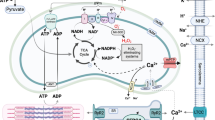
Abstract
The review addresses the Ca2+-dependent mitochondrial mechanisms of adaptation of cardiomyocytes to hypoxia and ischemia and analyzes signaling mechanisms responsible for expression of “antioxidant” genes and the formation of tolerance to hypoxia and ischemia. A special attention is paid to the role of the transcription factor Nrf2, reactive oxygen species and large-conductance Ca2+-activated K+ channels (BKCa channels) in the regulation of adaptive responses of cardiomyocytes in myocardial ischemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Under physical overload and numerous pathological conditions, intracellular physiological and metabolic processes get disrupted due to oxygen deficiency. Primarilly, this concerns metabolically active tissues functioning of which relies on mitochondria playing a key role in cellular energy metabolism. Mitochondrial dysfunction causes a reduced performance of cardiomyocytes (CM), as well as neurodegenerative pathologies and so-called mitochondrial diseases.
Since the discovery of mitochondrial respiration in 1949, these intracellular organelles are intensely being explored all over the world. However, despite numerous studies, the mechanisms of mitochondria-mediated cytoprotection, particularly cardioprotection, remain far from being completely understood. Recent observations have demonstrated that CM mitochondria, apart from their pivotal energy-producing function, are actively involved in intracellular signaling and regulation of specialized CM functions. In doing this, an important role the implementation of intracellular signaling and proper mitochondrial functions is played by calcium ions. The Ca2+ imbalance in CM mitochondria leads to impair cardiac activity and develop cardiovascular pathology [1]. Previously, we already noted a pathogenetic significance of Ca2+ in the development of post-ischemic myocardial injuries and gave examples of the involvement of Ca2+ in the processes influencing CM energetics and myocardial contractility [2]. In experiments on mitochondria isolated from the rat heart, it was established that Ca2+ ions are involved in the regulation of the inner mitochondrial membrane (IMM) permeability and that mitochondrial Ca2+-activated nonspecific (or mitochondrial permeability transition, MPT) pores are implicated in the induction of apoptosis and necrosis in CM [3–5]. However, while the mechanisms of the mitochondrial involvement in the development of functional and structural disorganization of CM are well covered in the literature, the information about the mitochondrial mechanisms (including Ca2+-dependent) of cardioprotection is very sparse, indicative of an insufficient elaboration of this problem [1]. Currently, mitochondria are considered as objects of targeted medicinal therapy of many diseases, including cardiovascular, and from this point of view, the task of generalizing and summarizing the knowledge accumulated in this field of cell biology appears important and quite timely.
EFFECT OF HYPOXIA ON ACTIVITY OF CARDIOMYOCYTES AND KEY FUNCTIONS OF MITOCHONDRIA
Hypoxia is the central pathogenetic factor of multiple pathological processes and chronic diseases, including those of the cardiovascular system (CVS), among which ischemic heart disease is most widespread.
The molecular basis of pathogenetic disorders in hypoxia and ischemia is mainly represented by various disturbances of the key mitochondrial functions (ATP-generating and Ca2+-depositing), leading to energy dysfunction of CM, impaired myocardial excitability and the impossibility for ventricular CM to implement their contractile function [2, 6].
In studies carried out on a model of isolated mitochondria, the following timing of intracellular changes caused by the cessation of oxygen supply was established [17]:
0–5 min: a 2–4-fold decrease in the ATP level despite activated glycolysis.
5–15 min: an increase in the intracellular Ca2+ concentration; activation of hydrolytic enzymes, including mitochondrial phospholipase A2; Ca2+ overloading of mitochondria while preserving their key functions (mitochondria are not yet damaged).
15–30 min: hydrolysis of mitochondrial phospholipids by phospholipase A2 and damage to the barrier properties of the mitochondrial membrane; tissue reoxygenation at this stage leads to active mitochondrial swelling; mitochondrial respiratory control is impaired; oxidative phosphorylation is uncoupled; the ability of mitochondria to accumulate Ca2+ is reduced.
30–60 min: partial restoration of mitochondrial functions; temporary increase in the respiratory control and ability to accumulate Ca2+; the mechanism of compensatory processes leading to a temporary amelioration of mitochondrial functions is unknown, but it is clearly associated with the function of the cell as a whole, since during anaerobic incubation of isolated mitochondria this phenomenon is not observed.
60–90 min: irreversible damage to mitochondria and cell death.
It is important to point out that the sequence of intracellular changes due to anoxia is identical in a variety of tissues, as demonstrated in experiments on tissue sections, isolated cells and isolated mitochondria [7].
The heart is the most energy-consuming organ, with ATP accumulated in mitochondria during respiration being a major source of its energy [8]. Providing the myocardium with enough ATP requires a huge number of functionally active mitochondria which under normoxic conditions occupy about one third of the total CM volume and, accordingly, more than half the volume of myofibrils. In hypoxia, the number of mitochondria in CM increases many times [1]. Mitochondrial dysfunction induced by progressing hypoxia or ischemia/reperfusion leads to impair cellular respiration which, in turn, results in arrhythmias and reduced myocardial contractility [8].
Presently, the influence of the impaired mitochondrial ATP synthesis on functional activity of CM is best studied. It was established that when the intracellular ATP level decreases by 10–20%, the activity of all energy-dependent processes drops by 70–80%. Suppression of the mitochondrial energy production is accompanied by an attenuation of lipid β-oxidation leading to impaired lipid metabolism in CM, as well as accumulation of acyl-CoA-thioesters, acylcarnitines, ceramides and triglycerides having potential cytotoxicity. At the same time, there occurs a suppression of anabolic processes, impairment of ion pumping and, accordingly, ion homeostasis, leading to a disruption of specialized cellular functions and a reduction in the vital activity of CM [1, 7–9]. The effect of hypoxia on energy-dependent processes in CM was described in more detail in our previous work [2].
Another crucial factor that gains a pathogenetic significance during the progression of hypoxia is oxidative stress, which is induced by reactive oxygen species (ROS) generated due to disruption of the mitochondrial respiratory chain in CM. It is well known that during functioning of the respiratory chain under normoxic conditions, there occurs a production of small amounts of the superoxide radical, which is a byproduct of respiratory complexes. In functionally robust mitochondria, the effect of the mitochondrial antioxidant system (AOS), comprising glutathione, thioredoxin-2, glutathione peroxidase, phospholipid-hydroperoxide glutathione peroxidase and Mn-superoxide dismutase, prevents the ROS-induced impairment to mitochondrial structures [9]. In case of disrupted electron transfer between components of the respiratory chain under oxidative stress conditions during hypoxia, the superoxide radical production in mitochondria appreciably increases. In this situation, functional insufficiency of the mitochondrial AOS promotes the progression of oxidative stress, activation of self-sustaining processes of lipid peroxidation (LPO), oxidative damage to proteins and nucleic acids. Eventually, these events culminate in a reduction of cellular functions and accumulation of mutations in mitochondrial and nuclear DNA. In turn, damage to mitochondrial genes promotes the disruption of electron transfer in the respiratory chain, resulting in a further enhancement of the free radical production in mitochondria [9, 10].
By now, it has been demonstrated that ROS generated in mitochondria, by affecting the intracellular signaling mechanisms, can initiate the development of myocardial hypertrophy and fibrosis. An excessive ROS level can also promote a reduction of the CM contractility and an increase in the sensitivity of microfilaments to Ca2+ due to impaired ion exchange in CM, as well as damaged cell membranes and ion channels [10].
