The transmission spectra of femtosecond laser pulses in the mid-IR range (5–6.6 μm) were investigated of submonolayer of Staphylococcus aureus and Pseudomonas aeruginosa bacteria on a silicon plate in the region of characteristic absorption bands of proteins and lipids, as well as stationary spectra after irradiation. Invertible “translucence” of samples in the region of characteristic bands of bacteria and blue shift these bands indicating the destruction of hydrogen bonds were discovered. The possibility of inactivation of pathogenic bacteria by selective IR laser denaturation of functional proteins by resonance irradiation of low average power is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1. Besides traditionally used thermal and UV treatment methods, mid-IR radiation is also considered for inactivation of pathogenic bacteria method [1, 2]. One of the possible ways of IR inactivation of bacteria is denaturation of functional proteins (polypeptides) as a result of destruction of hydrogen bonds, providing proteins a secondary and tertiary structure [2, 3]. Proteins in cells have an important metabolic function, that realized through activation of biocatalytic reactions, electrons transfer and conformational transformations [4–7]. Thus, migration and relaxation of vibrational excitation energy in the structure of proteins determinate the directions, rates and efficiency of the above-mentioned processes. Subpicosecond laser pulses (∼102 fs) are used for real-time scale investigation by ultrafast spectroscopy methods [6, 7], as well as for multiphoton vibrational excitation [5].
Modification of IR absorption spectra of oligomers of amino acids (peptides) and simple molecules similar to them in aqueous solutions under the action of femtosecond laser pulses in the mid-IR range in the context of transformation of a secondary and tertiary structure was studied in works [6, 7] (a broader and more general overview [4, 8–10]). Differential absorption and intramolecular relaxation rate for O–H, N–H and C–H vibrations, as well as amid vibrations C=O (type I) and C–N (type II), associated with the anharmonicity of higher vibrationally excited states and the resulting red spectral shift of absorption bands, were measured in the IR range near 3 and 6 μm [6]. However, directly in bacteria, where the bands of amide vibrations of proteins overlap with bands analogical vibrations in lipids and other molecules, optical investigations haven’t yet been carried out. Thus, IR irradiation such complex biological systems as bacteria, may have specific character by the existence of hydrogen bonds. Even in simple water molecules, a blue spectral shift of vibrational absorption bands as the result of change surroundings of O–H fragments due to destruction of hydrogen bonds under the action of femtosecond IR radiation was observed [11]. It is important to note that in complex protein molecules, the destruction of a certain critical fraction of hydrogen bonds actually means their denaturation, i.e., inactivation of their function.
In this study, to determine the possibility of bacterial inactivation by the mechanism of selective denaturation of proteins, we experimentally investigated the change in the IR absorption of pathogenic bacteria samples under their intense vibrational excitation by ultrashort pulses in the region of characteristic vibrations of functional proteins and lipids. The measurements were made during multiple pulses irradiation with exposition 20 s, as well as in a stationary mode of exposure.
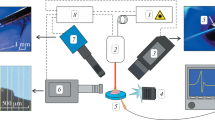
2. In our experiments, IR transmittances of pure (reference) and covered by a submonolayer of bacteria silicon wafers that were arranged in front of the IR spectrometer slit were measured (Fig. 1a). Bacterial isolates of cultures of Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) (took from the collection of the Gamaleya National Research Center for Epidemiology and Microbiology) were placed on 0.5‑mm-thick n-doped (phosphorus, 1017 cm–3) silicon wafers as a submonolayer with known optical density spectra in the IR range and were excited in the region C–N and C=O vibrations of amide groups (≈1500 and 1750 cm–1, Fig. 2). Stationary spectra of the optical density in the range of 2.5–25 μm (400–4000 cm–1) minus the optical density of a pure silicon wafer were recorded by a Bruker Vertex 70v FT IR spectrometer.
