Abstract
A model of focal seizures with automatisms is being developed on a strain of rats selected for enhanced pendulum-like movements (PM). At a definitive age, 90% of animals of the PM strain generate seizures to audiogenic stimulation. In contrast to Krushinsky–Molodkina and GEPR-9 (genetically epilepsy-prone rats) strains, populations of which demonstrate generalized seizures, PM rats generate a clonic cascade of seizures—the stereotyped jumps reaching a height of 0.5 m at a speed of one jump per second. The PM strain is a new model of epileptiform responses, and it has not actually been studied in early ontogeny. To detect prodromal symptoms of epilepsy, we set a goal to study behavioral and hormonal/metabolic characteristics of the PM model during the early neonatal period. Wistar rats, the initial strain for selection, served as a control. Prodromal symptoms experimentally detected the PM strain included such characteristics as a slowdown in locomotor activity, decreased body and testes weights, an inhibited rise in plasma cholesterol levels on days 10 and 14. PM rats showed a peak shift in circular movements and an increased manifestation of excitable responses, such as vocalizations and paroxysms. A phase change was detected in the plasma triglyceride level. Correlation analysis revealed that in male Wistar rats, the testosterone level correlates negatively with body and testes weights and positively with the blood triglyceride level. By contrast, in PM rats the same correlations were statistically insignificant. These findings indicate a destabilization of the development of morphophysiological characters in PM rats compared to the control Wistar strain. Considering the revealed prodromal symptoms characterizing enhanced pendulum-like hyperkinesis (retarded developmental rate, phase-shifted circular movements and plasma triglyceride levels, altered relationships between correlated phenotypic characters), the PM rat strain can be considered as a model for further investigating the biological basis of epileptiform pathology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In studies conducted on human populations, the signs of a “high alert” of the nervous system for epileptic disorders have been revealed in infants. These signs include motor reactions with clonic, tonic and myoclonic seizures, dysplastic processes, motor response developmental delays, delayed body/organ weight gain [1−3], and metabolic imbalance considered as a comorbid manifestation [4−6]. All these deviations indicate early shifts in neurohormonal and metabolic processes in the organism and can be considered as markers of genetically determined epileptic disorders during early ontogeny.
Focal motor seizures with typical automatisms represent one of the types of epilepsy. The mechanisms behind this syndrome and its relationship with the functioning of physiological systems are normally studied on animal models. V.G. Kolpakov generated two rat models of catatonic responses: GC (genetic catatonia) strain [7] and PM strain with pendulum-like movements [7−10]. In terms of general phenotypic manifestations, both strains are distinguished by increased stress responses to external impacts and an associated reduced birthrate. During selection, the known adrenal-gonadal antagonism leads to the fact that stress suppresses sexual activity [11]. The mechanisms of this early ontogenetic phenomenon in the PM strain have not been studied.
Animals with pendulum-like (hereinafter pendulum) movements (PM) are characterized by rhythmic side-to-side rocking of the head and forebody in the absence of locomotion (Fig. 1).
PM occur not only in mice and rats, but also in albino rabbits and scorpion hamsters [cited in 10, 12]. In rats selected for an increased amplitude of PM (PM strain), after the 40th generation, audiogenic seizures began to develop in 80−90% of cases, in contrast to the initial (control) Wistar rat population, in which epileptic seizures were observed only in 25−30% of animals [13]. To date, according to our data, unselected Wistar rats and WAG (Wistar Albino Glaxo) rats with PM develop seizures in response to audiogenic stimulation in 10% of cases. The facts of differential susceptibility to audiogenic seizures in different generations of selection indicate the important role of genetic variation in selection on behavior [11]. PM rats demonstrated a shift from a catatonic to an epileptiform type of responses. Appropriate deviations in brain levels of neurotransmitters ensued accordingly [13, 14]. In PM rats, these deviations proved to be similar to those in Krushinsky–Molodkina rats and GEPR-9 (genetically epilepsy-prone rats) [13]. Currently, the following facts support the use of inbred rats with PM as a model of focal motor seizures with typical automatisms. Animals of this strain, in contrast to control Wistar rats, exhibited 50% of abortive audiogenic seizures [13, 15], a reduced taurine level in the hippocampus [14], and a positive correlation between the intensity of seizures and the duration of postictal catalepsy [15]. It was also shown that during selection for enhanced PM, the body weight in sexually mature animals, as well as the fertility rate and the proportion of missed females, decreased [15].