Currently, we know that mitochondria play a central role in the implementation of programmed cell death of CM caused primarily by an unregulated elevation of the intramitochondrial Ca2+ concentration [11, 12].
Ca2+overload in mitochondria. Multiple studies revealed that hypoxia evokes Ca2+ overloading of mitochondria, which has a number of negative consequences for CM. Among the most significant is uncoupling of oxidative phosphorylation that reduces the ATP output and prevents the restoration of an adequate energy supply to mitochondria; it also impairs passive ion transport to mitochondria, causes mitochondrial swelling, and disintegrates the IMM.
Accumulation of Ca2+ in the mitochondrial matrix passes in two phases, as demonstrated on a model of isolated mitochondria. The first phase covers an energy-dependent Ca2+ influx to the matrix, stimulation of respiration, and proton extrusion from the matrix. During the second phase, due to opening of MPT pores in the IMM, Ca2+ ions accumulated in the matrix begin to come out from the matrix, and in a while there occur three parallel events: a rapid increase in the IMM permeability, proton reuptake, and K+ release from the matrix. The progression of Ca2+ overload of isolated mitochondria is aggravated by an impairment of the mechanism of passive ion transport across the outer mitochondrial membrane [12].
The processes of free oxidation that promotes Ca2+ accumulation in the mitochondrial matrix of CM are thought to dominate in these organelles under conditions of ischemia and ischemia/reperfusion, as indicated by a cessation of mitochondrial Ca2+ uptake and even a release of already accumulated Ca2+ ions upon suppression of free oxidation. At the same time, the damaging effect of excess Ca2+ accumulation in CM intensifies under oxidative stress conditions initiated by hypoxia [10]. Modeling ischemia and reperfusion in experiments on rats led simultaneously to a reduction of the Ca2+-binding ability of mitochondria and the transmembrane potential, a decrease in the ADP/O coefficient, which is a ratio of added ADP and oxygen consumed in mitochondrial respiratory state 3, and a slowdown of mitochondrial state 3 respiration [13].
At the same time, it should be kept in mind that mitochondria are important Ca2+-sequestering organelles of CM, which by this capacity are only insignificantly inferior to the sarcoplasmic reticulum [3, 12]. They are directly involved in the regulation of Ca2+ homeostasis in CM and can be recruited in the mechanisms of CM adaptation to oxidative stress during reperfusion.
In general, an idea has taken shape to date that mitochondria represent primary targets under conditions of hypoxia and ischemia/reperfusion and that processes occurring in them can lead both to dysfunction and injury of CM and their post-ischemic adaptation [1, 6, 9, 13]. That is why many studies aim to explore the role of mitochondria, mitochondrial ROS and Ca2+ in the cardioprotective mechanism of ischemic and pharmacological preconditioning.
ROLE OF THE MITOCHONDRIAL RESPIRATORY CHAIN IN ADAPTATION TO HYPOXIA
The systemic response of an organism includes various adaptive reactions that result in an increased volume of alveolar ventilation, elevated blood oxygen capacity, centralized blood flow, increased cardiac output, etc. At the cellular level, these processes aim primarily to preserve the energy-producing function of mitochondria. In doing this, two types of mechanisms are employed: (a) an immediate compensatory mechanism aimed at preventing the consequences of acute hypoxia at the molecular level and rapid restoring the vital activity of cells in the post-hypoxic period, and (b) long-term mechanisms that form during a longer period and promote an increase in nonspecific cell resistance to oxygen deficiency. These mechanisms are based on regulatory reprogramming the activity of mitochondrial enzyme complexes (MEC) [14].
Under normoxic conditions, the performance of the mitochondrial respiratory chain, as a rule, depends on oxidation of NAD-dependent substrates, which is a main provider of the reducing equivalents from the cytosol to the respiratory chain via MEC I. Nonetheless, 25–30% of mitochondrial respiration in these conditions are associated with MEC II [9, 14] and oxidation of succinate the level of which in the mitochondrial matrix is quite low (0.2–0.4 mmol/L) [15].
At the early stage of hypoxia, there occurs an activation of electron transport by MEC I that promotes enhanced ATP synthesis. This reflects the primary compensatory mechanism of mobilizing the basic energy resources of the cell under conditions of relatively weak external exposures. With increasing hypoxic exposure, the redox-potential of MEC I and, accordingly, oxidation of NAD-dependent substrates, as well as compensatory activation of alternative pathways of oxidation of NAD- and FAD-dependent substrates supplying the reducing equivalents to MEC II–IV, decreases. Among the MECs, a special role is played by MEC II, the succinate oxidase oxidation pathway. Under hypoxia, it has thermodynamic advantages over oxidation of NAD-dependent substrates of the Krebs cycle, and despite preserving only two oxidation–phosphorylation coupling sites, high reaction rates ensure sufficient energy efficiency of the process in general [16]. Activation of the alternative metabolic pathways, which function as immediate compensatory mechanisms, allows preservation of the supply of reducing equivalents to the cytochrome segment of the respiratory chain, due to which the electron transport function of MEC III and IV, as well as ATP synthesis, at this segment remain undamaged, thus ensuring the retention of the energy-synthesizing function. This process is aimed at using the succinate–oxidase pathway of respiratory substrate oxidation, which is more efficient under hypoxic conditions, due to which there occurs a compensation of the decreased rate of oxidative transformations and ATP synthesis. Besides, this process prevents metabolic acidosis, which is characteristic of hypoxia, and therefore increases the myocardial tolerance to oxygen deficiency [14, 16]. Furthermore, since MEC II activation determines Ca2+ influx to mitochondria, intramitochondrial reserves of this cation increase to be used under conditions of hypoxia and reduced myocardial efficiency [17].
If hypoxia evokes no functional reorganization of the respiratory chain’s substrate segment, then there occurs a drastic mitochondrial de-energization: a decrease in the membrane potential, ATP loss, changes in the adenine nucleotide pool, impaired respiration associated with oxidation of NAD-dependent substrates serving as electron donors for MEC I. For this reason, switching of the mitochondrial substrate oxidation pathways is concomitant to virtually any types of hypoxia or ischemia/reperfusion [14, 16, 18, 19].
According to the L.D. Lukyanova’s opinion [9], reprogramming of the mitochondrial respiratory chain under hypoxia, which leads to switching of metabolic pathways from oxidation of NAD-dependent substrates to succinate oxidation, is due to the following reasons:
– sensitivity of succinate dehydrogenase (MEC II) to the redox state of pyridine nucleotides and the Fe–S cluster (MEC I + coenzyme Q), as well as the ability of MEC II to be activated upon an increase in their reduction degree under hypoxia and to be inhibited upon their oxidation;
– limitation of oxalic acid inhibition which suppresses the succinate-dependent respiration, because under conditions of a high reduction degree of the respiratory chain oxaloacetate (a competitive SDG inhibitor) is reduced to malate and its inhibitory effect on the enzyme decreases;
– kinetic advantage of succinate (a FAD-dependent substrate) oxidation under conditions of high NADH reduction (hypoxia) over NAD-dependent substrates due to the fact that in this context flavins are preserved in a more oxidized state;
– ability of succinate-dependent oxidation to maintain a high delivery rate of reducing equivalents to the respiratory chain and to provide a maximum output of energy-rich compounds per time unit; for the biological system, this is more important than a high coefficient of performance, as evaluated by the number of oxidation–phosphorylation coupling sites, which in this case decreases.