During the experiments, the sample was placed in front of spectrometer slit along the normal to the optical axis of radiation focused on the slit by a spherical mirror (focus length 150 mm). Mid-IR laser pulses radiation (central wavelength 5.8 μm, FWHM 0.6 μm), pulse duration 130 fs, pulse energy 2 μJ, frequency 1 kHz was obtained by parametric generation of radiation of Ti: sapphire laser (Spitfire HP, Spectra-Physics, central wavelength 800 nm, frequency 1 kHz, half-height pulse duration 50 fs) in parametric amplifier with difference-frequency generation “OPA TOPAS-C + nDFG” (Light Conversion) [12]. Peak intensity of radiation on sample surface achieved 5 GW/cm2 without filters and 0.3 GW/cm2 after attenuation by a set of neutral metallized filters on BaF2 substrates. The obtained spectra of transmitted IR radiation for bacterial samples were normalized on spectra of transmitted irradiation for pure silicon wafer (Fig. 1b), that gave a dynamic transmission spectrum of bacterial coating as a result (Figs. 3, 4).
(Color online) Dynamic transmission spectra of bacterial coating of Staphylococcus aureus (SA) with characteristic bands (stationary reference spectra for “zero intensity”) at different peak power laser pulses: (a) 0.3 GW/cm2 (fresh sample spot, weak pump); (b) 5 GW/cm2 (irradiation of the spot in panel (a), strong pump), (с) 0.3 GW/cm2 (panel (а), weak pump) and 0.3 GW/cm2 (probing of the point in panel (b), weak probe). The femtosecond laser pulse spectrum is shown below for comparison.
(Color online) Dynamic transmission spectra of bacterial coating of Pseudomonas aeruginosa (PA) with characteristic bands (stationary reference spectra for “zero intensity”) at different peak power laser pulses: (a) 0.3 GW/cm2 (fresh sample spot, weak pump), (b) 5 GW/cm2 (irradiation of the spot in panel (a), strong pump), (с) 0.3 GW/cm2 (panel (а), weak pump) and 0.3 GW/cm2 (probing of the point in panel (b), weak probe). The femtosecond laser pulse spectrum is shown below for comparison.
3. Comparison of stationary and dynamically transmission spectra of the bacterial coating of Staphylococcus aureus and Pseudomonas aeruginosa for different values of peak intensity of laser radiation is shown in Figs. 3, 4. It shows characteristic bands of absorption of bacteria, associated with stretching C=O vibrations of ester and carboxyl groups of fatty acids of the lipid layer of the bacterial membrane, nucleic acids, amide groups α-, β- and antiparallel secondary structures of proteins in the range of ≈1650–1750 cm–1 [13]. The adjacent band of C–N vibrations of amide groups in the region of about ≈1520–1550 cm–1 is associated mainly with proteins. There are C–H vibrations of hydrocarbon skeleton (≈1450 cm–1), as well as symmetric stretching С=О vibrations of free carboxyl groups [13].
In the case of both types of bacteria, 20-seconds exposure to low-intensity irradiation of ultrafast laser pulses (“weak pump,” peak intensity 0.3 GW/cm2) leads to an amplification of characteristic absorption in the range of 1550–1900 cm–1 and to pronounced spectral blue shift of bands by 100 cm–1 (Figs. 3a, 4a), that can be associated with destruction of hydrogen bonds, and that can achieve 300 cm–1 for water [11]. Strong modification of spectra (curve “strong pump” in Figs. 3b, 4b, that has not yet been explained, was observed in the case more intense 20-seconds exposure (peak intensity 5 GW/cm2) same area of bacterial coating. However, this modification is inversible, because after repeated 20-seconds low-intensity ultrafast laser pulses exposure of the same area of the bacterial coating (“weak probe,” Figs. 3с, 4с, peak intensity 0.3 GW/cm2) the spectrum of the initial low-intensity irradiation is well reproduced in Figs. 3a and 4a.
Subsequent stationary IR spectroscopy of the exposed samples show a noticeable modification of the characteristic absorption bands for the coating of Staphylococcus aureus and almost no change for the coating of Pseudomonas aeruginosa. Moreover, in the first case, changes take place in a wider range of 1000–3800 cm–1 (Fig. 5), than the relatively narrow laser action range of 1500–1900 cm–1 (Fig. 2). It may indicate the expected intramolecular energy transfer [4–12], which spatially expands the area of laser action beyond the borders of amide groups. Detailed studies of causes of changes in exposed samples and the viability of irradiated bacteria will be presented in subsequent works. However, it can already be noticed, that observed final modification of chemical structure may indicate laser inactivation of bacteria. Note, that previous studies of stationarity IR treatment of bacteria also demonstrated a significant inactivation effect in the 6 μm region while it was absent in the of 3 and 4.5 μm regions [2].
