Abstract
The Caribbean king crab Maguimithrax spinosissimus is an omnivorous crustacean with high feeding plasticity. We characterized the main digestive enzymes of this crab species. Alpha-amylase and esterase activities were higher in the hepatopancreas, while those of trypsin and chymotrypsin were higher in the gastric fluid. Only one α-amylase isoform of 40 kDa generates a high α-amylase activity in this crab with a maximum at pH 5.5, 40–60°C, and 2.5 mM CaCl2. A high proteolytic activity was observed in the gastric chamber, while the activity of these enzymes was low in hepatopancreas extracts due to the presence of an inhibitor that was further demonstrated by enzyme assays and reverse zymography. Several proteases from 10 to 45 kDa are synthesized in the hepatopancreas and selectively secreted into the gut. Trypsin-like and chymotrypsin-like activities were highest at pH 7, 60°C, 0.02–1.5 M NaCl, 25 mM CaCl2. These activities were poorly influenced by Ca2+. Both enzymes were stable at a neutral to alkaline pH and a temperature up to 40°C. Esterase activity peaked at pH 9.0, 37°C, 2 M NaCl, being also poorly affected by Ca2+ but temperature sensitive. In summary, high α-amylase and protease activities, a high protease abundance, as well as a tight control over their activity by an unknown inhibitor-based mechanism appear suited for M. spinsissimus to feed opportunistically on a diet of benthic algae and epifauna with eventual carnivorous preferences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Crabs (Infraorder Brachyura) are a highly successful group of crustaceans, comprising 7250 species with a wide variation in feeding habits, including herbivores, carnivores, scavengers, deposit and filter feeders, although most of them can be considered opportunistic omnivores [1, 2]. Therefore, crabs are suited for studying the evolution of feeding habits and, particularly, adaptations (e.g., behavioral, morphological, biochemical, physiological) to their diet. Regarding biochemical adaptations, studies have been performed on the digestive enzymes of different crab species [1, 3–7]. In general, carnivorous crabs produce high amounts of such proteinases as trypsin and chymotrypsin, herbivorous species exhibit high cellulase activity, whereas omnivorous species show high activity of proteinases and amylase, while also exhibiting activities of cellulase to digest plants and chitinase to break down chitin of invertebrate shells [1]. This link between digestive enzymes and dietary preferences, however, was not observed in one study comparing the digestive enzymes of four sympatric land crab species with detritivorous or omnivorous feeding habits [8]. Likewise, a relatively high α-amylase activity in carnivorous larvae of the crab Hyas araneus does not correspond to a low carbohydrate level in its food and may be a phylogenetic remnant from ancestor species with partly herbivorous larvae [9]. Among different feeding habits, omnivory is perhaps the most interesting from an evolutionary perspective because differences between plants and animals force to seek trade-offs in the traits required to use these feeds [10]. Moreover, the fact that the capacity of consuming plant materials had independently arisen in several crustaceans’ lineages [11] makes the differences to arise in biochemical adaptations to plant-rich diets among groups/species.
The Majoidea superfamily includes six families (Epialtidae, Inachidae, Inachoididae, Majidae, Mithracidae, Oregoniidae) [2], with a wide geographical distribution [12]. Some species, such as Mithraculus coryphe and M. sculptus, have a commercial value as ornamental species [13], while larger species sustain commercial fisheries [14], such as the Caribbean king crab Maguimithrax spinosissimus [15, 16]. This crab is distributed throughout the tropical Atlantic Ocean from the Southwest of the United States of America to Venezuela, and lives on coral and rocky reefs from 3 m down to 40 m [17], although it has been found at an almost 200 m depth [18]. M. spinosissimus forages on benthic algae (i.e. macroalgae and algal turf) and associated epifauna (i.e. nematodes polychaetes, microcrustaceans) [16, 18, 19]. Indeed, it has been reported that in captivity, no meat supplements to this crab’s diet affect the growth rate negatively [20]. Moreover, it is well known that under certain conditions (e.g. high population densities and poor nutrition in captivity) this species can exhibit cannibalistic behavior [15]. This observation suggests that the Caribbean king crab has the enzymatic capabilities to digest quite different feeds. Digestive plasticity is known in other crabs too. For instance, in the crab Neohelice granulate, trypsin, chymotrypsin, and cellulolytic activities were higher in crabs fed on leaves than in those fed on sediments [21]. In addition, a dietary shift has been documented in some species both throughout ontogeny [22] and during habitat changes (e.g., estuarine, marine) [23]. However, there is no information on the biochemistry of digestion in M. spinosissimus and, accordingly, on biochemical adaptations that allow this crab to behave opportunistically as a herbivore, omnivore and/or carnivore. Thus, this study aimed to characterize the main digestive enzymes in the Caribbean king crab with a special focus on proteinases and α-amylase because of their key roles in digestion in carnivorous and omnivorous crabs, respectively [1]. The results to be obtained were supposed to promote our understanding the adaptations of this crab species to omnivory.
MATERIALS AND METHODS
1. Collection of samples and preparation of extracts
Crabs were collected (n = 10) in June 2017 in the North coast of Playa, Havana, Cuba (23°7.733' N–82°25.409' W). Animals were transported alive to the Center for Marine Research at the University of Havana, Cuba. This study did not involve endangered or protected species. The animals were anesthetized by immersing into ice-cold water before hepatopancreas extraction. Gastric fluid samples were obtained through the oral cavity using disposable insulin syringes with a plastic cannula over the sharp end of the needle as described previously [24]. Enzymatic extracts were obtained by homogenizing the samples with silver 1 mM diethyldithiocarbamate (a phenoloxidase inhibitor) to avoid melanization [25]. The gastric fluid and hepatopancreas extracts were centrifuged at 8000× g for 25 min at 4°C, and the supernatants were immediately frozen in liquid nitrogen and then stored at –80°C until use. The pH of the gastric fluid was measured after extraction, whereas pH of the hepatopancreas after homogenization. Thus, the pH of the hepatopancreas extract did not necessarily reflect the pH in the gland microtubules, where extracellular digestion took place.
2. Enzyme assays
Crude extracts were diluted to concentrations at which initial velocity conditions were met, i.e. the enzyme activity depended on the enzyme concentration and was independent of the substrate concentration. Assays were always run in triplicate, and activities were expressed as a variation in the absorbance per minute, as specific activity per milligram of protein (ΔAbs min–1 mg protein–1), or as a percentage. The soluble protein content of the extracts was measured by the Bradford protein assay [26] using bovine serum albumin as a standard.