During long-term adaptation to hypoxia, there occurs transcriptional remodeling of properties of the main MEC I subunits, as a result of which MEC I recovers its ability to transfer electrons and catalyze oxidative phosphorylation, which was lost during immediate adaptation. While doing this, the prevailing influence of succinate oxidase oxidation on energy production and mitochondrial respiration gradually decreases [14, 16, 19].
SIGNALING AND CARDIOPROTECTIVE ROLES OF SMALL CONCENTRATIONS OF MITOCHONDRIAL REACTIVE OXYGEN SPECIES
It is quite evident that the dysfunction of CM mitochondria in hypoxia is in the focus of attention of contemporary medicine. However, when tackling the problems of pharmacological correcting CM disorders induced by a hypoxic or reperfusion injury to mitochondria, it should not be forgotten that these organelles are highly adapted to endogenous oxidative stress induced by periodical drops in the blood oxygen level and can thus resist physiological loads associated with short-term hypoxia [20]. During this process, the mechanisms of antioxidant defense and mitochondrial DNA (mtDNA) protection in CM are activated, and the mitochondrial respiratory control is enhanced. At the same time, ROS production increases, promoting mitochondrial biogenesis, increased resistance of CM to extreme exposures, and the induction of systemic metabolic cardioprotection [1].
Intracellular ROS homeostasis is an indispensable requirement of normal proceeding of multiple physiological and signaling processes, while the excess intracellular level of free oxygen radicals leads to oxidative stress, reduced cell viability and even cell death [21–23].
When hypoxia and ischemia/reperfusion progress during myocardial reoxygenation, ROS production in CM drastically intensifies [23, 24]. This occurs due to a decrease in the ADP concentration and impairment of the mitochondrial electron transport chains (mainly at the MEC I and III level [25]), as well as decrease in the production of mitochondrial antioxidants, intramitochondrial accumulation of glutathione [26] and synthesis of frataxin, a protein involved in the regulation of mitochondrial iron transport [27] and responsible for the formation of the mitochondrial Fe–S cluster. Decreased frataxin synthesis leads to an increase in the intracellular concentration of Fe2+, in the presence of which endogenous hydrogen peroxide can generate a highly active hydroxyl radical having a maximum cytotoxicity among other ROS [28]. A decrease in the partial pressure of oxygen in CM during ischemia, which is accompanied by a conversion of oxidized Fe3+ into reduced Fe2+ [29], also promotes hydroxyl radical production.
It is noteworthy that in many cases ROS-induced dysfunction of CM and vascular endothelial cells is accompanied or preceded by a disturbance of Ca2+ homeostasis [30]. In these cells, the major Ca2+ storage depot is the sarco/endoplasmic reticulum which contains up to 75% of all intracellular Ca2+ reserves; the second most important Ca2+ stores are mitochondria which substantially contribute to agonist-induced Ca2+ mobilization [31]. Ca2+ ions enter mitochondria mainly via an IMM uniporter and are extruded via a Na+/Ca2+ exchanger (NCX) [31].
In vascular endothelial cells, mitochondria tightly contact Ca2+ channels in the endoplasmic reticulum (ER) and plasma membrane (PM). It appears quite evident that endothelial mitochondria, unlike their counterparts in CM, should be of no considerable importance for cells, energy demands of which are about 2/3 covered by anaerobic glycolysis [32]. However, experimental results indicate that mitochondria in endothelial cells, exactly like in CM, perform important regulatory and signaling functions implemented with the involvement of ROS, and that these functions are disrupted during the development of cardiovascular pathology and surgical interventions. Most apparent ROS-induced pathological alterations in cells of the cardiovascular system arise under hypoxia, and in case of ischemia under reperfusion. The main sources of ROS during vascular reperfusion are NADPH oxidases, xanthine oxidase and the respiratory chain of mitochondria themselves (MEC IV) [33]. Enhancement of free-radical processes in endothelial mitochondria can result in pathological mitochondrial division and long-term elevation of the intramitochondrial Ca2+ level associated with ROS-induced inhibition of the mitochondrial NCX exchanger and structural/functional uncoupling of mitochondria and the ER [34, 35].
The role of mitochondrial calcium in damaging endothelial cells during reperfusion, likewise the calcium-ROS interrelationship in endothelial cells in general, represents an important, albeit poorly studied, problem [29]. The available data suggest that during reoxygenation there arise ROS-initiated oscillations in cytosolic Ca2+ [36], which, in turn, influence the mitochondrial state, enhancing thereby the secondary generation of ROS [37] and exocytosis of adhesive molecules that aggravate endothelial dysfunction due to leukocyte infiltration [38].
The negative influence of ROS on the vascular endothelium is largely determined by a damaging effect of oxygen radical on cell membranes. In doing this, ROS can activate some ER and PM channels, including IP3- and ryanodine-sensitive ones, as well as a number of uncontrolled (non-voltage-gated) Ca2+ entry channels of the TRP superfamily, leading thereby to Ca2+ overload of cells, increased IMM permeability, and eventually to programmed death of endothelial cells [39–43].
However, as has already been mentioned many times, in the cardiovascular system, ROS act not only as damaging factors under conditions of excessive intensification of free-radical oxidation, but also play a protective role as inducers of redox-sensitive cardiporotective signaling pathways, the key components of which are the transcription factors HIF-1 (hypoxia inducible factor-1) and Nrf2 (nuclear factor erythroid-derived 2-related factor 2). The structure and functions of HIF-1 as a signaling molecule in adaptation to hypoxia was described in our previous work [20]. Here we consider the components of another, less studied, Nrf2-dependent signaling pathway that is activated under conditions of hypoxia and ischemia/reperfusion, being targeted at cell protection from injuries associated with hypoxia [44].
The transcription factor Nrf2 refers to a family of DNA-binding proteins containing the leucine motif, a so-called leucine zipper, and thus represents a basic leucine zipper transcription factor. It is a major molecular regulator in the process of intracellular antioxidant defense. Under oxidative or electrophilic stress conditions, Nrf2 is translocated to the nucleus [44], where it binds to the antioxidant response regulators in promoters of multiple genes responsible for the synthesis of antioxidant defense enzymes and detoxication, such as superoxide dismutase-1, glutathione transferase, glutamate cysteinlygase, heme oxigenase-1 (HO-1), and others [45]. It was shown in some studies that Nrf2 overexpression has a cytoprotective effect, while the absence of its expression, by contrast, increases tissue sensitivity to oxidative stress [45–50]. Despite the fact that many types of structural abnormalities in cells and tissues induced by oxidative stress relate to mitochondrial dysfunction, it was unknown until recently whether Nrf2 protects mitochondria form ROS-induced injuries. In this regard, the studies which revealed the effect of Nrf2 on the biogenesis and functions of mitochondria in CM under oxidative stress conditions arouse special interest. In these works, it was demonstrated that Nrf2 overexpression prevents the development of ROS-induced mitochondrial lesions, such as a disruption of mitochondrial networks, loss of the mitochondrial membrane potential, decrease in cytochrome c expression. The localization of Nrf2 to the outer mitochondrial membrane suggests a direct interaction of this transcription factor with mitochondrial structural components in preserving their integrity and functional activity [44, 51, 52].