The perinatal period of ontogeny is critical for the formation of the nervous system and the development of postural/motor responses. In classic studies, a special focus is laid on the formation of motor behavior as a spatio-temporal process [16−18]. In motor behavior, researchers identify its constituent components, analyze their appearance, frequency, and degree of manifestation. These behavioral components include head movements, trunk turns, locomotion, upright rearings, grooming. Based on a detailed analysis of motor behavior, 4 critical points in neonatal development were identified: day 1 (head movements and crawling), day 7 (maturation of the corticospinal tract), day 10 (standing on four legs and quadrupedal locomotion), day 14 (opening of the ears and eyes, forming of the range of vision) [16−18].
The rat reproductive system develops in the neonatal period. A key challenge is to elucidate the physiological mechanisms that underlie the transformation of reproductive function due to behavioral selection, because the very existence of a species and its evolution depend on the successful reproduction of healthy offspring. A comparative analysis of morphological and hormonal indicators of testis development in early ontogeny allows identification of those deviations in PM rats that could be used as predictors of decreased fertility in this experimental strain as compared to control Wistar rats. Testosterone, which is synthesized in testicular Leydig cells, affects the development and accumulation of the musculoskeletal mass [19], regulation of spermatogenesis and sexual behavior [20]; it interacts with lipids, such as cholesterol and triglycerides [21, 22]. Lipids play a polyfunctional role in ontogeny: they make up an energy reserve for a developing organism, stabilize cell membranes, serve as precursors of biologically active substances, corticosteroid and sex hormones.
PM rats with 95% frequency of a stereotyped hyperkinesis in the form of pendulum-like movements is a new inbred strain. Animals of this strain have not actually been examined in early ontogeny. The goal of this work was to study the development of motor responses and to characterize morphological and hormonal functions of the gonadal system and lipid metabolism at critical points of early ontogeny in PM rats. An additional task was to carry out a comparative analysis of the data to be obtained in the present work and those for GC rats, which represent a model of the catatonic type of responses. Such a comparison will enable the determination of the origin mechanisms of epileptiform responses in PM rats, as well as the identification of the predictive signs of epilepsy in PM rats at the definitive age.
MATERIALS AND METHODS
Animals. Experiments were carried out on inbred PM rats (n = 116) and outbred Wistar rats (n = 126), a total of 242 animals. Rats were kept under standard vivarium conditions with a free access to food and water. All experimental procedures complied with the rules and regulations formulated in the EU Council Directive 1986 (86/609/EEC) and the Declaration of Helsinki on the protection of vertebrate animals used in experimental research.

To obtain offspring of the required age, males were mated to females in the PM and Wistar strains. In the last week of gestation, females were placed into individual cages, and the day of birth was considered as the first day of rat pups’ life. The number of rat pups was recorded at birth in each litter. Immediately before decapitation, males were weighed, while after decapitation, blood was collected and the testes were excised and weighed.
Test for the activity of motor subsystems [16−18]. Testing was carried out on an open platform (20 × 30 cm) heated to 30−35°C; the temperature was maintained at the level of a nest temperature. Rat behavior was recorded on a Panasonic video camera for 1 min. The rat pup was taken by the skin of the dorsal part of the neck and placed in the middle of the platform with its tail toward the experimenter. The following parameters were recorded: the number of head motor responses, circular movements, pseudo-swimming movements and locomotions, as well as immobility time (s). Each pup was tested no more than 3 times out of 14 days.
Test for pinch-induced catalepsy [23]. Pinch-induced catalepsy was tested in 2-week-old rat pups by raising them slightly by the skin of the dorsal part of the neck. The following parameters were recorded: immobility time (s), number of paroxysms, vocalization time and eyes-closed time (s). Rat behavior was recorded on a Panasonic video camera for 2 min.