2.1. Trypsin and chymotrypsin-like activities
Trypsin-like (EC 3.4.21.4) activity was measured using 1.25 mM N-benzoil-DL-Arg-p-nitroanilide (BApNA) in 200 mM Tris-HCl containing 20 mM CaCl2 (pH 7.0) as described elsewhere [27]. Chymotrypsin-like (EC 3.4.21.1) activity was measured using 0.1 mM Suc-Ala-Ala-Pro-Phe-p-nitroanilide (SApNA) in the same buffer [27]. Substrate stock solutions of BApNA (125 mM) and SApNA (10 mM) were prepared in DMSO and brought to the working concentration by diluting with buffer prior to the assay. The assays were performed in 96-well microplates, and reaction mixtures consisted of 50 µL of enzyme extract, 150 µL of the buffer and 50 µL of the substrate working solution. Released p-nitroanilide (pNA) was detected kinetically every 15 s for 10 min at 405 nm and 37°C, using a Biotek ELx808IU microplate reader. The speed of the reaction was determined by the slope of the linear portion of the absorbance versus time plot, using the KC4 software (BioTek).
2.2. Alpha-amylase activity
Alpha-amylase (α-1,4-alpha-D-glucan glucanohydrolase; EC 3.2.1.1) activity was measured using 1.125 mM 2-Chloro-4-nitrophenyl-a-D-maltotrioside (CNP-G3) as a substrate in assay buffer (50 mM MES [2-(N-morpholino) ethanesulfonic acid], pH 5.5) [28]. The assay was performed in 96-well microplates, and the reaction mixture consisted of 50 µL of enzyme extract in 200 µL of the buffer with CNP-G3. The released 2-chloro-4-nitrophenol (CNP) was measured kinetically at 405 nm for 10 min at 37°C.
2.3. Esterase activity
Esterase activity (EC 3.1.11) was measured using 0.3 mM p-nitrophenyl butyrate (pNPB) as described previously [29]. The stock solution of pNPB substrate (10 mM) was prepared in 1 mL of acetone and brought to the working concentration by diluting with buffer (50 mM Tris-HCl, 6 mM CaCl2, 2 M NaCl, pH 9). The assay was performed in 96-well microplates, and the reaction mixture consisted of 10 µL of enzyme extract, 190 µL of the buffer and 50 µL of the working substrate solution. Released p-nitrophenol (pNP) was detected as described above.
3. Effect of ionic strength and calcium on enzymatic activity
The effect of NaCl and CaCl2 on the activity of different enzymes was evaluated by using different concentrations of NaCl (0.025, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 1, 1.5, 2 M; for esterase activity, the range was extended up to 3 M) and CaCl2 (0, 1, 2.5, 6, 12.5, 25, 50 mM) in the assay buffers. Negative controls were carried out using buffer solutions without the enzyme. The optimum concentrations of NaCl and CaCl2 for each enzyme were always used in subsequent assays.
4. Effects of pH and temperature on the activity and stability of enzymes
The optimum pH for the enzymes studied was determined by evaluating their activities at different pH, using the following buffer solutions: 50 mM sodium citrate (pH 2–4), Tris-HCl 50 mM (pH 5–8) and 50 mM Glycine-NaOH (pH 9–10). Enzyme assays were performed as described above. The optimum temperature was determined by measuring the activity of the enzymes in the range from 10 to 70°C. The effects of pH and temperature on the stability of the digestive enzymes were assessed by preincubating the enzyme extracts at different pH and temperature for 60 min. The mixtures were then brought to the optimum pH of the enzyme before performing the enzyme assay at 37°C. The stability results were expressed as a residual activity (% of the maximum activity). Activation energy (E a) and Q10 values were calculated for the range from 10 to 37°C. E a was obtained from the Arrhenius plot of log activity versus 1/T.
5. Zymograms of digestive proteases and α-amylase
Substrate-SDS-PAGE (5% stacking gel, 15% separating gel) was used to determine the composition of digestive proteases [30]. The samples were neither heated nor treated with β-mercaptoethanol. Gels were run at 30 mA constant current and 4°C in a vertical electrophoresis device (P81 Puffin™, Owl, 8 × 10 × 0.75 cm). The gel was then immersed in a 3% casein solution for 45 min at 4°C to allow the diffusion of casein into the gel and incubated at 37°C for 1 h to allow the proteases to digest gel-embedded casein. The gel was washed with distilled water and stained with 0.1% Coomassie Brilliant Blue in 40% methanol with 10% acetic acid and finally distained with the same solution without the dye. The gel stains blue due to the presence of casein except the areas with proteolytic activity. Unreduced molecular weight markers (15–175 kDa, NIPPON Genetics) were used to determine apparent molecular weights. In addition, for protease classification, the same substrate-SDS-PAGE was used [27], but the enzyme extract was incubated with different inhibitors for 60 min at 25°C before electrophoresis. The inhibitors employed were the soybean trypsin inhibitor (SBTI) and phenylmethane sulfonyl fluoride (PMSF) for serine proteases inhibition, NEM for cysteine protease inhibition, EDTA for metalloprotease inhibition, Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK) for trypsin-like inhibition, N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) for chymotrypsin-like inhibition. The absence of bands in the presence of specific inhibitors indicated a specific type of protease.
Substrate-SDS-PAGE for α-amylase was performed in a 5% stacking gel and a 12% separating gel as described previously [27]. Electrophoresis was carried out under the same conditions as for proteases. The gel was immersed in a starch solution (1%, p/v) at pH 6 for 60 min. Subsequently, the gel was washed and stained with a iodine/KI solution (10%) until clear bands were visualized on the dark background. Molecular weight markers (15–175 kDa, NIPPON Genetics) without a reducing agent were used to determine apparent molecular weight.
5.1. Reverse zymography
In order to determine the protease inhibitory activity of hepatopancreatic extracts from the king crab, a reverse zymography was used [31]. To do this, a Substrate-SDS-PAGE (5% stacking gel, 15% separating gel) was performed. The separating gel was co-polymerized with 1% gelatin as a substrate. The run was performed as mentioned above. Then, the gel was washed six times every 10 min with 100 mL of 2.5% Triton X-100 to eliminate SDS. Subsequently, the gel was incubated at 37°C for 1 h in 50 mM Tris-HCl (pH 7.5), bovine trypsin (0.01 mg/mL). The gel was then stained with Coomassie brilliant blue 0.1% in a solution of 40% methanol and 10% acetic acid for 1 h and distained with the same solution without dye until bands with inhibitory activity were visible.