In in vitro and in vivo experiments on rats and rabbits, the effect of Nrf2 on oxidative stress-initiated mitophagy and cell death was investigated both under acute myocardial injury and developing heart failure [44, 53–57]. It was established that Nrf2 protects from destruction mtDNA and proteins involved in mitochondrial biogenesis, such as Drp1, Drp2, hFis-1, mitofusin 1, mitofusin 2, OPA1 [44, 53, 57], and also suppresses proteotoxic necrosis of CM through promoting autophagic clearance of toxic ubiquitized proteins [58]. It was also established that Nrf2 expression in CM decreases the degree of desadaptive left ventricular remodeling in response to blood pressure overload. At the same time, at the systemic level, Nrf2 deficiency was accompanied by the early onset of myocardial remodeling and subsequent development of heart failure [59, 60]. It is worth noting that heart failure and other cardiovascular disorders could also result from mutations in the Nrf2 gene itself or polymorphism of Nrf2-regulated detoxication- and AOS-related genes (its attenuated polymorphic forms) due to long-term oxidative stress. This can serve yet another proof of the importance of this transcription factor for providing normal vital activity of CM and mitochondria themselves under conditions of hypoxia and ischemia/reperfusion [61, 62].
As a signaling molecule, Nrf2 can be involved in signal transmission along the redox-dependent signaling pathways, primarily the Akt/PKB/mTOR (mammalian target rapamycin) pathway, which plays a key role in cell physiology of the cardiovascular system in the norm and in pathology [63, 64]. Activation of mTOR complexes is of great importance for myocardial cell survival under ischemia. Specifically, there is evidence that phosphorylated mTOR complexes, mainly mTORC1, exhibit cardioprotective properties due to suppression of autophagy and activation of restorative processes in the ischemic myocardium [64].
ROLE OF MITOCHONDRIAL POTASSIUM CHANNELS IN THE IMPLEMENTATION OF CARDIOPROTECTIVE EFFECTS OF ISCHEMIC PRECONDITIONING
In view of the critical role of potassium transport in providing normal performance of mitochondria in CM, there is no doubt that developing the measures to optimize intramitochondrial potassium transport under conditions of ischemia and ischemia/reperfusion is an important strategy in metabolic cardioprotection and molecular therapy. In normal physiology of CM, K+ transport in mitochondria is of great functional significance [65], but its role increases many times under hypoxia, when there occur drastic changes in cellular metabolism of CM and activation of the mechanisms of immediate cellular adaptation.
In mitochondria, there are several K+ transport systems, including passive transport, although considering a high electrochemical potential (~–200 mV at the mitochondrial matrix side), the rate of such a diffusion would be low [66]. As for the specific transport systems, there exists a system of electrogenic potassium entry into mitochondria through the specific K+ channels and K+/Н+ antiporter [65, 67, 68], of which K+ channels are considered not only as components of the mitochondrial transport system but also from the viewpoint of applied medicine as major targets of molecular therapy and prevention of aftereffects of ischemia and especially reperfusion that arises when blood supply returns to the ischemically injured myocardial zone [65]. First of all, it is about the mitochondrial ATP-sensitive K+ channels (mitoKATP), interest in which was incited by the discovery of the cardioprotective effect of intermittent hypoxia, a phenomenon that received the name of ischemic preconditioning [69].
Ischemic preconditioning (IP) is an adaptation of the myocardium to ischemia, which develops after several repeated transient episodes of ischemia/reperfusion and leads to the myocardial tolerance to a subsequent longer ischemic attack. According to the literature, in some cases IP allows preventing the development of myocardial infarction or, upon its development, providing a smaller area of the infarction zone; IP also decreases the probability of developing arrhythmias, including those induced by reperfusion, and prevents developing left ventricular dysfunction [2].
Studies conducted until recently demonstrate that the IP mechanism represents a cascade of metabolic and signaling events that begins from the activation of receptors by an ischemic stimulus and includes consecutive signal amplification and its transmission to the terminal effector targets, including the sarcolemmal and mitochondrial ATP-sensitive K+ channels [70].
When studying the molecular mechanisms of IP, it was established that KATP channels that open in the sarcolemma and mitochondria during ischemia play a central role in the implementation of protective effects of preconditioning. This was proved by the fact that the cardioprotective effect of IP can be completely blocked by KATP channel inhibitors, such as glibenclamide and 5-hydroxidecanoate [71]. Initially, it is exclusively the sarcolemmal KATP channels that were considered responsible for IP, but later on it has become obvious that a major role in the development of this process is played by mitoKATP [65, 72]. Mitochondrial KATP channels are specifically opened by pinacydil and diazoxide, while sarcolemmal ones are opened by pinacydil only and blocked by glibenclamide and 5-hydroxidecanoate, although, in contrast to the latter, mitoKATP channels are inhibited at far lower concentrations of the agents [65]. For more information about the role of mitoKATP channels in IP, their structure and regulation, see our previous work [2].
In this paper, we aim to attract attention to high-conductance Ca2+-activated K+ channels (BKCa) which represent another, poorly studied, type of mitochondrial K+ channels involved in the mechanism of cardioprotection [73].
Initially, studies were focused on the structure of BKCa channels localized on the plasma membrane. It was established that these channels are characterized by a large conductance and sensitivity to changes in the membrane potential and intracellular Ca2+ concentration [74]. A study of the structural and functional organization of BKCa channels demonstrated that the channel protein encoded by the Kcnma1 gene is formed by four α-subunits comprising the extracellular N-terminus, seven transmembrane domains (S0–S7), and intracellular C-terminus. The voltage-sensing domain includes S0–S4, while the channel pore and the selective filter are formed by the S5–S6 linker [75, 76].
The intracellular C-terminus, being a longest domain of the BKCa channel, contains two Ca2+-sensitive regions known as regulators of K+ conductance 1 and 2 (RCK1 and RCK2). Mutagenesis studies revealed that RCK1 contains two aspartate residues (D362/D367) crucial for Ca2+ regulation, while RCK2 comprises five consecutive aspartates in Ca2+-binding sites (“calcium bowl”). A combination of these components suffices for the activation of the BKCa channel at physiological Ca2+ concentrations [77, 78]. Besides Ca2+, BKCa channels can be activated by millimolar Mg2+ concentrations. This activity of the channel owes the presence of the Mg2+ sensor representing a constellation of amino acid residues (D99, N172 and E374, E399) localized at different channel α-subunits but interlinked by electrostatic interactions [79].
Importantly, the Kcnma1 gene can undergo extensive alternative splicing during transcription, yielding several BKCa channel isoforms with different functional characteristics, including voltage- and Ca2+ sensitivity, subcellular localization (including mitochondrial targeting), and responses to phosphorylation and modulation by arachidonic acid [80].
The first evidence that BKCa channels with a conductance of about 300 pS (in 150 mM KCl) are present on the IMM was obtained in the late 1990s [81]. The authors characterized the channel using mitoplasts, i.e. mitochondria deprived of the outer membrane and isolated from LN-229 glioma cells. Later on, mitochondrial BKCa channels (mitoBKCa) with similar conductances (within the range from 200 to 307 pS) were also found in other cell systems.