Measurement of serum testosterone in testicular homogenates was performed as described previously [24, 25]. In brief, peripheral blood was centrifuged at 3000 rpm and 4°C for 20 min. Both testes were homogenized in 500 µL of phosphate buffer and centrifuged, while the supernatant was stored at −40°C. Serum testosterone in testicular homogenates was assayed using Steroid IEA-testosterone-01 kits (Alkor Bio, St. Petersburg, Russia) according to a manufacturer’s instruction. The range of testosterone concentrations was 0.2−50 nmol/L, sensitivity 0.2 nmol/L. Serum testosterone concentration was expressed in nmol/L.
Measurements of lipid metabolism parameters were performed as described elsewhere [25]. Total cholesterol (TCh) and triglyceride (TG) levels were determined by the enzymatic colorimetric method on a tablet modification using standard Vector Best kits (Novosibirsk, Russia) according to the instruction attached. The range of concentrations was 0.2−50 nmol/L, sensitivity 0.2 nmol/L.
Statistics. The obtained data were processed using STATISTICA 10.0. In tables and figures, data are presented as mean ± SEM. When comparing the PM and Wistar strains, two-way ANOVA was used (genotype and age factors). Data for discrete time points (1, 7, 10, 14 days) were compared by the LSD post hoc test. Differences were considered statistically significant at p < 0.05. Coefficients of correlation (R) among the parameters of body and testes weights, testosterone, cholesterol and triglyceride levels were calculated using the Correlation Matrices computer program.
RESULTS
A reduced fertility was recorded in the PM strain (30 broods) compared to Wistar rats (20 litters): 7.0 ± 0.5 and 10.6 ± 0.9 pups per litters (p < 0.001), respectively.
Behavioral tests. Wistar and PM rats differed in the test for the activity of motor subsystems. The main parameters of this test were head movements, circular and pseudo-swimming movements, locomotion, and freezing (Table 1; Figs. 2, 3). Movements were considered pseudo-swimming, when pups moved in a crawling manner without lifting their abdomens from the platform, thus resembling true swimming movements. Particular attention was paid to neonatal day 1. Rat pups are born with eyes and ears closed, hence with limited abilities to perceive sensory information, and therefore the influence of the genotype factor can be tracked most clearly because of the absence of mature visual and auditory receptors when perceiving external stimuli. The results of testing are shown in Table 1.
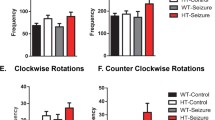
The number of circular movements in the Wistar and PM strain was influenced by the genotype factor (F(5, 256)= 4.62; p < 0.001). The pups differed during 2 weeks of testing in the dynamics of circular movement: the peak in Wistar rats fell on day 8, while in MD rats it was on day 10.
The parameter of locomotor activity was influenced by the genotype factor (F(5, 237) = 25.45; p < 0.001) in both rat strains. This influence was reflected in the retardation of the locomotor activity growth rate in PM strain compared to Wistar rats on days 12 and 14.
Test for pinch-induced catalepsy at the age of 2 weeks [23]. Data on pinch-induced catalepsy test differed in the Wistar and PM strains (Table 1). The parameters divided into “positive” and “negative”. The positive parameters, reflecting the excitability of the nervous system, included the increased number of paroxysms and the vocalization time. In both these parameters, PM rats were superior to Wistar rats. The eyes-closed parameter reflects a trans-like (dopey) condition characteristic of cataleptic responses [26]. Altogether, in terms of the negative parameters, such as the immobility time (Tables 1, 2) and the duration of the eyes-closed period, PM animals showed significant differences compared to the initial Wistar strain. Overall, catatonic traits, both positive and negative, can be tracked in the PM strain.
Morphological parameters. The inter-strain body weight parameters proved to be under the influence of the factors of genotype (F(1, 58) = 5.0; p < 0.03) and age (F(3, 58) = 104.1; p = 0.0000) (see Fig. 4). As a result, body weight gain in PM rats was progressively decreasing at the critical developmental points, on days 7, 10 and 14.
The testes weight was changing in a similar way. The influence of genotype (F(1, 58) = 60.3; p < 0.000) and age (F(3, 58) = 385.9; p < 0.000) was noted. Figure 5a shows that in terms of the testes weight, PM rats significantly lagged behind Wistar rats, beginning from day 10, and the difference between the testes weight in rats of different strains increased by day 14. However, the testes weight to body weight ratio was significantly higher in PM rats compared to Wistar rats at all four time points (Fig. 5b). These data indicate the presence of dysplastic signs and the destabilization effect during selection for enhanced pendulum movements in the inbred PM strain.