6. Inhibitory activity
The inhibitory activity in crude extracts of hepatopancreas was determined as described previously [32]. Briefly, the proteolytic activity of bovine trypsin was determined using BApNA as a substrate (final concentration, 0.9 mM). A bovine trypsin solution was prepared at a concentration of 1 mg/mL in trypsin-stabilizing buffer (1 mM HCl, 20 mM CaCl2). The nominal trypsin concentration was determined at 280 nm using an extinction coefficient (E280 1%) of 14.4 [33]. Then, different concentrations of crude hepatopancreatic extracts were used in a mixture composed of 50 µL trypsin 1 mg/mL; 10, 20, 30, 40, 50, 60 and 70 µL extract of hepatopancreas. The mixture was brought to a final volume of 200 µL with the buffer (50 mM Tris-HCl, 20 mM CaCl2, 20 mM NaCl, pH 7.0). The final mixture was incubated at 37°C for 10 min, and 50 µL of the substrate (BApNA) were added thereafter. Absorbance readings were made every 15 s for 10 min at 405 nm and 37°C, using a Biotek ELx808IU microplate reader. The data were recorded using the BioTek KC4 software. For a control reaction, the same mixture was used to replace the volume of the homogenate by the buffer solution. The percentage of inhibition was determined according to the formula:
where v i—initial velocity obtained for extracts and enzymes; v 0—initial velocity obtained for the enzyme.
RESULTS
1. Digestive enzymes in Maguimithrax spinosissimus
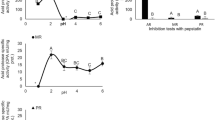
We first established the distribution of major digestive enzymes in the digestive tract of the Caribbean king crab Maguimithrax spinosissimus, and then determined their operational parameters. Table 1 shows the specific activities of digestive enzymes measured in the gastric fluid and hepatopancreas of M. spinosissimus. In the hepatopancreas, α-amylase and esterase activities were 2 and 14 times higher, respectively, than in gastric fluid. However, trypsin and chymotrypsin-like activities were mostly observed in gastric fluid, with trypsin activity doubling chymotrypsin activity. It is worth noting the low trypsin and chymotrypsin-like activities found in the hepatopancreas, where these enzymes are synthesized and stored (Table 1). This contrasted with the high number of protease bands observed in the hepatopancreas after electrophoresis (Fig. 1a). More than 12 bands between 45 and 10 kDa of the apparent molecular weight were detected. Some of these bands were absent from gastric fluid at the moment of sampling (Fig. 1a). To investigate the possible causes for low activities of trypsin and chymotrypsin-like enzymes in the hepatopancreas, we evaluated the protease inhibitory activity of crude hepatopancreatic extracts using bovine trypsin as an enzyme. Our results suggested the presence of a putative inhibitor of serine proteases, as we found a positive correlation between the hepatopancreatic crude extract concentration and inhibition of bovine trypsin (Fig. 1b). Moreover, by analyzing hepatopancreas crude extracts using a reverse zymography, an intense and diffuse band was observed below 10 kDa (Fig. 1c), corroborating the presence of at least one small serine protease inhibitor.
The use of specific inhibitors of proteolytic activity showed that PMSF is a poor inhibitor of serine proteases in this crab (Fig. 2a). However, the protease bands below 20 kDa were strongly inhibited by SBTI, indicating that they all are serine proteases (Fig. 2a). Three of these bands were also turned off by EDTA (Fig. 2a). Although TLCK was a weak inhibitor, it partially inhibited proteases smaller than 15 kDa, indicating that these are trypsin-like enzymes.
Since TPCK was unable to inhibit the observed bands, chymotrypsin-like enzymes remained unidentified (Fig. 2a). Two very active bands between 30–40 kDa were not inhibited by serine protease inhibitors or any other inhibitor used in this study and thus remained unclassified (Fig. 2a). Alpha-amylase exhibited high activity in gels both for the hepatopancreas and gastric fluid, where the activity is apparently produced by a single enzyme. The molecular weight of α-amylase in M. spinosissimus is approximately 40 kDa (Fig. 2b).
2. Optimal conditions for digestive enzyme activities
Trypsin and chymotrypsin-like enzymes showed high activities at pH between 6 and 7.5, with optimum at pH 7.0. No activity was found under acid conditions (pH ≤ 4.0 for trypsin-like and pH ≤ 5.0 for chymotrypsin-like enzymes) (Table 2). Alpha-amylase showed a highest activity at pH 5.5. Esterase activity was quite sensitive to acidic media, and the activity increased at pH ≥ 6 with optimal values at pH 9.0 (Table 2). The pH obtained for gastric fluid was 5.6 and 4.8 for the hepatopancreas. Trypsin and chymotrypsin-like enzymes exhibited maximum activities at 60°C, α-amylase between 40–60°C, and esterase at 37°C (Table 2). Activation energy (E a) and Q10 values were calculated for the range from 10 to 37°C (Table 3).
Trypsin and chymotrypsin-like enzymes exhibited maximum activities at 0.02 and 1.5 M NaCl, respectively. Chymotrypsin activity was more affected by the ionic strength than trypsin activity. CaCl2 exerted a positive effect on these activities up to 25 mM (Figs. 3a–3b; Figs. 4a–4b). In the case of α-amylase, NaCl did not affect the activity, but it increased at CaCl2 concentrations with maximum values at 2.5 mM (Figs. 5a–5b). CaCl2 in the reaction media poorly affected esterase activity. However, this activity was enhanced by NaCl concentrations up to 2M.
3. pH and temperature stability of digestive enzymes
Trypsin and chymotrypsin-like activities were not stable at pH ≤ 4.0 (Figs. 3c, 4c). However, after 1 h at pH 5.0, 80% of trypsin and 100% of chymotrypsin activity were maintained. Alpha-amylase was the most stable enzyme as it maintained more than 50% of its activity at pH 3.0 (Fig. 5c), while esterase activity showed only 20% of its maximum activity in acidic media. In alkaline buffers, however, all enzymes sustained more than 70% of their activity until pH 10. Regarding thermal stability, trypsin-like enzymes were shown to be slightly less thermostable than chymotrypsin-like enzymes. The former lost their activity above 37°C, while the latter above 40°C (Figs. 3d, 4d). Remarkably, α-amylase activity showed a great thermal stability. After 1 h at 70°C, only 35% of the activity were compromised (Fig. 5d). Conversely, esterase enzymes appeared to be most thermally unstable as they lost stability above 20°C; esterase enzymes maintained 30% of their initial activity at 40°C.