To date, it is well known that mitoBKCa and pore-forming α-subunits of plasmalemmal BKCa channels are encoded by the same gene (Kcnma1) [82]. This explains why they share common basic biophysical properties, including large conductance and similar responses to changes in the membrane potential and [Ca2+]i, although it is obvious that concrete values of their parameters may differ. A comparison of the properties of plasmalemmal BKCa channels and mitoBKCa in human LN-229 glioma cells showed that conductance was 199 ± 8 pS in the former and 278 ± 10 pS in the latter. Although both channel types are voltage- and Ca2+-dependent, their sensitivities were different [83]. In the inside-out configuration, plasmalemmal BKCa channels displayed low sensitivity to voltage, indicative of a low degree of their opening (Po) even at high voltage (Po < 0.1 at +80 mV and ~0.4 at 100 mV) and application of 400 µM Ca2+ from the cytosolic channel side, whereas the threshold (Po) of mitoBKCa opening in the on-mitoplast configuration and at the same Ca2+ concentration in the medium reached Po ~0.6 already at a negative voltage (–40 mV) [83]. Subsequently, it was shown that different types of cells express mitoBKCa with different voltage- and Ca2+-sensitivity.
For example, mitoBKCa channels in guinea pig CM displayed a highest Po ~0.9 at quite a broad input voltage range (from –60 to +60 mV) and Ca2+ concentration of 0.5 µM, which suggests that their molecular composition (e.g., association with auxiliary subunits) may considerably differ from the analogs expressed in glioma mitochondria, in which Po is 0.5 at a Ca2+ concentration of 1 µM and input voltage of +41 mV (half-activation voltage V1/2 = 41 mВ). The same variability also characterizes plasmalemmal BKCa channels even within the same cell type [84]. Obviously, in order to understand what underlies the variability and heterogeneity of BKCa channels, their biophysical and molecular characterization needs to be further detailed [80].
As mentioned above, interest in mitoBKCa channels is associated mainly with the discovery of their protective role in ischemia and reperfusion [73]. The authors managed to demonstrate that pharmacological preconditioning with the use of the NS1619 agent, a BKCa channel activator, at a concentration of 10–30 µM before the onset of reperfusion led to ameliorate diastolic filling of the left ventricle and to reduce the myocardial infarction zone. Both effects were abolished by 1 µM paxilline, a selective BKCa channel inhibitor. The viewpoint that activators of BKCa channels act exactly on mitochondrial BKCa channels in CM is supported by the following arguments: (1) NS1619 cannot interact with plasmalemmal BKCa channels because mature cardiomyocytes are well known not to express sarcolemmal BKCa [82, 85]; (2) K+ influx to mitochondria is accelerated in the presence of NS1619 and decelerated in the presence of 100 nM iberiotoxin, a selective BKCa blocker; (3) the protective effect of preconditioning with NS1619 is not associated with myorelaxation of vascular smooth muscle cells, as the myorelaxation effect triggered by BKCa activation is part of the natural ion mechanism regulating vascular tone under hypoxia. Subsequent studies confirmed the above viewpoint [80]. Moreover, in a number of studies it was proved that the protective effect of preconditioning mediated by activation of BKCa channels is due to the role of the latter in maintaining the normal (physiological) level of ROS and hence preventing the development of oxidative stress and disturbance of Ca2+ homeostasis [82, 85]. A direct measurement of the mitochondrial Ca2+-binding ability revealed that the protective effect of pharmacological preconditioning by NS1619 was lacking in Kcnma1 knockout mice [82]. In other experiments, it was shown that the application of iberiotoxin also led to decrease the mitochondrial Ca2+-binding ability [87]. These results imply that activation of BKCa channels can to a certain extent protect mitochondria from uncontrolled opening of MPT pores under hypoxia [87]. The mechanism of coupling BKCa channels with nonselective Ca2+ pores in the IMM is still unclear, the more that on mitochondria such studies have not ever been conducted. However, as has been shown in studies conducted on mitochondria from brain cells (close to CM in the intensity of energy metabolism and the level of oxygen consumption), inhibition of mitoBKCa by iberiotoxin decreased the amount of Ca2+ needed to depolarize mitochondria and thereby increased the degree of MPT pore opening [88]. At the same time, the release of cytochrome c, a marker of MPT pore opening and apoptosis, from mitochondria increased [87]. It was suggested that a key protein that drives the interaction of mitoBKCa channels and MPT pores is a proapoptotic protein Bax (Bcl-2 associated protein X) which directly binds to mitoBKCa channels, decreasing thereby their activity and triggering opening of MPT pores [87].
Summarizing the above-said, it is safe to say that mitoBKCa channels play an important role in cardioprotection and, along with the more studied mitoKATP channels, should be considered as promising molecular objects for cardioprotection and target therapy of ischemic heart disease.
Abbreviations
- AOS:
-
antioxidant system
- IP:
-
ischemic preconditioning
- CVS:
-
cardiovascular system
- CM:
-
cardiomyocytes
- mitoKATP :
-
mitochondrial ATP-sensitive K+ channels
- MPT pore:
-
mitochondrial permeability transition pore
- mtDNA:
-
mitochondrial DNA
- NCX:
-
Na+/Ca2+ ex-changer
- HIF-1:
-
hypoxia inducible factor-1
- HO-1:
-
heme oxigenase-1
- Nrf2:
-
nuclear factor erythroid-derived 2-related factor 2
- RCK:
-
regulators of K+ conductance
- BKCa channels:
-
high-conductance Ca2+-activated K+ channels
- IMM:
-
inner mitochondrial membrane
- MEC:
-
mitochondrial enzyme complexes
- PM:
-
plasma membrane
- SDG:
-
succinate dehydrogenase
REFERENCES
Pohjoismäki, J.L. and Goffart, S., The role of mitochondria in cardiac development and protection, Free Radic. Biol. Med., 2017, vol. 106, pp. 345–354. https://doi.org/10.1016/j.freeradbiomed.2017.02.032
Shemarova, I.V., Nesterov, V.P., Korotkov, S.M., and Sobol, K.V., Involvement of Ca2+ in the development of ischemic disorders of myocardial contractile function, J. Evol. Biochem. Physiol., 2017, vol. 53, pp. 368–379. https://doi.org/10.1134/S0022093017050027
Han, X., Xu, J., Xu, S., Sun, Y., He, M., Li, X., Li, X., Pi, J., Yu, R., and Tian, W., Role of mitochondrial permeability transition pore in mediating the inhibitory effect of gastrodin on oxidative stress in cardiac myocytes in vitro, Nan Fang Yi Ke Da Xue Xue Bao, 2018, vol. 38, pp.1306–1311. doi: 10.12122/j.issn.1673-4254.2018.11.05
Purroy, R., Britti, E., Delaspre, F., Tamarit, J., and Ros, J., Mitochondrial pore opening and loss of Ca2+ exchanger NCLX levels occur after frataxin depletion, Biochim. Biophys. Acta, 2018, vol. 1864, pp. 618–631. https://doi.org/10.1016/j.bbadis.2017.12.005
Wacquier, B., Romero Campos, H.E., González-Vélez, V., Combettes, L., and Dupont, G., Mitochondrial Ca2+ dynamics in cells and suspensions, FEBS J., 2017, vol. 284, pp. 4128–4142. https://doi.org/10.1111/febs.14296
Tsyplenkova, V.G., Sutyagin, P.V., Suslov, V.B., and Ettinger, A.P., Features of the mitochondrial apparatus of cardiomyocytes in various heart diseases and experiment, Mezhd. Zh. Priklad. Fundam. Issl., 2014, vol. 8(2), pp. 53–56.
Egorova, M.V., The role of fatty acids in adaptive reactions of cardiomyocytes under metabolic ischemia, Doctorate Sci. Diss., Tomsk, 2014.
