitude of LN capsule strips incubated in F-10 Ham medium without LPS. * Differences are significant vs. the value of LN capsule strip reduction in PSS, p < 0.05.
DISCUSSION
To date, it is well known that H2S is involved in the functional regulation of the cardiovascular system, gastrointestinal tract, nervous system, and kidneys, and is also an important participant of the inflammatory process [25]. In addition, H2S is able to influence various aspects of the immune response, both congenital (functional regulation of neutrophils, macrophages and mast cells [26]) and acquired (stimulation of T cell differentiation [27]). As for the effect of H2S on the organs of the lymphatic system that provide the transport of lymph (lymphatic vessels and LN), such studies are scarce, and it is only the effects of exogenous H2S and the putative signaling pathways involved in the response to H2S exposure that have been demonstrated [21, 28].
As mentioned above, the goal of our study did not include a study of LN immune cells and the regulation of their relationships with “dendritic” cells. We set ourselves a simpler, albeit less studied, task to assess the role of endogenous H2S in the relaxation of the LN capsule during its inflammatory remodeling. LNs are very dynamic structures that must be able to rapidly increase in size in the process of adaptive reactions in order to recruit lymphocytes and dendritic cells while maintaining their structural integrity. Such a unique property of the LN is provided by a network of fibroblastic reticular cells (FRC) that form quite a rigid framework to support the internal structure of the LN [29]. In addition to FRC, the process of rapid and extensive LN remodeling recruits other stromal cells that make up a structural basis of LNs. The main causes that lead to an increase in the LN size immediately after infection or immunization are the expansion of afferent lymphatic vessels, leading to an increased recruitment of antigen-presenting DCs from the periphery [30], and an increase in the number, size and permeability of high-endothelium veins to facilitate the penetration of naive lymphocytes into LN tissue [31]. An increase in the LN size is also associated with blocking the exit of lymphocytes from LN [32, 33]. As a result, the number of antigen-presenting cells and lymphocytes in draining LNs increases considerably. The dense rigid network of FRCs in the LN during inflammatory remodeling has only been partially reconstructed [30]. As for the changes in the structure and function of the LN capsule during inflammatory remodeling, there are practically no relevant data to date.
Our present results show that a short-term (over 1 h) LPS exposure does not affect the contractile parameters of the bovine mesenteric LN capsule (tonic tension level and the phasic contraction parameters remained practically intact). At the same time, 6-h LPS exposure led to the inhibition of phasic contractions and a pronounced decrease in the level of tonic tension of the capsule. The data obtained suggest that during the 6-h incubation of preparations in a medium with LPS, a number of genes are expressed under its influence, leading to the synthesis of proteins having an inhibitory effect on the contractile function of SMCs in the LN capsule. This assumption is supported by the results of experiments with the addition of actinomycin D (an RNA synthesis inhibitor) or cycloheximide (an protein synthesis inhibitor) into the incubation medium. The presence of inhibitors in the incubation medium prevented the development of LPS-induced inhibition of the contractile function of SMCs in the LN capsule (Table 2).
What proteins are expressed in the LN capsule under the effect of LPS? In our previous study, it was shown that these may be inducible NO synthase and cyclooxygenase-2 [20]. The data of the present study, obtained using a specific iNOS inhibitor 1400W, also confirm that LPS promotes the expression of inducible NO synthase in the LN capsule, which is able to produce large amounts of NO [34] causing to a strong relaxation of SMCs in the LN capsule.
Since we have previously proved that, under physiological conditions, H2S has a pronounced relaxing effect on the bovine LN capsule [21], it was logical to assume that this gas transmitter is involved in the regulation of functions of SMCs in the LN capsule during inflammation. Hence, in the next series of experiments, we assessed the contractile properties of SMCs in the LN capsule after exposure to inhibitors of H2S-synthesizing enzymes. Initially, we used a mixture of inhibitors (PPD + AOAA + L-Asp). In the presence of these inhibitors, the amplitude of the PE-induced contractile response of LN capsule strips incubated in F-10 Ham + LPS increased, thus confirming the implication of H2S in the LPS-induced relaxation of LN capsule SMCs. Similar results were also obtained in an analogous study using LN capsule strips with the removed SS, suggesting that the main source of endogenous H2S is not endothelial cells of the SS, but SMCs of the LN capsule.
As the presence in the solution of a mixture of inhibitors of three enzymes catalyzing H2S production in the LN capsule significantly reduced the relaxation amplitude of the capsule, the question naturally arose on the significance of each of the enzymes in this process. The next series of experiments was set up to obtain an answer to this question. The application of the inhibitors in effective concentrations, one at a time on different preparations to rule out cumulative and other undesirable effects, showed that all three H2S-synthesizing enzymes are expressed in the LN capsule when modeling inflammation: cystathionine-γ-lyase (CSE), cystathionine-β-synthase (CBS) and 3-mercaptopurivatesulfurtransferase (3-MST). The data of our experiments demonstrate that the role of each of them in the LPS-induced relaxation of the LN capsule was approximately identical. An interesting fact should be noted: the LN capsule strips that were not exposed to LPS reacted most actively to DL-propargylglycine application, suggesting that CSE is most active in the LN capsule under physiological conditions.