Proteolytic activity and endogenous serine protease inhibitor in hepatopancreas extract of M. spinosissimus. (a) Zymogram (15% SDS-PAGE) shows the proteolytic activity in hepatopancreas (HP) extracts and gastric fluid (GF); (b) Inhibition assay of bovine trypsin activity with increasing concentration of hepatopancreas extract; (c) Reverse zymogram (15% SDS-PAGE) of trypsin activity showing the inhibitory activity in hepatopancreas extract. MW: molecular weight. Inhibitor is marked with arrow.
Enzymatic activity in hepatopancreas extracts of M. spinosissimus. (a) Proteolytic activity and inhibition by specific inhibitors. Zymogram (SDS-PAGE 15% acrylamide). The following inhibitors were used: soybean trypsin inhibitor (SBTI) and phenylmethane sulfonyl fluoride (PMSF) for serine proteases, NEM for cysteine protease, EDTA for metalloprotease, Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK) for trypsin, N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) for chymotryosin. The absence of spots in the presence of specific inhibitors indicates a specific type of protease. Arrows show when there was a clearance or disappearance of the bands. (b) Amylolytic activity (SDS-PAGE 12% acrylamide) in hepatopancreas (HP) and gastric fluid (GF). MW: molecular weight.
Characterization of chymotrypsin-like activity in crab M. spinosissimus. Effect of CaCl2 (a), NaCl (b) on chymotrypsin activity and stability of the enzyme under different pH (c) and temperature (d). The enzyme activity was measured using 0.1 mM Suc-Ala-Ala-Pro-Phe-p-nitroanilide (SApNA) as a substrate, n = 5.
Characterization of α-amylase activity in Maguimithrax spinosissimus. Effect of CaCl2 (a), NaCl (b) on α-amylase activity and stability of the enzyme under different pH (c) and temperature (d). The enzyme activity was measured using 0.5 mM 2-Chloro-4-nitrophenyl-a-D maltotrioside (CNP-G3) as a substrate, n = 5.
DISCUSSION
Digestive enzymes of crustaceans are produced in the hepatopancreas. Among them, carbohydrases, such as α-amylase, peptidases, esterases, and lipases, are the main enzymes involved in food digestion [27]. The type of digestive enzymes in the gut, as well as their activities, reflect the ability of the animal to use different components of the diet. This issue has been addressed in different crustacean groups, such as penaeid shrimps [34, 35], spiny lobsters [36, 37] and crabs [3, 4, 5, 6, 38]. This is the first study on the digestive biochemistry of M. spinosissimus, a crab species that exhibits a high feeding plasticity.
Proteases, and especially enzymes with trypsin- and chymotrypsin-like activities, have been the subject of a large number of studies due to their central role in the digestion of proteins [36, 39]. The contribution of trypsin activity to total proteolysis in different crustaceans varies from 33 to 60%, whereas chymotrypsin-like activity seems to be more important for the caridean shrimps [27, 34, 35]. Our results indicate a predominance of trypsin activity in M. spinosissimus, which doubles the chymotrypsin activity in gastric fluid (Table 1). High proteolytic activity in gastric fluid, especially of trypsin, has been observed previously in carnivorous species, such as the spiny lobsters [27, 36] and other crustaceans. It is well known that M. spinosissimus consumes large amounts and a great variety of algae [40, 18, 41]. Thus, the high protease activity found in M. spinosissimus gastric fluid may represent an adaptive mechanism to maximize the digestion of low protein diet (i.e., vegetable material) but may also allow this crab to display an opportunistic feeding behavior when the animal material becomes abundant in the environment. Indeed, it is known that M. spinosissimus can behave like an opportunistic carnivore [15, 19, 42, 43]. Also, activation energy (E a) and Q10 values were calculated for the range from 10 to 37°C, covering the full range of temperatures this species may face in its natural environment. No substantial differences were found in E a between α-amylase and proteases. However, the Q10 obtained for α-amylase was higher than for trypsin and chymotrypsin, indicating that amylase activity is more thermally sensitive than protease activity. This result suggests that specimens found in deeper waters may exhibit a higher decrease in amylase than in protease activity, which may be of adaptive value in an environment where algae became sparse. On the other hand, given the high proteolytic activity (both of trypsin and chymotrypsin) observed in gastric fluid of M. spinosissimus, our results indicate that protein digestion begins in the gastric chamber as soon as the food is ingested. The degradation of ingested proteins can be further aided by the secretion of trypsin, chymotrypsin and other proteases from the hepatopancreas into the gastric chamber.
The proteolytic activity in M. spinosissimus hepatopancreas was very low (Table 1). When proteolytic activity was studied using a protein fraction separation technique (i.e. zymography), the presence of several proteolytic enzymes was observed in this organ (Fig. 1a). The presence of twelve bands with proteolytic activity, from 10 to 45 kDa, in the M. spinosissimus hepatopancreas (Fig. 1a) agrees with studies in other crustaceans. In particular, this protease pattern is similar to that described in the hepatopancreas of juveniles and gastric fluid of the adult southern king crab Lithodes santolla [44], although few differences are evident. Low-molecular-weight protease (12–20 kDa) patterns are quite similar in both species, including different bands of trypsin-like enzymes according to our in-gel inhibitor assays. However, we found a greater number of proteases of high molecular weight (20–60 kDa) in M. spinosissimus, suggesting that this species may be better equipped for digesting a wide variety of protein substrates. The protease abundance in other animals, such as insects, has been related with functional diversification as an adaptation to plant feeding [45]. Notably, some proteases found in the M. spinosissimus hepatopancreas were absent from gastric fluid. This is not common amid crustaceans, especially in species that routinely ingest more animal items. Indeed, it is different to that observed in a strict carnivore, the spiny lobster Panulirus argus [27], and in the scavenger and omnivorous predator L. santolla [44]. Moreover, the composition of proteinases in the hepatopancreas and feces are identical in an omnivorous/opportunistic predator, the shrimp Penaeus vannamei [46]. Thus, our results suggest that protease secretion from the hepatopancreas into gastric fluid of M. spinosissimus is a selective process, and this mechanism may have evolved as an adaptation to a specific diet. Two explanations seems likely for this observation. Firstly, “leading” proteases may be responsible the first steps of protein digestion, while other proteases may be collectively secreted postprandially. Secondly, some proteases in the hepatopancreas are only secreted if specific nutrient signaling occurs after the ingestion of a specific diet. Adding a complexity to the regulation of these enzymes in M. spinosissimus, they appear to be tightly controlled by an inhibitor-based mechanism in the digestive gland.