Brown, D.A. and O’Rourke, B., Cardiac mitochondria and arrhythmias, Cardiovasc. Res., 2010, vol. 88, pp. 241–249. https://doi.org/10.1093/cvr/cvq231
Lukyanova, L.D., Disregulation of aerobic energy metabolism: a standard pathological process, Dizregulyatsionnaya patologiya (Disregulational Pathology), Moscow, 2002, pp. 188–215.
Giordano, F.J., Oxygen, oxidative stress, hypoxia, and heart failure, J. Clin. Invest., 2005, vol. 115, pp. 500–508. https://doi.org/10.1172/JCI200524408
Guimaraes, C.A. and Linden, R., Programmed cell death: apoptosis and alternative deathstyles, Eur. J. Biochem., 2004, vol. 217, pp. 1638–1650. https://doi.org/10.1111/j.1432-1033.2004.04084.x
Saris, N.E. and Carafoli, E., A historical review of cellular calcium handling, with emphasis on mitochondria, Biochem., 2005, vol. 70, pp. 187–194. https://doi.org/10.1007/s10541-005-0100-9
Lishmanov, Yu.B., Naryzhnaya, N.V., Maslov, L.N., Prokudina, E.S., Gorbunov, A.S., and Tsibulnikov, S.Yu., Functional state of myocardial mitochondria under cardiac ischemia-reperfusion in rats adapted to hypoxia, Byull. Eksp. Biol. Med., 2013, vol. 156(11), pp. 589–592.
Kirova, Yu.I., Regulatory role of succinate-dependent signaling systems (HIF-1 and GPR91) in adaptation to hypoxia, Doctorate Diss., Moscow, 2016.
Komaromy-Hiller, G., Sundquist, P.D., Jacobsen, L.J., and Nuttall, K.L., Serum succinate by capillary zone electrophoresis: marker candidate for hypoxia, Ann. Clin. Lab. Sci., 1997, vol. 27, pp. 163–168.
Lukyanova, L.D., Bioenergetic hypoxia: notion, mechanisms, correction, Byull. Eksp. Biol. Med., 1997, vol. 124(9), pp. 244–254.
Levrat, C. and Louisot, P., Study of the succinate dehydrogenase activation in permeabilized mitochondria through the Ca2+-stimulated phospholipase A2, Biochem. Mol. Biol. Int., 1994, vol. 34, pp. 569–578.
Dudchenko, A.M. and Lukyanova, L.D., The triggering role of energy metabolism in cascade of functional and metabolic disorders under hypoxia, Problemy gipoksii: molekulyarnye, fiziologicheskie i klinicheskie aspekty (Problems of Hypoxia: Molecular, Physiological and Clinical Aspects), Moscow, 2004, pp. 51–83.
Kondrashova, M.N., Transaminaznyi tsikl okisleniya substratov v kletke kak mekhanizm adaptatsii k gipoksii. Farmakologicheskaya korrektsiya gipoksicheskikh sostoyanii (Transaminase Cycle of Cellular Substrate Oxidation as a Mechanism of Adaptation to Hypoxia. Pharmacological Correction of Hypoxic Conditions), Moscow, 1989, pp. 51–66.
Shemarova, I.V., Nesterov, V.P., Korotkov, S.M, and Sylkin, Yu.A., Evolutionary aspects of cardioprotection, J. Evol. Biochem. Physiol., 2018, vol. 54(1), pp. 8–21. https://doi.org/10.1134/S0022093018010027
Gamaley, I.A., Role of reactive oxygen species in the regulation of cellular functions, Doctorate Sci. Diss., St. Petersburg, 1999.
Dröge, W., Free radicals in the physiological control of cell function, Physiol. Rev., 2002, vol. 82, pp. 47–95. https://doi.org/10.1152/physrev.00018.2001
Baines, C.P., How and when do myocytes die during ischemia and reperfusion: the late phase, J. Cardiovasc. Pharmacol. Ther., 2011, vol. 16, pp. 239–243. https://doi.org/10.1177/1074248411407769
Grebenchikov, O.A., Likhvantsev V.V., and Plotnikov, E.Yu., Molecular developmental mechanisms and target therapy of the ischemia-reperfusion syndrome, Anest. Reanim., 2014, vol. 3, pp. 59–67.
Herrero, A. and Baria, G., Localization of the site of oxygen radical generation inside the mitochondria, J. Bioenerg. Biomembr., 2000, vol. 32, pp. 609–615.
Kulinsky, V.I. and Kolesnichenko, L.S., Metabolism of glutathione, Usp. Biol. Khim., 1990, vol. 31, pp. 157–179.
Pandolfo, M., Frataxin deficiency and mitochondrial dysfunction, Mitochondrion, 2002, vol. 2, pp. 87–93. https://doi.org/10.1023/A:1005626712319
Vladimirov, Yu.A., Free radical and antioxidants, Vestnik RAMN, 1998, vol. 7, pp. 43–51.
Nadeev, A.D. and Goncharov, N.V., Reactive oxygen species in cells of the cardiovascular system, Kompl. Probl. Serd.-Sosud. Zabol., 2014, vol. 4, pp. 80–94.
Dhalla, N.S., Temsah, R.M., and Netticadan, T., Role of oxidative stress in cardiovascular diseases, J. Hypertens., 2000, vol. 18, pp. 655–673. https://doi.org/10.1097/00004872-200018060-00002
Malli, R., Frieden, M., Trenker, M., and Graier, W.F., The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum, J. Biol. Chem., 2005, vol. 280, pp. 12114–12122. https://doi.org/10.1074/jbc.M409353200
Culic, O., Gruwel, M.L., and Schrader, J., Energy turnover of vascular endothelial cell, Am. J. Physiol., 1997, vol. 273, pp. C205–C213. https://doi.org/10.1152/ajpcell.1997.273.1.C205
Pearlstein, D.P., Ali, M.H., Mungai, P.T., Hynes, K.L., Gewertz, B.L., and Schumacker, P.T., Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia, Arterioscler. Thromb. Vasc. Biol., 2002, vol. 22, pp. 566–573. https://doi.org/10.1161/01.ATV.0000012262.76205.6A
Paltauf-Doburzynska, J., Malli, R., and Graier, W.F., Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells, J. Cardiovasc. Pharmacol., 2004, vol. 44, pp. 423–436. https://doi.org/10.1097/01.fjc.0000139449.64337.1b
Jornot, L., Maechler, P., Wollheim, C.B., and Junod, A.F., Reactive oxygen metabolites increase mitochondrial calcium in endothelial cells: implication of the Ca2+/Na+ exchanger, J. Cell Sci., 1999, vol. 112, pp. 1013–1022.