Let us estimate the characteristic levels of intensity and average power of ultrashort laser pulses for bacteria inactivation. Since using spectrum of laser pulses with central wavelength 5.8 μm (wavenumber 1700 cm–1, Fig. 1b) allows to excite C=O (band B1) and C–N (band B2) vibrations of amide groups of proteins, the effective intensity in each specific spectral range differs from the entire peak intensity of pulse \({{I}_{0}} \approx 5\) GW/cm2 (Fig. 2). In particular, effective intensity \({{I}_{{{{{\text{B}}}_{{1,2}}}}}}\) is determined by integrating and normalizing the ultrashort laser pulses spectrum within the corresponding bands B1,2
According to numerical estimates, the values of \({{I}_{{{{{\text{B}}}_{{1,2}}}}}}\) are 2.5 and 0.84 GW/cm2 (50 and 17%, respectively) for Staphylococcus aureus and 2.4 and 0.35 GW/cm2 (48 and 7%, respectively) for Pseudomonas aeruginosa. The corresponding average radiation powers at a pulse repetition rate of 1 kHz are in the range of 0.01–1 mW, which suggests the effect of low-power laser action.
4. In conclusion, multipulse laser irradiation of submonolayer coating of bacteria Staphylococcus aureus and Pseudomonas aeruginosa on a silicon wafer with low-intensity (∼0.1–10 GW/cm2) femtosecond IR laser pulses (5–6.6 μm) demonstrates a reversible “translucence” of the samples for spectral intervals in the range of characteristic absorptions bands of proteins, nucleotides and lipids of bacteria and blue shift of this absorption bands, potentially indicating a destruction hydrogen bonds. Significant changes in the stationary transmission spectra of exposed samples in the mid-IR range suggest the possibility of inactivation of pathogenic bacteria by the selective IR laser disruption of a critical number of hydrogen bonds and the associated irreversible denaturation of functional organelles by low-power resonance irradiation.
REFERENCES
D. Hamanaka, T. Uchino, N. Furuse, W. Han, and S. I. Tanaka, Int. J. Food Microbiol. 108, 281 (2006).
A. A. Oduola, R. Bowie, S. A. Wilson, Z. Mohammadi Shad, and G. G. Atungulu, J. Food Saf. 40, e12764 (2020).
W. Elliott and D. C. Elliott, Biochemistry and Molecular Biology (Oxford Univ. Press, Oxford, 2001).
D. Laage, T. Elsaesser, and J. T. Hynes, Chem. Rev. 117, 10694 (2017).
C. Kolano, J. Helbing, M. Kozinski, W. Sander, and P. Hamm, Nature (London, U. K.) 444 (7118), 469 (2006).
L. P. de Flores, Z. Ganim, S. F. Ackley, H. S. Chung, and A. Tokmakoff, J. Phys. Chem. B 110, 18973 (2006).
E. H. Backus, P. H. Nguyen, V. Botan, R. Pfister, A. Moretto, M. Crisma, C. Toniolo, G. Stock, and P. Hamm, J. Phys. Chem. B 112, 9091 (2008).
V. N. Bagratashvili, V. S. Letokhov, A. A. Makarov, and E. A. Ryabov, Multiple Photon Infrared LaserPhotophysics and Photochemistry (Harwood Academic, Chur, 1985).
A. A. Makarov, A. L. Malinovsky, and E. A. Ryabov, Phys. Usp. 55, 977 (2012).
E. T. Nibbering, H. Fidder, and E. Pines, Ann. Rev. Phys. Chem. 56, 337 (2005).
H. K. Nienhuys, S. Woutersen, R. A. van Santen, and H. J. Bakker, J. Chem. Phys. 111, 1494 (1999).
V. O. Kompanets, V. N. Lokhman, D. G. Poydashev, S. V. Chekalin, and E. A. Ryabov, J. Exp. Theor. Phys. 122, 621 (2016).
K. Maquelin, C. Kirschner, L. P. Choo-Smith, N. van den Braak, H. P. Endtz, D. Naumann, and G. J. Puppels, J. Microbiol. Methods 51, 255 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kompanets, V.O., Kudryashov, S.I., Totordava, E.R. et al. Femtosecond Infrared Laser Spectroscopy of Characteristic Molecular Vibrations in Bacteria in the 6-µm Spectral Range. Jetp Lett. 113, 365–369 (2021). https://doi.org/10.1134/S0021364021060060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364021060060