Hormonal and lipid parameters. The effect of the genotype (F(1, 58) = 7.37; p < 0.01) and age (F(3, 58) = 54.3; p < 0.000) factors on cholesterol level was found (Fig. 6a). In contrast, the plasma triglyceride level was unaffected by these factors, although two-way ANOVA revealed a significant influence of the interaction of the genotype (F(3, 58) = 6.2; p < 0.001) and age (F(3, 58) = 54.3; p < 0.000) factors, depending on the critical point of the neonatal period (Fig. 6b).
In terms of the plasma and testicular testosterone levels, no differences were found between Wistar and PM rats: F(1, 58) = 1.6; p > 0.05 and F(1, 58) = 1.0; p > 0.05, respectively.
Correlation analysis included the following parameters: body and testes weights, plasma and testicular testosterone levels, plasma cholesterol and triglyceride levels. The correlation coefficient was denoted as R. The highest values in both strains, R = 0.72 and R = 0.64, were established between the cholesterol level and body and testicular weights, respectively. A maximum number of significant correlations was revealed in the relationship between the plasma testosterone level and the above parameters in Wistar rats, but not for the testicular level of this hormone (Fig. 7). These parameters, showing a significant R with the plasma testosterone level in Wistar rats, included positive (R testicular testosterone = 0.44, Rtriglycerides = 0.56) and negative (Rbody weight = −0.38; R testes weight = −0.48) correlations. For the PM strain, correlation coefficients between the plasma testosterone level and the above parameters showed low and nonsignificant values: Rcholesterol = 0.11, Rtriglycerides = 0.01, R body weight = −0.06; R testes weight = −0.21. The latter facts indicate the effect of destabilization of organismic systems, which resulted from the selection for enhanced hyperkinesis in the PM rat strain.
Testes weight (a) and relative testes weight index (b) in Wistar and PM rats. Differences are significant for the influence of the genotype (F (1, 58) = 60.3; p < 0.000) and age (F (3, 58) = 385.9; p < 0.000) factors. * p < 0.05, ** p < 0.01, *** p < 0.001—differences are significant according to LSD post hoc test.
Plasma levels of cholesterol (a) and triglycerides (b) in Wistar and PM rats. Differences are significant for the influence of the genotype factor (a): F(1, 58) = 7.37; p < 0.01 and the genotype-age relationship (b): F(3, 58) = 6.2; p < 0.001. * p < 0.05, ** p < 0.01, *** p < 0.001—significant differences and # p = 0.07—trend according to LSD post hoc test.
DISCUSSION
In the present work, we tracked the development of some phenotypic characteristics of PM rats in the neonatal period, which became predictors of epileptiform responses in animals at a definitive age. These responses were divided into “negative” and “positive” indices. The “negative” indices included those that were delayed in ontogeny or showed inhibited manifestations. These parameters reflect the rate of genetic variation and, as a result, can lead to the appearance of pathological signs in the PM line. These signs included a delayed development of locomotor responses, increased immobility, a longer eyes-closed period of time, a shift in circular movements, a lag in body weight gain, a slower cholesterol elevation in PM rats compared to control. Those signs that had the property of increased excitability were considered “positive”. These are a larger number of stereotypic paroxysms and a longer period of vocalization. Both categories of signs, negative and positive, are observed to a large extent in PM rats and show catatonic manifestations in the PM strain.
It was important for us to compare the phenotypic traits in the PM and GC strains, which was an additional task in this study. At the early stages of selection, the responses of animals with PM were referred by researchers to a catatonic type of response, because these animals were characterized by pendulum hyperkinesis not accompanied by locomotions [27–29]. However, after the 40th generation of selection, in rats with PM, the proportion of audiogenic seizures sharply increased (up to 83%) [13]. The authors of PM strain selection began to classify the responses of animals as epileptiform. If at the first stages of PM strain selection a positive correlation was shown between PM and spontaneous catalepsy, then at the last stages, the correlation turned out to be negative and shaped as an inverted letter U [9]. Currently, GC (genetic catatonia) rats do not generate PM, while PM rats show audiogenic seizures in 90% of cases.