Hu, Q. and Ziegelstein, R.C., Hypoxia/reoxygenation stimulates intracellular calcium oscillations in human aortic endothelial cells, Circulation, 2000, vol. 102(20), pp. 2541–2547. https://doi.org/10.1161/01.CIR.102.20.2541
Camello-Almaraz, C., Gomez-Pinilla, P.J., Pozo, M.J., and Camello, P.J., Mitochondrial reactive oxygen species and Ca2+ signaling, Am. J. Physiol., 2006, vol. 291, pp. 1082–1088. https://doi.org/10.1152/ajpcell.00217.2006
Ichimura, H., Parthasarathi, K., Quadri, S., Issekutz, A.C., and Bhattacharya, J., Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries, J. Clin. Invest., 2003, vol. 111, pp. 691–699. https://doi.org/10.1172/JCI17271
Hecquet, C.M. and Malik, A.B., Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury, Thromb. Haemost., 2009, vol. 101, pp. 619–625. https://doi.org/10.1160/TH08-10-0641
Hu, Q., Zheng, G., Zweier, J.L., Deshpande, S., Irani, K., and Ziegelstein, R.C., NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells, J. Biol. Chem., 2000, vol. 275, pp. 15749–15757. https://doi.org/10.1074/jbc.M000381200
Poteser, M., Graziani, A., Rosker, C., Eder, P., Derler, I., Kahr, H., Zhu, M.X., Romanin, C., and Groschner, K., TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3–TRPC4 heteromeric channels in endothelial cells, J. Biol. Chem., 2006, vol. 281, pp. 13588–13595. https://doi.org/10.1074/jbc.M512205200
Stoyanovsky, D., Murphy, T., Anno, P.R., Kim, Y.M., and Salama, G., Nitric oxide activates skeletal and cardiac ryanodine receptors, Cell Calcium, 1997, vol. 21, pp. 19–29. https://doi.org/10.1016/S0143-4160(97)90093-2
Sun, L., Yau, H.Y., Wong, W.Y., Li, R.A., Huang, Y., and Yao, X., Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells, PLoS One, 2012, vol. 7, p. 43186. https://doi.org/10.1371/journal.pone.0043186
Strom, J., Xu, B., Tian, X., and Chen, Q.M., Nrf2 protects mitochondrial decay by oxidative stress, FASEB J., 2016, vol. 30, pp. 66–80. https://doi.org/10.1096/fj.14-268904
Narasimhan, M. and Rajasekaran, N.S., Exercise Nrf2 and antioxidant signaling in cardiac aging, Front. Physiol., 2016, vol. 7, p. 241. https://doi.org/10.3389/fphys.2016.00241
Lee, J.M., Calkins, M.J., Chan, K., Kan, Y.W., and Johnson, J.A., Identification of the NF–E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis, J. Biol. Chem., 2003, vol. 278, pp. 12029–12038. https://doi.org/10.1074/jbc.M211558200
Nguyen, T., Nioi, P., and Pickett, C.B., The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress, J. Biol. Chem., 2009, vol. 284, pp. 13291–13295. https://doi.org/10.1074/jbc.R900010200
Li, W. and Kong, A.N., Molecular mechanisms of Nrf2-mediated antioxidant response, Mol. Carcin., 2009, vol. 48, pp. 91–104. https://doi.org/10.1002/mc.20465
Ma, Q., Role of nrf2 in oxidative stress and toxicity, Annu. Rev. Pharmacol. Toxicol., 2013, vol. 53, pp. 401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Kensler, T.W., Wakabayashi, N., and Biswal, S., Cell survival responses to environmental stresses via the Keap1–Nrf2–ARE pathway, Annu. Rev. Pharmacol. Toxicol., 2007, vol. 47, pp. 89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046
Piantadosi, C.A., Carraway, M.S., Babiker, A., and Suliman, H.B., Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1, Circ. Res., 2008, vol. 103, pp. 1232–1240. https://doi.org/10.1161/01.RES.0000338597.71702.ad
Suliman, H.B., Carraway, M.S., Tatro, L.G., and Piantadosi, C.A., A new activating role for CO in cardiac mitochondrial biogenesis, J. Cell Sci., 2007, vol. 120, pp. 299–308. https://doi.org/10.1242/jcs.03318
Dominic, E.A., Ramezani, A., Anker, S.D., Verma, M., Mehta, N., and Rao, M., Mitochondrial cytopathies and cardiovascular disease, Heart, 2014, vol. 100, pp. 611–618. https://doi.org/10.1136/heartjnl-2013-304657
Muthusamy, V.R., Kannan, S., Sadhaasivam, K., Gounder, S.S., Davidson, C.J., Boeheme, C., Hoidal, J.R., Wang, L., and Rajasekaran, N.S., Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium, Free Radic. Biol. Med., 2012, vol. 52, pp. 366–376. https://doi.org/10.1016/j.freeradbiomed.2011.10.440
Erkens, R., Kramer, C.M., Lückstädt, W., Panknin, C., Krause, L., Weidenbach, M., Dirzka, J., Krenz, T., Mergia, E., Suvorava, T., Kelm, M., and Cortese-Krott, M.M., Left ventricular diastolic dysfunction in Nrf2 knock out mice is associated with cardiac hypertrophy, decreased expression of SERCA2a, and preserved endothelial function, Free Radic. Biol. Med., 2015, vol. 89, pp. 906–917. https://doi.org/10.1016/j.freeradbiomed.2015.10.409
Ying, Z., Chen, M., Xie, X., Wang, X., Kherada, N., Desikan, R., Mihai, G., Burns, P., Sun, Q., and Rajagopalan, S., Lipoicmethylenedioxyphenol reduces experimental atherosclerosis through activation of Nrf2 signaling, PLoS One, 2016, vol. 11, p. e0148305. https://doi.org/10.1371/journal.pone.0148305
Dickinson, S.E., Lin, Y., Dedek, M., Morrissy, S., Johnson, J., and Chen, Q.M., Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor, J. Mol. Cell Cardiol., 2007, vol. 42, pp. 159–176. https://doi.org/10.1016/j.yjmcc.2006.09.012
Wang, W., Li, S., Wang, H., Li, B., Shao, L., Lai, Y, Horvath, G., Wang, Q., Yamamoto, M., Janicki, J.S., Wang, X.L, Tang, D., and Cui, T., Nrf2 enhances myocardial clearance of toxic ubiquitinated proteins, J. Mol. Cell Cardiol., 2014, vol. 72, pp. 305–315. https://doi.org/10.1016/j.yjmcc.2014.04.006
Korshunova, A.Yu., Pathogenetic features of cell death in myocardial alteration of varied genesis, Candidate Sci. Diss., Moscow, 2016.
Xing, Y., Niu, T., Wang, W., Li, J., Li, S., Janicki, J.S., Ruiz, S., Meyer, C.J., Wang, X.L., Tang, D., Zhao, Y., and Cui, T., Triterpenoid dihydro-CDDO-trifluoroethyl amide protects against maladaptive cardiac remodeling and dysfunction in mice: a critical role of Nrf2, PLoS One, 2012, vol. 7.(9), pp. 1–8. https://doi.org/10.1371/journal.pone.0044899
Suzuki, T., Shibata, T., Takaya, K., Shiraishi, K., Kohno, T., Kunitoh, H., Tsuta, K., Furuta, K., Goto, K., Hosoda, F., Sakamoto, H., Motohashi, H., and Yamamoto, M., Regulatory nexus of synthesis and degradation deciphers cellular Nrf2 expression levels, Mol. Cell Biol., 2013, vol. 33, pp. 2402–2412. https://doi.org/10.1128/MCB.00065-13
Morozova, K.V., Gene polymorphism of detoxication, antioxidant defense and DNA reparation enzymes in the genesis of noncarrying of pregnancy, Candidate Sci. Diss., Moscow, 2014.