Indeed, our observation of low protease activity in the hepatopancreas and the high number of bands in zymograms suggested the presence of a protease inhibitor in the hepatopancreas. We further verified this hypothesis by determining the inhibitory activity of the M. spinosissimus hepatopancreas crude extract toward the bovine trypsin (Fig. 1b), confirming thereby the presence of a serine protease inhibitor. The presence of protease inhibitors in the hepatopancreas of crustaceans has been documented previously; they can inhibit bovine trypsin by 64 to 73% [30]. The molecular mass of the described inhibitors ranges from 5.9 to 13.2 kDa [30, 39], similar to the mass of 15 kDa that we estimated for the M. spinosissimus inhibitor (Fig. 1c). In general, the role of proteinase inhibitors in the gut of crustaceans is poorly understood from a functional and evolutionary perspective.
While other carbohydrases, such as laminarinase, play key roles in the digestion of marine macroalgae, alpha-amylase is essential for the digestion of starch. Starch is a major storage carbohydrate in various species of seaweeds [47], often reaching more than 20% of their dry matter [48]. Molecular weights of alpha-amylases range from 29 to 68 kDa in crustaceans [5, 49–51, 27, 37, 49, 38]. For M. spinosissimus α-amylase, an approximate molecular weight of 40 kDa was obtained. Interestingly, in spite of the high α-amylase activity found in this crab species, only one isoform was observed in zymograms, so it is anticipated that this single enzyme exhibits a very high catalytic activity. This digestive feature matches the herbivorous feeding habitat of this crab. However, glycogen is a storage polysaccharide in marine invertebrates, e.g., mussels, and it is also a suitable substrate for alpha-amylase. Thus, high amylase activity in this crab may also represent an adaptation to opportunistic carnivore behavior. On the other hand, the high esterase activity observed in the M. spinosissimus hepatopancreas would be related with extracellular and intracellular digestion, as well as metabolic processes in the gland, such as detoxification [27, 52].
Usually, the optimum pH of an enzyme approximately reflects the conditions that prevail in the medium in which it works [53]. In crustaceans, pH values between 4.0 and 5.5 were reported for the hepatopancreas [54], while for gastric fluid, pH varies from 5.0 to 7.0 [55], matching the results obtained in this study: pH 4.8 and 5.6 for the hepatopancreas and gastric fluid, respectively. In general, the optimum pH for the enzymes studied showed that they are suited to exhibit an efficient activity at the pH found in the crab gut. Also, our results on the optimum pH for different enzymes are consistent with those reported for proteases [27, 56], α-amylases [37, 51, 57] and esterases [4, 27, 58] in other crustaceans. In addition, we showed that digestive enzymes in M. spinosissimus are stable at pH values found in the digestive tract, hence they are suited for extended digestion if transit time decreases as an adaptation to a low-quality diet.
All the enzymes studied enhanced their activity with increments in temperature, although optimal temperatures varied. The optimal temperature was 60°C for trypsin- and chymotrypsin-like enzymes, 40–60°C for α-amylase, and 37°C for esterases; all these values are consistent with the data on other crustacean trypsins [27, 56] and α-amylases [5, 27, 37, 59], while values for esterases are more variable across species [4, 27]. Accordingly, given the tropical habitat of M. spinosissimus, thermal stability of trypsin and chymotrypsin activities (up to 40°C) was higher than in the temperate lobster P. interruptus [60], being at the same time similar to that in the tropical lobster P. argus [27]. While the thermal stability of α-amylases is compromised above 30–37°C in some crustacean species [37, 57, 59], we showed that α-amylase in M. spinosissimus was very stable at high temperatures (Fig. 5d). In general, these results relate with structural features of the enzymes, and it is not clear if there is some relation with a high temperature and thermal stability of the environment M. spinosissimus has evolved in.
Trypsin and chymotrypsin-like enzymes in M. spinosissimus increased their activity with the ionic strength until maximum activity at 0.02 M and 1.5 M, respectively, as detected in other crustaceans [27]. However, α-amylase activity does not seem to be affected by NaCl, and this differs from what have been observed in other species [37, 61]. Whether these features have evolved as a result of the stable (i.e., marine) environment of M. spinosissimus is unknown. In euryhaline crustaceans, such as the crab N. granulate, α-amylase and trypsin gene expression and activity are more affected by environmental salinities [6].
We also found that the presence of CaCl2 only slightly enhanced trypsin-like and chymotrypsin activities. Contradictory results have been obtained in crustaceans and other invertebrates regarding the requirement of calcium for trypsin activity. Unlike mammalian trypsins, many invertebrate trypsins do not seem to require calcium for their activity or stability. However, trypsins from crustaceans, such as the shrimp P. vannamei [62], crayfish A. leptodactylus [63] and spiny lobster P. argus [64], have calcium binding sites. In this study, the loss of some bands (in zymograms) with serine protease activity in the presence of EDTA indicated that a minimum amount of calcium is mandatory for the activity of some M. spinosissimus proteases. On the other hand, at least one calcium binding site occurs in α-amylases [53, 65], while studies on the crab C. maenas [66], spiny lobster P. argus [37] and other invertebrates [67, 68] showed an enhancement of α-amylase activity as the CaCl2 concentration increased. Similarly, M. spinosissimus α-amylase activity increased as the concentration of CaCl2 rose up to 2.5 mM but then started to decline. It is well known that some members of the α-amylase family are also inhibited by Ca2+. This has been reported for enzymes with several Ca2+ binding sites, such as in Aspergillus niger (two binding sites) [65, 69]. This type of behavior has been reported previously for three penaeid shrimps, where α-amylase activity was inhibited at CaCl2 concentrations higher than 1 mM [59].