The results of a comparative phenotypic analysis of PM and GC strains at a definitive age, bred from the same Wistar population and kept under the same conditions at the vivarium of the Institute of Cytology and Genetics (Novosibirsk), showed the following. Both of these rat strains are supposed to be a model of a catatonic type of response [9, 27–30]. Between the catatonia model (GC strain) and the epilepsy model (PM strain), there are multidirectional deviations in phenotypic characteristics. The list of the oppositely directed parameters included audiogenic seizures [15, 31], the number of stereotypes [9, 32, 33], startle reflex [9, 15, 31], blood pressure level [15, 33], spontaneous catalepsy [7, 15, 31]. Similar characteristics were found in animals of the GC and PM strains. They manifest themselves in responses of increased excitability in different test situations. These are epileptiform responses revealed in GC rats by EEG [34], aggression toward humans [29, 35], impulsivity and reduced fertility [36, 37], lower body weight [15, 31]. All the above characteristics, both antagonistic and alike, are observed in epileptic people [38–40].
In the present work, we studied phenotypic characteristics of rats with PM in the neonatal period, and revealed a number of similarities with the GC strain. During this ontogenetic period, rats of both strains demonstrated an increase in the immobility time, a decrease in locomotor responses, and a decrease in the birth rate [15, 31]. A distinctive behavioral feature revealed on the GC model during early ontogenesis were such predictors of catatonic behavior as dyskinetic movements and asymmetric postures [41], which was not shown on the PM model.
When addressing the shifts in the hormonal/metabolic system, we can see that the endophenotypes of the GC and PM strains developed in different directions. The total plasma cholesterol index showed multidirectional deviations in GC [36] and PM rats (reported in this article) as compared to the control.
In the neonatal period, there occurs male sexual differentiation; during this period, neurosecretory hypothalamic centers, forward and backward connections in the hypothalamic-pituitary-testicular system are formed [42, 43]. In rats, under normal conditions, the blood level of steroid sex hormones (including testosterone) drops by the end of this period due to a decrease in their secretion in testicular Leydig cells and a replacement of the embryonic cell by a definitive one. The general trend in the plasma testosterone dynamics in Wistar and PM rats proved to be downward by the end of the neonatal period. Previously, it was established that the testosterone peak shifts to a later time in GC males compared to Wistar males at an early age [36], whereas in PM rats, differences in testosterone levels were found neither in blood plasma nor in testicular Leydig cells. A change in the level and production of testosterone in the developing male organism leads to subsequent changes in metabolism, decreased muscular and physical strength, and reduced sexual function in adults [44–46].
Nevertheless, in PM rats, by the results of correlation analysis in the neonatal period, a destabilizing effect of testosterone was revealed. The blood level of this androgen in PM rats does not correlate with such parameters as cholesterol and triglyceride levels, as well as a testicular weight. In the same experiment, significant correlative levels were established in Wistar rats for all of the above parameters, indicating the integratedness of the endocrine system in animals of the initial (Wistar) strain. The fact of destabilization of morphophysiological parameters was also confirmed in adult PM rats compared to animals from the Wistar population [15].
At the time of writing this article, we tested outbred Wistar and WAG (Wistar Albino Glaxo) rats with stereotyped head shaking to identify seizures, and obtained a total of 10% of audiogenic seizures in these strains. In the group of unselected rodents, the amplitude of head and shoulder girdle shaking was 0.5 cm in both directions. At the same time, in rats of the selected PM group, the amplitude of pendulum-like movements reached up to 4 cm in both directions. Previous studies showed an increase in the frequency of seizures during selection for enhanced PM in this strain from 41% (in the 5th generation) to 91% (in the 23rd generation) [9, 13, 30, 31]. Thus, by the 50th generation of selection, a new endophenotype with an altered hormonal and metabolic basis formed in PM rats. To its prodromal characteristics, we referred such parameters as a slowdown in the growth rate of locomotions and in body and testis weight gain, delayed appearance of circular movements, the greater manifestation of excitable reactions (vocalizations and paroxysms), a decrease in cholesterol levels, a disruption of correlations between the plasma testosterone level and morpho-lipid parameters. The selection of animals for an increase in the amplitude of PM led from the catatonic signs (immobility and stereotypes) to an enhancement of epileptiform responses in adults. The prodromal abnormalities revealed in the neonatal period are nonspecific and represent a basis for the appearance of pendulum-like movements in the prepubertal and definitive developmental periods. In the future, it is planned to influence these signs, including by pharmacological methods, to decrease the risk of epileptiform pathology.