With all the importance and diversity of the mechanisms of action of H2S in the LN capsule, it is important not to forget about NO synthesized in the same structure. Coexpression of H2S and NO in the LN capsule makes it possible to significantly enhance the relaxation effect as it promotes the production of biologically active hybrid S/N forms, H2S induces the release of NO from its various stable “pools” and increases the specific activity of NO synthases. H2S stabilizes soluble guanylate cyclase in its reduced (NO-sensitive) form, and also inhibits vascular phosphodiesterase 5 (PDE5), extending thereby the biological half-life of cGMP. Finally, polysulfides derived from H2S directly activate cGMP-dependent protein kinase (PKG). As a result, H2S, being itself a powerful relaxant, is also an important endogenous enhancer of NO signaling, contributing to further relaxation [35]. Thus, the presence of two gas transmitters in the capsule of the inflamed LN promotes its quite rapid and strong relaxation, allowing the LN to increase in volume and begin to retain dendritic cells and lymphocytes in LN sinuses, creating conditions for the development of immune responses.
REFERENCES
von der Weid PY, Muthuchamy M (2010) Regulatory mechanisms in lymphatic vessel contraction under normal and inflammatory conditions. Pathophysiology 17: 263–276. https://doi.org/10.1016/j.pathophys.2009.10.005
Liao S, von der Weid PY (2015) Lymphatic system: an active pathway for immune protection. Semin Cell Dev Biol 38: 83–89. https://doi.org/10.1016/j.semcdb.2014.11.012
Wülfing C, Günther HS (2015) Dendritic cells and macrophages neurally hard-wired in the lymph node. Sci Rep 19;5: 16866. https://doi.org/10.1038/srep16866
Willard-Mack CL (2006) Normal structure, function, and histology of lymph nodes. Toxicol Pathol 34(5): 409–424. https://doi.org/10.1080/01926230600867727
Lucas ED, Tamburini BAJ (2019) Lymph Node Lymphatic Endothelial Cell Expansion and Contraction and the Programming of the Immune Response. Front Immunol 10: 36. https://doi.org/10.3389/fimmu.2019.00036
Jalkanen S, Salmi M (2020) Lymphatic endothelial cells of the lymph node. Nat Rev Immunol 20(9): 566–578. https://doi.org/10.1038/s41577-020-0281-x
Bonavita O, Mollica Poeta V, Setten E, Massara M, Bonecchi R (2017) ACKR2: An Atypical Chemokine Receptor Regulating Lymphatic Biology. Front Immunol 7: 691. https://doi.org/10.3389/fimmu.2016.00691
Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH (2010) Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aireindependent direct antigen presentation. J Exp Med 207(4): 681–688. https://doi.org/10.1084/jem.20092465.9
Saxena V, Li L, Paluskievicz C, Kasinath V, Bean A, Abdi R, Jewell CM, Bromberg JS (2019) Role of lymph node stroma and microenvironment in T cell tolerance. Immunol Rev 292(1): 9–23. https://doi.org/10.1111/imr.12799
Reynoso ED, Lee JW, Turley SJ (2009) Peripheral tolerance induction by lymph node stroma. Adv Exp Med Biol 633: 113–112 https://doi.org/10.1007/978-0-387-79311-5_10
Thierry GR, Gentek R, Bajenoff M (2019) Remodeling of reactive lymph nodes: Dynamics of stromal cells and underlying chemokine signaling. Immunol Rev 289(1): 42–61. https://doi.org/10.1111/imr.12750
Acton SE, Reise Sousa C (2016) Dendritic cells in remodeling of lymph nodes during immune responses. Immunol Rev 271(1): 221–229. https://doi.org/10.1111/imr.12414
Zhu M, Fu YX (2011) The role of core TNF/LIGHT family members in lymph node homeostasis and remodeling. Immunol Rev 244(1): 75–84. https://doi.org/10.1111/j.1600-065X.2011.01061.x
Köhler CN (2010) The actin-binding protein caldesmon is in spleen and lymph nodes predominately expressed by smooth-muscle cells, reticular cells, and follicular dendritic cells. J Histochem Cytochem 58(2): 183–193. https://doi.org/10.1369/jhc.2009.954651
Breslin JW, Yang Y, Scallan JP, Sweat RS, Adderley SP, Murfee WL (2018) Lymphatic Vessel Network Structure and Physiology. Compr Physiol 9(1): 207–299. https://doi.org/10.1002/cphy.c180015
Koning JJ, Mebius RE (2012) Interdependence of stromal and immune cells for lymph node function. Trends Immunol 33(6): 264–270. https://doi.org/10.1016/j.it.2011.10.006
Yang CY, Vogt TK, Favre S, Scarpellino L, Huang HY, Tacchini-Cottier F, Luther SA (2014) Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci U S A 111(1): E109–E118. https://doi.org/10.1073/pnas.1312585111