In the present study, the key digestive enzymes in the Caribbean king crab M. spinosissimus were characterized as the first step to understand biochemical adaptations to its feeding habits. In summary, we showed that this crab species exhibits a high proteolytic activity both in the hepatopancreas and the gastric chamber, that secretion of proteases in this crab is a selective process, and that the activity of serine proteases is tightly controlled by at least one endogenous inhibitor in the hepatopancreas. Other distinctive features of this crab’s digestive biochemistry are a great number of proteinase enzymes and isoenzymes, a single but very active α-amylase, and a high thermal stability both of proteases and α-amylase. These features of M. spinosissimus digestive biochemistry relate with its great feeding plasticity [15, 19, 42, 43] and its evolution in the tropical environment. Further studies are required to address, under controlled feeding conditions, the physiological significance and adaptive value both of the selective secretion and the inhibitor-based control over proteases.
REFERENCES
Johnston, D. and Freeman, J., Dietary preference and digestive enzyme activities as indicators of trophic resource utilization by six species of crab, Biol. Bull., 2005, vol. 208, no. 1, pp. 36–46.
Davie, P.J.F., Guinot, D., and Ng, P.K.L., Systematics and classification of Brachyura. Treatise on zoology–anatomy, taxonomy, biology, The Crustacea, 2015, vol. 9, pp. 1049–1130.
Brun, G. and Wojtowicz, M., A comparative study of the digestive enzymes in the hepatopancreas of Jonah crab (Cancer borealis) and rock crab (Cancer irroratus), Comp. Biochem. Physiol. B, 1976, vol. 53, pp. 387–391.
Smichi, N., Fendri, A., Zarai, Z., Bouchaala, E., Chérif, S., Gargouri, Y., and Miled, N., Lipolytic activity levels and colipase presence in digestive glands of some marine animals, Fish Physiol. Biochem., 2012, vol. 38, pp. 1449–1458.
Asaro, A., Paggi, R.A., De Castro, R., and Lopez-Mañanes, A.A., Amylase in the hepatopancreas of a euryhaline burrowing crab: characteristics and modulation, Turk. J. Zool., 2017, vol. 41, pp. 443–453.
Asaro, A., Martos-Sitcha, J.A., Martínez-Rodríguez, G., Mancera, J.M., and López Mañanes, A.A., In silico analysis and effects of environmental salinity in the expression and activity of digestive α-amylase and trypsins from the euryhaline crab Neohelice granulate, Can. J. Zool., 2017, vol. 96, pp. 127–139.
Karasov, W.H. and Douglas, A.E., Comparative digestive physiology, Compr. Physiol., 2013, vol. 3, no. 2, pp. 741–783. doi: 10.1002/cphy.c110054
Linton, S.M., Saborowski, R., Shirley, A.J., and Penny, J.A., Digestive enzymes of two brachyuran and two anomuran land crabs from Christmas Island, Indian Ocean, J. Comp. Physiol. B, 2014, vol. 184, no. 4, pp. 449–468. doi: 10.1007/s00360-014-0815-2
Hirche, H.J. and Anger, K., Digestive enzyme activities during larval development of Hyas araneus (Decapoda, Majidae), Comp. Biochem. Physiol. B, 1987, vol. 87, pp. 297–302.
Roitberg, B.D., Gillespie, D.R., Quiring, D.M., Alma, C.R., Jenner, W.H., Perry, J., Peterson, J.H., Salomon, M., and Van Laerhoven, S., The cost of being an omnivore: mandible wear from plant feeding in a true bug, Naturwiss., 2005, vol. 92, no. 9, pp. 431–434.
Poore, A.G.B., Ahyong, S.T., Lowry, J.K., and Sotka, E.E., Plant feeding promotes diversification in the Crustacea, Proc. Natl. Acad. Sci. USA, 2017. vol. 114, no. 33, pp. 8829–8834. doi: 10.1073/pnas.1706399114
Hultgren, K.M. and Stachowicz, J.J., Molecular phylogeny of the brachyuran crab superfamily Majoidea indicates close congruence with trees based on larval morphology, Mol. Phylogenet. Evol., 2008, vol. 48, pp. 986–996.
Windsor, A.M. and Felder, D.L., Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda: Brachyura: Majoidea), Invertebrate Systematics, 2014, vol. 28, no. 2, pp. 145–173.
Orensanz, J.M., Armstrong, J., Armstrong, D., and Hilborn, R., Crustacean resources are vulnerable to serial depletion–the multifaceted decline of crab and shrimp fisheries in the Greater Gulf of Alaska, Rev. Fish Biol. Fisheries, 1998, vol. 8, pp. 117–176.
Creswell, R.L., The cultivation of marine invertebrates indigenous to the Wider Caribbean Region: established culture techniques and research needs for crustacean, A Regional Shellfish Hatchery for the Wider Caribbean, 2010, p. 105.
Hurtado-Alarcón, J.C., Campos Campos, N.H., Bermúdez Tobón, A., and Márquez, E.J., Phylogeographic patterns in Maguimithrax spinosissimus (Decapoda: Mithracidae) from Colombian Caribbean, New Zealand J. Marine Freshwater Res., 2018, vol. 52, no. 1, pp. 118–137.
Humann, P., Deloach, N., and Wilk, L., Reef Creature Identification: Florida. Caribbean, Bahamas, New World Publications, Jacksonville, FL, 1992, vol. 328.
Butler, M.J. and Mojica, A.M., Herbivory by the Caribbean king crab on coral patch reefs, Mar. Biol., 2012. vol. 159, no. 12, pp. 2697–2706.
Wilber, D.H. and Wilber, T.P. Jr., The effects of holding space and diet on the growth of the West Indian spider crab Mithrax spinosissimus (Lamarck), J. Exp. Mar. Biol. Ecol., 1989, vol. 131, no. 3, pp. 215–222.
Wilber, D.H. and Wilber, T.P. Jr., The effects of holding space and diet on the growth of the West Indian spider crab Mithrax spinosissimus, J. Exp. Mar. Biol. Ecol., 1989, vol. 131, pp. 215–222.
Lancia, J.P., Fernandez-Gimenez, A., Bas, C., and Spivak, E., Adaptive differences in digestive enzyme activity in the crab Neohelice granulata in relation to sex and habitat, J. Crust. Biol., 2012, vol. 32, no. 6, pp. 940–948.
Serrano, A., Ontogenetic changes in the activity of chymotrypsin and carboxypeptidases A and B in mud crab, Scylla serrata, Isr. J. Aquacult-Bamid, 2013, vol. 65, pp. 1–6.
Wolcott, D.L. and O’Connor, N.J., Herbivory in crabs: adaptations and ecological considerations, Amer. Zoologist, 1992, vol. 32, pp. 370–381.