REFERENCES
Sculiera C, Gasparda N (2019) New onset refractory status epilepticus (NORSE). Seizure: European Journal of Epilepsy 68: 72–78. https://doi.org/10.1111/epi.14022
Gataullina S, Dulac O, Bulteau C (2015) Temporal lobe epilepsy in infants and children. Rev neurologique 171: 252–253. https://doi.org/10.1016/j.neurol.2015.01.559
Camfield P, Camfield C (2019) Regression in children with epilepsy. Neurosci Biobehav Rev 96:210–218. https://doi.org/10.1016/j.neubiorev.2018.12.008
Park JT, Shahid AM, Jamoul A (2015) Common Pediatric Epilepsy Syndromes. Pediatric Annals 44(2): e30–e35. https://doi.org/10.3928/00904481-20150203-09
Harrison VS, Oatman O, Kerrigan JF (2017) Hypothalamic hamartoma with epilepsy: Review of endocrine comorbidity. Epilepsia 58(2): 50–59. https://doi.org/10.1111/epi.13756
Kaprara A, Huhtaniemi IT (2018) The hypothalamus-pituitary-gonad axis: Tales of mice and men. Metabolism 86:3–17. https://doi.org/10.1016/j.metabol.2017.11.018
Kolpakov VG, Richner MS, Kornetov NA, Samoshvalov VP, Zalevskii GV, Korolenko CP (1985) Genetic and evolutionary problems of psychiatry. Ed. Timofeeva. Nauka 256.
Kolpakov VG, Alekhina TA, Barykina NN, Chugui VF, Popova NK (2000) Physiological manifestations of action of a gene regulating predisposition to a pendulum-like movements in rodents. Russ J Physiol 86(1):33–40.
Kolpakov VG, Barykina NN, Alekhina TA, Chugui VF, Popova N.K, Zorkol’steva IV, Aksenovich TI (1999) Genetic relationships between catalepsy and pendulum movements in rats. Genetika 35(8): 1118–1123.
Kolpakov VG, Borodin PM, Barykina NN (1977) Catatonic behaviour in the Norway rat. Behaviour 62: 190–208.
Belyaev DK, Borodin PM (1982) The impact of stress on the genetic variability and its role in evolution. Evolutionary genetics. L. 35–59.
Kolpakov VG, Barykina NN, Borodin PM, Serova LI (1976) Jactatio capitis—movement stereotypy in rodents. Genetika 12(1): 71–77.
Alekhina TA, Prokudina OI, Ryazanova MA, Ukolova TN, Barykina NN, Kolpakov VG (2007) Typological Characteristics of Behavior in Strains of Rats Bred for Enhancement and Absence of Pendulum Movements. Association with Brain Monoamines. Zh Vyssh Nerv Deiat 57(3): 336–343.
Akulov AE, Alekhina TA, Meshkov IO, Petrovskii ED, Prokudina OI, Koptiug IV, Savelov AA, Moshkin MP (2014) Selection for catatonic reaction in rats: a study of interstrain differences by magnetic resonance imaging. Zh Vyssh Nerv Deiat 64(4):439–447.