Demeulder, B., Zarrinpashneh, E., Ginion, A., Viollet, B., Hue, L., Rider, M.H., Vanoverschelde, J.L., Beauloye, C., Horman, S., and Bertrand, L., Differential regulation of eEF2 and p70S6K by AMPKalpha2 in heart, Biochim. Biophys. Acta, 2013, vol. 1832, pp. 780–790. https://doi.org/10.1016/j.bbadis.2013.02.015
Sciarretta, S., Yee, D., Ammann, P., Nagarajan, N., Volpe, M., Frati, G., and Sadoshima, J., Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes, Clin. Sci. (Lond), 2015, vol. 128, pp. 387–403. https://doi.org/10.1042/CS20140336
Garlid, K.D., Dos Santos, P., Xie, Z.J., Costa, A.D., and Paucek, P., Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K+ channel in cardiac function and cardioprotection, Biochim. Biophys. Acta, 2003, vol. 1606, pp. 1–21. https://doi.org/10.1016/S0005-2728(03)00109-9
Mitchell, P. and Moyle, J., Translocation of some anions cations and acids in rat liver mitochondria, Eur. J. Biochem., 1969, vol. 9, pp. 149–155. https://doi.org/10.1111/j.1432-1033.1969.tb00588.x
Chaves, E., Yung, D., and Brierley, G., Energy-dependent exchange of K+ in heart mitochondria K+ efflux, Arch. Bioch. Biophys., 1977, vol. 183, pp. 460–470. https://doi.org/10.1016/0003-9861(77)90381-2
Diwan, J.J., Haley, T., and Sanadi, D.R., Reconstitution of K+ transport with 53 kDa mitochondrial protein, Biochem. Biophys. Res. Commun., 1988, vol. 153, pp. 224–230. https://doi.org/10.1016/S0006-291X(88)81212-9
Murry, C.E., Jennings, R.B., and Reimer, K.A., Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium, Circulation, 1986, vol. 74, pp. 1124–1136. https://doi.org/10.1161/01.CIR.74.5.1124
Santillo, E., Migale, M., Postacchini, D., Balestrini, F., and Incalzi, R.A., Cardioprotection by сonditioning mimetic drugs, Antiinflamm. Antiallergy Agents Med. Chem., 2016, vol. 15, pp. 15–30. https://doi.org/10.2174/1871523015666160719155122
Likhvantsev, V.V., Moroz, V.V., Grebenchikov, O.A., Gorokhovatsky, Yu.I., Zarzhetsky, Yu.V., Timoshin, S.S., Levikov, D.I., and Shaibakova, V.L., Ischemic and pharmacological preconditioning, Obshchaya Reanimatol., 2011, vol. 7(6), pp. 59–65. https://doi.org/10.15360/1813-9779-2011-6-59
Downey, J.M., Davis, A.M., and Cohen, M.V., Signaling pathways in ischemic preconditioning, Heart Fail. Rev., 2007, vol. 12, pp. 181–188. https://doi.org/10.1007/s10741-007-9025-2
Xu, W., Liu, Y., Wang, S., McDonald, T., Van Eyk, J.E., Sidor, A., and O'Rourke, B., Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane, Science, 2002, vol. 298, pp. 1029–1033. https://doi.org/10.1126/science.1074360
Contreras, G.F., Castillo, K., Enrique, N., Carrasquel-Ursulaez, W., Castillo, J.P., Milesi, V., Neely, A., Alvarez, O., Ferreira, G., González, C., and Latorre, R., ABK(Slo1)channel journey from molecule to physiology, Channels (Austin.), 2013, vol. 7, pp. 442–458. https://doi.org/10.4161/chan.26242
Gao, Y.D. and Garcia, M.L., Interaction of agitoxin 2, charybdotoxin, andiberiotoxin with potassium channels: selectivity between voltage-gated and maxi-K+ channels, Proteins, 2003, vol. 52, pp. 146–154. https://doi.org/10.1002/prot.10341
Banerjee, A., Lee, A., Campbell, E., and MacKinnon, R., Structure of apore-blocking toxin in complex with a eukaryotic voltage-dependent K+ channel, Elife, 2013, vol. 2, e00594. https://doi.org/10.7554/eLife.00594
Schreiber, M. and Salkoff, L., A novel calcium-sensing domain in the BK-channel, Biophys. J., 1997, vol. 73, pp. 1355–1363. https://doi.org/10.1016/S0006-3495(97)78168-2
Xia, X.M., Zeng, X., and Lingle, C.J., Multiple regulatory sites in large- conductance calcium-activated potassium channels, Nature, 2002, vol. 418, pp. 880–884. https://doi.org/10.1038/nature00956
Yang, H., Shi, J., Zhang, G., Yang, J., Delaloye, K., and Cui, J., Activation of Slo1BK-channels by Mg2+ coordinated between the voltage sensorand RCK1 domains, Nat. Struct. Mol. Biol., 2008, vol. 15, pp. 1152–1159. https://doi.org/10.1038/nsmb.1507
Balderas, E., Zhang, J., Stefani, E., and Toro, L., Mitochondrial BKCa channel., Front. Physiol., 2015, vol. 6, p. 104. https://doi.org/10.3389/fphys.2015.00104
Siemen, D., Loupatatzis, C., Borecky, J., Gulbins, E., and Lang, F., Ca2+-activated K+ channel of the BK-type in the inner mitochondrial membrane of a human glioma cell line, Biochim. Biophys. Res. Comm., 1999, vol. 257, pp. 549–554. https://doi.org/10.1006/bbrc.1999.0496
Singh, H., Lu, R., Bopassa, J.C., Meredith, A.L., Stefani, E., and Toro, L., mitoBK Ca is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location, Proc. Natl. Acad. Sci. U.S.A., 2013, vol. 110, pp. 10836–10841. https://doi.org/10.1073/pnas.1302028110
Gu, X.Q., Pamenter, M.E., Siemen, D., Sun, X., and Haddad, G.G., Mitochondrial but not plasmalemmal BK channels are hypoxia-sensitive in human glioma, Glia, 2014, vol. 62, pp. 504–513. https://doi.org/10.1002/glia.22620
Tanaka, Y., Meera, P., Song, M., Knaus, H.-G., and Toro, L., Molecular constituents of maxiKCa channels in human coronary smooth muscle: predominant alpha + beta subunit complexes, J. Physiol., 1997, vol. 502, pp. 545–557. https://doi.org/10.1111/j.1469-7793.1997.545bj.x
Schmitt, N., Grunnet, M., and Olesen, S.P., Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia, Physiol. Rev., 2014, vol. 94, pp. 609–653. https://doi.org/10.1152/physrev.00022.2013
Kulawiak, B., Kudin, A.P., Szewczyk, A., and Kunz, W.S., BK-channel openers inhibit ROS production of isolated rat brain mitochondria, Exp. Neurol., 2008, vol. 212, pp. 543–547. https://doi.org/10.1016/j.expneurol.2008.05.004
Cheng, Y., Gulbins, E., and Siemen, D., Activation of the permeability transition pore by Bax via inhibition of the mitochondrial BKchannel, Cell. Physiol. Biochem., 2011, vol. 27, pp. 191–200. https://doi.org/10.1159/000327944
Cheng, Y., Gu, X.Q., Bednarczyk, P., Wiedemann, F.R., Haddad, G.G., and Siemen, D., Hypoxia increases activity of the BK-channel in the inner mitochondrial membrane and reduces activity of the permeability transition pore, Cell. Physiol. Biochem., 2008, vol. 22, pp. 127–136. https://doi.org/10.1159/000149790
Funding
This work was supported by state budget funding (reg. no. АААА-А18-118012290142-9).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shemarova, I.V., Korotkov, S.M. & Nesterov, V.P. Ca2+-Dependent Mitochondrial Mechanisms of Cardioprotection. J Evol Biochem Phys 56, 304–317 (2020). https://doi.org/10.1134/S002209302004002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002209302004002X