Perera, E., Rodriguez-Casariego, J., Rodriguez-Viera, L., Calero, J., Perdomo-Morales, R., and Mancera, J.M., Lobster (Panulirus argus) hepatopancreatic trypsin isoforms and their digestion efficiency, Biol. Bull., 2012, vol. 222, no. 2, pp. 158–170.
Hata, S., Azomi, K., and Yokosawa, H., Ascidian phenoloxidase: its release from hemocytes, isolation, characterization and physiological roles, Comp. Biochem. Physiol. B, 1998, vol. 119, pp. 769–776.
Bradford, M., A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, vol. 72, pp. 248–254.
Perera, E., Moyano, F.J., Díaz, M., Perdomo-Morales, R., Montero-Alejo, V., Alonso, E., Carrillo, O., and Galich, G.S., Polymorphism and partial characterization of digestive enzymes in the spiny lobster Panulirus argus, Comp. Biochem. Physiol. B, 2008, vol. 150, pp. 247–254.
Gella, F.J., Gubern, G., Vidal, R., and Canalias, F., Determination of total and pancreatic α-amylase in human serum with 2-chloro-4-nitrophenyl-α-D-maltotrioside as substrate, Clinica Chimica Acta, 1997, vol. 259, no. 1, pp. 147–160.
Perera, E. and Yúfera, M., Effects of soybean meal on digestive enzymes activity, expression of inflammation-related genes, and chromatin modifications in marine fish (Sparus aurata L.) larvae, Fish Physiol. Biochem., 2017, vol. 43, no. 2, pp. 563–578.
García-Carreño, F.L., Dimes, E.N., and Haard, F., Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors, Anal. Biochem., 1993, vol. 214, pp. 65–69.
Hanspal, J.S., Bushell, G.R., and Ghosh, P., Detection of protease inhibitors using substrate-containing sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Anal. Biochem., 1983, vol. 132, no. 2, pp. 288–293.
Perdomo-Morales, R., Montero-Alejo, V., Corzo, G., Besada, V., Vega-Hurtado, Y., et al., The trypsin inhibitor panulirin regulates the prophenoloxidase-activating system in the spiny lobster Panulirus argus, J. Biol. Chem., 2013, vol. 288, no. 44, pp. 31867–31879.
Labouesse, J., and Gervais, M., Preparation of chemically defined ε N-acetylated trypsin, Eur. J. Biochem., 1967, vol. 2, pp. 215–223.
Galgani, F., Benyamín, Y., and Ceccaldi, H., Identification of digestive proteinasas of Penaeus kerathurus: a comparison with Penaeus japonicas, Comp. Biochem. Physiol. B, 1984, vol. 78, pp. 355–361.
Tsai, I.H., Lien, K.C., and Chuang, J.L., Chymotrypsins in digestive tracts of crustacean decapods (shrimp), Comp. Biochem. Physiol. B, 1986, vol. 85, no. 1, pp. 235–239.
Perera, E. and Simon, C., Digestive physiology of spiny lobsters: implications for formulated diet development, Rev. Aquacult., 2014, vol. 7, no. 4, pp. 243–261.
Rodríguez-Viera, L., Perera, E., Martos-Sitcha, J.A., Perdomo-Morales, R., Casuso, A., Montero-Alejo, V., et al., Molecular, biochemical, and dietary regulation features of α-amylase in a carnivorous crustacean, the spiny lobster Panulirus argus, PLoS ONE, 2016, vol. 11, no. 7, e0158919.
Andrés, M., Gisbert, E., Díaz, M., Moyano, F.J., Estévez, A., and Rotllant, G., Ontogenetic changes in digestive enzymatic capacities of the spider crab, Maja brachydactyla (Decapoda: Majidae), J. Exp. Mar. Biol. Ecol., 2010, vol. 389, nos. 1–2, pp. 75–84.
Albuquerque-Cavalcanti, C., García-Carreño, F.L., and del Toro, M., Trypsin and trypsin inhibitors from Penaeid shrimp, J. Food Biochem., 2002, vol. 26, no. 3, pp. 233–251.
Wilber, D., Wilber, T.P.Jr., Iglehart, J., and Adey, W., Culture of the Caribbean king crab on Grand Turk, Turks and Caicos Islands, BWI, 1992, pp. 588–591.
Butler IV, J.M. and Kintzing, M.D., An exception to the rule: top–down control of a coral reef macroinvertebrate community by a tropical spiny lobster, Bull. Mar. Sci., 2016, vol. 92, no. 1, pp. 137–152.
Winfree, R.A. and Weinstein, S., Food habits of the Caribbean king crab Mithrax spinosissimus (Lamarck), Proc. 39th Gulf and Caribbean Fisheries Institute, 1989.
Tunberg, B.G. and Creswell, R.L., Development, growth, and survival in the juvenile Caribbean king crab Mithrax spinosissimus (Lamarck) reared in the laboratory, J. Crust. Biol., 1991, vol. 11, no. 1, pp. 138–149.
Saborowski, R., Thatje, S., Calcagno, J.A., Lovrich, G.A., and Anger, K., Digestive enzymes in the ontogenetic stages of the southern king crab, Lithodes santolla, Mar. Biol., 2006, vol. 149, no. 4, pp. 865–873.
Srinivasan, A., Giri, A.P., and Gupta, V.S., Structural and functional diversities in lepidopteran serine proteases, Cell Mol. Biol. Lett., 2006, vol. 1, no. 1, pp. 132–154.
Córdova-Murueta, J.H., García-Carreño, F.L., and Navarrete-del-Toro, M.A., Digestive enzymes present in crustacean feces as a tool for biochemical, physiological, and ecological studies, J. Exp. Mar. Biol. Ecol., 2003, vol. 297, pp. 43–56.
Cian, R.E., Drago, S.R., De Medina, F.S., and Martínez-Augustin, O., Proteins and carbohydrates from red seaweeds: evidence for beneficial effects on gut function and microbiota, Mar. Drugs, 2015, vol. 13, pp. 5358–5383.
Prabhu, M., Chemodanov, A., Gottlieb, R., Kazir, M., Nahor, O., Gozin, M., Israel, A., Livney, Y.D., and Golberg, A., Starch from the sea: the green macroalga Ulva sp. as a potential source for sustainable starch production from the sea in marine biorefineries, Algal Res., 2019, vol. 37, pp. 215–227.