Alekhina TA, Kozhemyakina RV (2019) Modeling of focal seizures with automatisms in rats with pendulum movements. Bull exp biol med 168(2): 300–303. https://doi.org/10.1007/s10517-019-04695-7
Clarac F, Vinay L, Cazalets JR, Fady JC, Jamon M (1998) Role of gravity in the development of posture and locomotion in the neonatal rat. Brain Res Rev 28(1–2): 35–43. https://doi.org/10.1016/S0165-0173(98)00024-1
Eilam D, Golani I (1988) The ontogeny of exploratory behavior in the house rat (Rattus rattus): the mobility gradient. Develop Psychobio 21(7): 679–710. https://doi.org/10.1002/dev.420210707
Schank C (2008) The development of locomotor kinematics in neonatal rats: an agent-based modeling analysis in group and individual contexts. J Theor Biol 254: 826–842. https://doi.org/10.1016/j.jtbi.2008.07.024
Manolagas SC, O’Brien CA, Almeida M (2013) The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 9(12): 699–712. https://doi.org/10.1038/nrendo.2013.179
Smith LB, Walker WH (2014) The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13. https://doi.org/10.1016/j.semcdb.2014.02.012
Saad F, Gooren L (2009) The role of testosterone in the metabolic syndrome: a review. J Steroid Biochem Mol Biol 114(1–2):40–43. https://doi.org/10.1016/j.jsbmb.2008.12.022
Beverlya BEJ, Furra JR, Lambrighta CS, Wilsona VS, McIntyrec BS, Fosterc PMD, Travlosc G, Gray Jr LE (2019) In utero exposure to simvastatin reduces postnatal survival and permanently alters reproductive tract development in the Crl:CD(SD) male rat. Toxicol Appl Pharmacol 365: 112–123. https://doi.org/10.1016/j.taap.2019.01.001
Korczynski R, Korda P (1988) Immobility reflex evoked by vertical lifting of the rat. Acta Neurobiol Exptl. 48:145–159.
Osadchuk LV (2010) Testicular function in mice of inbred strains BALB/cLac, PT, and CBA/Lac. Russ J Physiol 96(2):183–190.
Osadchuk LV, Kleschev MA, Baklanov AV, Bazhan NM (2016) Testicular function and lipid in male mice with hereditary predisposition to obesity. Russ J Physiol 102(3): 340–350.
McGrow CP, Klemm WR (1973) Genetic differences in susceptibility of rats to the immobility reflex (“Animal Hypnosis”). Behav Genetics 3(2): 155–162. https://doi.org/10.1007/BF01067655
Kolpakov VG, Barykina NN, Alekhina TA, Chepkasov IL (1985) Catalepsy in rats: Its inheritance and relationship to pendulum movements and audiogenic epilepsy. Behav Processes 10(1–2): 63–76. https://doi.org/10.1016/0376-6357(85)90118-4
Kolpakov VG, Alekhina TA, Barykina NN, Chugui VF, Popova NK (2011) Some Physiological Manifestations of the Activity of the Gene Controlling the Predisposition to Pendulum-Like Movements in Rats. Neurosci Behav Physiol 31(3): 311–316. https://doi.org/10.1023/a:1010390719547
Barykina NN, Chuguy VF, Prokudina OI, Plusnina IP, Kolpakov VG (2007) Confirmation of a positive genetic relationship between pendulum movements, audiogenic epilepsy, catalepsy and “nervousness” in rats. Genetica 48(7):987–993.
Kolpakov VG, Barykina NN, Chugui VF, Alekhina TA (1999) Relationship between certain forms of catalepsy in rats. An attempt at genetic analysis. Genetika 35(6):807–810.
Alehina TA, Kozhemjakina RV (2018) Interlinear differences in emotional and weight parameters in rats with a catatonic type of response and Wistar. Vavilov Journal of Genetics and Breeding 22(4):452-458. https://doi.org/10.18699/VJ18.382
Barykina NN, Chuguy VF, Alekhina TA, Ryazanova MA, Ukolova TN, Saharov DG, Kolpakov VG (2009) Learning of rats predisposed to catalepsy in Morris water test. Zhurn vish nerv deyat 59(6): 728–735.
Barykina NN, Chepkasov IL, Alekhina TA, Kolpakov VG (1983) Breeding of Wistar rats for predisposition of catalepsy. Genetica 19(12):2014–2021.
Petrova EV, Luchkova TI, Pirozhenko AV (1992) The ECoG of rats with genetic catalepsy. Zh Vyssh Nerv Deiat Im I P Pavlova 42(5):1009–1017.
Alekhina TA, Palchikova NA, Kozhemyakina RV, Prokudina OI (2016) Destabilization signs in behavioral and somatovegetable parametres of rats selected for catatonia. Vavilov Journal of Genetics and Breeding 20(1):74–82. https://doi.org/10.18699/VJ16.103
Osadchuk LV, Alekhina TA (2018) Developmental profiles of hormonal and metabolic parameters in male rats selected for catatonic type of response. Zh Evol Biokhim Fiziol 54(1):52–59.