Lobov GI, Pan’kova MN (2011) Mechanical properties of lymph node capsule. Bull Exp Biol Med 151(1): 5–8. https://doi.org/10.1007/s10517-011-1246-7
Lobov GI, Pan’kova MN, Dvoretsky DP, Sergeev IV (2010) Characteristic of the active and passive mechanical properties of the lymph node capsule. Dokl Biol Sci 434: 310–312. https://doi.org/10.1134/S0012496610050054
Lobov GI, Unt DV (2018) Protective Effect of Dexamethasone on Lipopolysaccharide-Induced Inhibition of Contractile Function of Isolated Lymphatic Vessels and Nodes. Bull Exp Biol Med 165(5): 602–605. https://doi.org/10.1007/s10517-018-4222-7
Lobov GI (2020) Relaxing Effect of Hydrogen Sulfide on Isolated Bovine Mesenteric Lymph Nodes. Bull Exp Biol Med 169(2): 192–196. https://doi.org/10.1007/s10517-020-04848-z
Sun HJ, Wu ZY, Nie XW, Bian JS (2020) Role of Endothelial Dysfunction in Cardiovascular Diseases: The Link Between Inflammation and Hydrogen Sulfide. Front Pharmacol 10: 1568. https://doi.org/10.3389/fphar.2019.01568
Greiner R, Pálinkás Z, Bäsell K, Becher D, Antelmann H, Nagy P, Dick TP (2013) Polysulfides link H2S to protein thiol oxidation. Antioxid Redox Signal 19(15): 1749–1765. https://doi.org/10.1089/ars.2012.5041
Lechuga TJ, Chen DB (2019) Analysis of Vascular Hydrogen Sulfide Biosynthesis. Methods Mol Biol. 2007: 19–36. https://doi.org/10.1007/978-1-4939-9528-8_3
Chen CQ, Xin H, Zhu YZ (2007) Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol Sin 28(11): 1709–1716. https://doi.org/10.1111/j.1745-7254.2007.00629.x
Roviezzo F, Bertolino A, Sorrentino R, Terlizzi M, Matteis M, Calderone V, Mattera V, Martelli A, Spaziano G, Pinto A, D’Agostino B, Cirino G (2015) Hydrogen sulfide inhalation ameliorates allergen induced airway hypereactivity by modulating mast cell activation. Pharmacol Res100: 85–92. https://doi.org/10.1016/j.phrs.2015.07.032
Yang R, Yu T, Liu D, Shi S, Zhou Y (2018) Hydrogen sulfide promotes immunomodulation of gingiva-derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res Ther 9(1): 62. https://doi.org/10.1186/s13287-018-0804-6
Lobov GI (2020) The Role of Hydrogen Sulfide in the Dilatation of Mesenteric Lymphatic Vessels in Bulls. Bull Exp Biol Med 169(3): 302–305. https://doi.org/10.1007/s10517-020-04874-x
Yang CY, Vogt TK, Favre S, Scarpellino L, Huang HY, Tacchini-Cottier F, Luther SA (2014) Trapping of naive lymphocytes triggers rapid growth and remodeling of the fibroblast network in reactive murine lymph nodes. Proc Natl Acad Sci USA 111(1): E109–E118. https://doi.org/10.1073/pnas.1312585111
Tan KW, Yeo KP, Wong FH, Lim HY, Khoo KL, Abastado JP, Angeli V (2012) Expansion of cortical and medullary sinuses restrains lymph node hypertrophy during prolonged inflammation. J Immunol 188(8): 4065–4080. https://doi.org/10.4049/jimmunol.1101854
Girard JP, Moussion C, Förster R (2012) HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 11: 762–773. https://doi.org/10.1038/nri3298
Hess E, Duheron V, Decossas M, Lézot F, Berdal A, Chea S, Golub R, Bosisio MR, Bridal SL, Choi Y, Yagita H, Mueller CG (2012) RANKL induces organized lymph node growth by stromal cell proliferation. J Immunol 188(3): 1245–1254. https://doi.org/10.4049/jimmunol.1101513
Cyster JG, Schwab SR (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30: 69–94. https://doi.org/10.1146/annurev-immunol-020711-075011
Förstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33(7): 829–37, 837a-837d. https://doi.org/10.1093/eurheartj/ehr304
Szabo C (2017) Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: mechanisms and implications. Am J Physiol Cell Physiol 312(1): C3–C15. https://doi.org/10.1152/ajpcell.00282.2016
Funding
This study was supported by the State Program 47 GP “Scientific and Technological Development of the Russian Federation” (2019–2030), theme reg. no. 0134-2019-0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The author declares that he has no conflict of interest related to the publication of this material.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2021, Vol. 107, No. 11, pp. 1372–1384https://doi.org/10.31857/S0869813921110066.
Rights and permissions
About this article
Cite this article
Lobov, G.I. Role of Endogenous Hydrogen Sulfide in Relaxation of the Lymph Node Capsule in LPS-induced Inflammation. J Evol Biochem Phys 57, 1363–1372 (2021). https://doi.org/10.1134/S0022093021060156
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021060156