Van Wormhoudt, A., Bourreau, G., and Lemoullac, G., Amylase polymorphism in Crustacea Decapoda electrophoretic and immunological studies, Biochem. Syst. Ecol., 1995, vol. 23, no. 2, pp. 139–149.
Asaro, A., Paggi, R.A., del Valle, J.C., and López-Mañanes, A.A., Glucose homeostasis in the euryhaline crab Cytograpsus angulatus: Effects of the salinity in the amylase, maltase and sucrase activities in the hepatopancreas and in the carbohydrate reserves in different tissues. Comp. Biochem. Physiol. B, 2018, vol. 216, pp. 39–47.
Wojtowicz, M.B. and Brockerhoff, H., Isolation and some properties of the digestive amylase of the American lobster (Homarus americanus), Comp. Biochem. Physiol. B, 1972, vol. 42, no. 2, pp. 295–298.
Perera, E., Moyano, F. J., Díaz, M., Perdomo-Morales, R., Montero, V., Rodríguez-Viera, L., Alonso, E., Carrillo, O., and Galich, G., Changes in digestive enzymes through developmental and molt stages in the spiny lobster, Panulirus argus, Comp. Biochem. Physiol. B, 2008, vol. 151, pp. 250–256.
Delkash-Roudsari, S., Zibaee, A., and Mozhdehi, M.R.A., Digestive α-amylase of Bacterocera oleae Gmelin (Diptera: Tephritidae): Biochemical characterization and effect of proteinaceous inhibitor, J. King Saud Univ. Scienc., 2014, vol. 26, pp. 53–58.
Bickmeyer, U., Lüders, A.K., and Saborowski, R., pH measurements in midgut gland cells of crustaceans, Comp. Biochem. Physiol. A, 2008, vol. 151, no. 1, S48.
Dall, W. and Moriarty, D.J.W., Functional aspects of nutrition and digestion, The Biology of Crustacea, Internal Anatomy and Physiological Regulation, vol. 5, Mantel, L.H., ed., Academic Press, New York, 1983, pp. 215–261.
Michiels, M.S., del Valle, J.C., and López-Mañanes, A.A., Trypsin and N-aminopeptidase (APN) activities in the hepatopancreas of an interticial euryhaline crab: biochemical characteristics and differential modulation by histamine and salinity, Comp. Biochem. Physiol. A, 2017, vol. 204, pp. 228–235.
Dutta, T.Kr., Jana, M., Pahari, P.R., and Bhattacharya, T., The effect of temperature, pH, and salt on amylase in Heliodiaptomus viduus (Gurney) (Crustacea: Copepoda: Calanoida), Turk. J. Zool., 2006, vol. 30, no. 2, pp. 187–195.
López-López, S., Nolasco, H., and Vega-Villasante, F., Characterization of digestive gland esterase-lipase activity of juvenile redclaw crayfish Cherax quadricarinatus, Comp. Biochem. Physiol. B, 2003, vol. 135, no. 2, pp. 337–347.
Castro, P.F., Freitas, A.C.V., Santana, W.M., Costa, H.M.S., Carvalho, L.B., and Bezerra, R.S., Comparative study of amylases from the midgut gland of three species of penaeid shrimp, J. Crust. Biol., 2012, vol. 32, pp. 607–613.
Celis-Gerrero, L.E., García-Carreno, F.L., and Navarrete del Toro, M.A., Characterization of proteases in the digestive system of spiny lobster (Panulirus interruptus), Mar. Biotech., 2004, vol. 6, pp. 262–269.
Vega-Villasante, F., Nolasco, H., and Civera, R., The digestive enzymes of the Pacific brown shrimp Penaeus californiensis: I-Properties of amylase activity in the digestive tract, Comp. Biochem. Physiol. B, 1993, vol. 106, no. 3, pp. 547–550.
Klein, B., Le Moullac, G., Sellos, D., and Van Wormhoudt, A., Molecular cloning and sequencing of trypsin cDNA from Penaeus vannamei (Crustacea, Decapoda): use in assessing gene expression during the moult cycle, Int. J. Biochem. Cell Biol., 1996, vol. 28, pp. 551–563.
Fodor, K., Harmat, V., Hetényi, C., Kardos, J., Antal, J., Perczel, A., Patthy, A., Katona, G., and Gráf, L., Extended intermolecular interactions in a serine protease–canonical inhibitor complex account for strong and highly specific inhibition, J. Molec. Biol., 2005, vol. 350, pp. 156–169.
Perera, E., Pons, T., Hernandez, D., Moyano, F.J., Martínez-Rodrıíguez, G., and Mancera, J.M., New members of the brachyurins family in lobster include a trypsin-like enzyme with amino acid substitutions in the substrate-binding pocket, FEBS J., 2010, vol. 277, pp. 3489–3501.
Janec̆ek, S., α-Amylase family: molecular biology and evolution, Prog. Biophys. Mol. Biol., 1997, vol. 67, no. 1, pp. 67–97.
Blandamer, A. and Beechey, R.B., The purification and properties of an alpha-amylase from the hepatopancreas of Carcinus maenas, the common shore crab, Biochimica et Biophysica Acta, 1966, vol. 118, pp. 204–206.
Žóltowska, K., The isoenzymes of o-amylase from the intestine of Ascaris suum, Helminthologia, 2001, vol. 38, no. 4, pp. 205–209.
Louati, H., Zouari, N., Fendri, A., and Gargouri, Y., Digestive amylase of a primitive animal, the scorpion: purification and biochemical characterization, J. Chromatography, B, 2010, vol. 878, pp. 853–860.
Dojnov, B., Božić, N., Nenadović, V., Ivanović, J., and Vujčić, Z., Purification and properties of midgut α-amylase isolated from Morimus funereus (Coleoptera: Cerambycidae) larvae, Comp. Biochem. Physiol. B, 2008, vol. 149, pp. 53–160.
ACKNOWLEDGMENTS
Special thanks to Lázaro Macias and Daylin Ortiz for technical support during experiments, and to Armando Perez for collaborating in animal’s collection. This work was partially supported by IFS grant F/5024-2.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chávez-Rodríguez, L., Rodríguez-Viera, L., Montero-Alejo, V. et al. A Very Active α-Amylase and an Inhibitor-Based Control of Proteinases Are Key Features of Digestive Biochemistry of the Omnivorous Caribbean King Crab Maguimithrax spinosissimus . J Evol Biochem Phys 56, 550–564 (2020). https://doi.org/10.1134/S0022093020060083
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093020060083