Kleshchev MA, Alekhina TA, Osadchuk LV (2018) The spermatogenic function testes in rats predisposed to the manifestation of catatonic reactions. Vavilov Journal of Genetics and Breeding 22(4):400–405. https://doi.org/10.18699/VJ18.375
Lenz H (1966) Pathologische EEG-Befunde bei epileptischen Psychosen, Depression and Schizophrenia. Arch Psychiatr Nervenkr 208(1):52-60. https://doi.org/10.1007/BF00341696
Tadokoro Y, Oshima T, Kanemoto K (2007) Interictal psychoses in comparison with schizophrenia—A prospective study. Epilepsy 48(12):2345–2351. https://doi.org/10.1111/j.1528-1167.2007.01230.x
Sakakibara E, Nishida T, Sugishita K, Jinde S, Inoue Y, Kiyoto Kasai K (2012) Acute psychosis during the postictal period in a patient with idiopathic generalized epilepsy: Postictal psychosis or aggravation of schizophrenia? A case report and review of the literature. Epilepsy Behav 24(3):373–376. https://doi.org/10.1016/j.yebeh.2012.04.127
Igonina TN, Alekhina TA, Palchikova NA, Prokudina OI (2016) Prodromal signs of catatonia are associated with hereditary dysfunction of body systems in rat pups. J Experimental and Integrative Medicine 6(3):99–108. https://doi.org/10.5455/jeim.270816.or.157
Angelopoulou R, Balla M, Lavranos G, Chalikias M, Kitsos C, Baka S, Kittas C (2008) Sertoli cell proliferation in the fetal and neonatal rat testis: a continuous phenomenon? Acta Histochem 110(4):341–347. https://doi.org/10.1016/j.acthis.2007.10.009
Chen H, Ge RS, Zirkin BR (2009) Leydig cells: From stem cells to aging. Mol Cell Endocrinol 306 (1–2): 9–16. https://doi.org/10.1016/j.mce.2009.01.023
Gehrand AL, Phillips J, Malott K, Raff H (2020) Corticosterone, Adrenal, and the Pituitary-Gonadal Axis in Neonatal Rats: Effect of Maternal Separation and Hypoxia. Endocrinology 161(7):bqaa085. https://doi.org/10.1210/endocr/bqaa085
Teerds KJ, Huhtaniemi IT (2015) Morphological and functional maturation of Leydig cells: from rodent models to primates. Hum Reprod Update 21(3):310–328. https://doi.org/10.1093/humupd/dmv008
Kilcoyne KR, Smith LB, Atanassova N, Macpherson S, McKinnell C, van den Driesche S, Jobling MS, Chambers TJ, De Gendt K, Verhoeven G, O’Hara L, Platts S, Renato de Franca L, Lara NL, Anderson RA, Sharpe RM (2014) Fetal programming of adult Leydig cell function by androgenic effects on stem/progenitor cells. Proc Natl Acad Sci USA 111(18):E1924–E1932. https://doi.org/10.1073/pnas.1320735111
Funding
This study was supported by the budget program no. 0259-2021-0016 with the use of the equipment of the CCU “Center of Genetic Resources of Laboratory Animals” at the Institute of Cytology and Genetics (Novosibirsk) (Unique Project Identifier RFMEFI62119X0023).
Author information
Authors and Affiliations
Contributions
T.A. Alekhina: planning of experiments, collection and processing of behavioral data, writing and editing the manuscript; V.S. Plekanchuk: collection and processing of behavioral data, editing of the manuscript; L.V. Osadchuk: collection and processing of biochemical data, writing and editing of the manuscript.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no evident or potential conflict of interest in relation to the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2021, Vol. 57, No. 3, pp. 240–249https://doi.org/10.31857/S0044452921030025.
Rights and permissions
About this article
Cite this article
Alekhina, T.A., Plekanchuk, V.S. & Osadchuk, L.V. Prodromal Characteristics of Epilepsy in Rats with Pendulum-Like Movements. J Evol Biochem Phys 57, 492–502 (2021). https://doi.org/10.1134/S0022093021030042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021030042