Abstract
Two microwave systems MARS-5 and UltraWAVE are compared in the efficiency with regard to the digestion of silicate rocks with subsequent determination of 32 elements (Sc, V, Cr, Co, Ni, Cu, Zn, Rb, Sr, Y, Nb, Ta, Cs, Ba, 14 REEs, Hf, Ta, Th, U) by inductively coupled plasma mass spectrometry (ICP-MS). The development of digestion methods and validation of the obtained results have been carried out using international reference materials—basalts BHVO-2 and BCR-2, serpentinite UB-N, and peridotite JP-1. Microwave digestion included a two-stage treatment of samples with a mixture of concentrated acids HF, HNO3, and HCl in MARS-5 (T = 190°C, P = 20 bar) and UltraWAVE (T = 240°C, P = 80 bar) with the distillation of excess fluorides in the form of SiF4 between microwave digestion stages. The determination of concentrations in the obtained solutions was carried out on an ELEMENT high-resolution mass spectrometer in low and medium resolution according to external calibration with the internal standard (In), taking into account the acid composition of the analyzed solutions. The detection limits of the analytes after acid digestion in MARS-5 and UltraWAVE are comparable and provide the determination of all specified elements, except for Ta in JP-1. The use of the developed sample preparation procedure in MARS-5 ensures the complete decomposition of BHVO-2, BCR-2, and UB-N followed by ICP-MS determination of 32 specified elements in the obtained solutions without additional preconcentration steps. The relative standard deviations for the determined elements are 2–9% for the reference materials BHVO-2 and BCR-2 and 3–12% for UB-N with an increase to 16–25% (Nb, Ta) due to the approach to the detection limit. The more efficient microwave digestion in UltraWAVE compared to MARS-5 was proved by the complete decomposition of JP-1 with the transfer of all the elements, including Cr, into the solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The application of ICP-MS for multielement analysis of silicate rocks requires the dissolution of all analytes, which is complicated at the sample preparation stage, especially for rocks containing refractory accessory minerals. When acid digestion is used at this stage, a low detection limit can be reached, which is essential to determine trace amounts. However, this long process does not always allow the complete transfer of the determined elements into solution [1–10]. Acid processing is frequently conducted in autoclaves under heating and high pressure to completely decompose geological samples with a silicate matrix [1–4]. The application of microwave systems is interesting because it dramatically reduces the decomposition time owing to fast and uniform heating of the reaction mixture and automatization [4–11].
Microwave digestion of silicate rocks was considered ineffective, and published results were contradictory. For example, the authors of [11, 12] mentioned difficulties with the complete dissolution of Zr and Hf for both granites and basalts, while their successful decomposition was discussed elsewhere [4, 7, 8, 10, 13]. At the same time, the authors of [14] claim that microwave acid processing can be applied to granites with subsequent determination of lanthanides, Th, and U. Modern studies conducted using powerful microwave systems (MARS-5, Multiwave, MileStone) show the efficiency of microwave acid digestion for rocks containing <60% silicon oxide and <1% refractory minerals such as chromite of zircon; in addition, such techniques are noticeably faster compared with other methods of acid digestion [4–8, 10, 13–16]. However, this area is under study because the developed techniques are not all-purpose, and microwave digestion with subsequent ICP-MS elemental analysis cannot be applied to some types of rocks [4, 7, 11, 13].
As in conventional acid digestion, mixtures of nitric and hydrofluoric acids taken in different ratios are used to destroy silicate matrix in microwave systems [4–6, 8, 10, 15, 16]. Then, excesses of HF and SiF4 are distilled, and fluorides are transformed into soluble nitrates and chlorides either by repeated acid treatment on a hot plate [8, 15] or in a microwave system [4, 7]. NH4HF2 can be used instead of HF [7], or boric acid and EDTA can be added to bind excess of HF [14]. However, evaporation is widely used because it allows reducing the total level of salts in a solution owing to removal of silicon as SiF4, as well as avoiding additional contamination of samples with reagent admixtures and line interferences of oxides, hydroxides, and argids of boron [1].
Recently, reactor microwave systems (Fig. 1) have been used in analytics, which allows one to reach higher pressure (up to 200 bar) in digestion vessels owing to the supply of inert gas in the reactor before heating. Comparing the MARS-5 and UltraWAVE microwave systems, higher power, pressure, and temperature used at sample preparation can be mentioned for the UltraWAVE microwave system (Table 1). These advantages can define the completeness of the digestion of refractory geological samples. In addition, the digestion time can be reduced owing to the absence of an autoclave assembling stage, automatic pressure relief, and efficient water cooling in the UltraWAVE microwave system [17, 18].
Despite emerging opportunities for the digestion of samples at higher temperatures and pressure, only a few studies are devoted to the application of reactor systems for geological samples [5, 19–23], and only several elements or isotopes have been determined: Sr [19], Mg [20], Tl, Fe, Mn, Ca [21], and Cr [22]. Only work [5] presents the results of the determination of 18 elements, including Nb, La, Ce, Dy, Hf, Th, and U, with confirmed completeness of reference material digestion, and study [23] shows data for 14 REEs, Y, and Sc in granites. In these studies, at the first digestion stage, acid mixtures with various ratios HNO3 : HF [19–21], HNO3 : HF : HCl [22], and HNO3 and hydrogen peroxide [23] were used. The construction of test tubes made of modified polytetrafluoroethylene of the UltraWAVE microwave system intended for digestion using hydrofluoric acid (Fig. 1) does not allow one to remove excess of HF from the test tubes. ICP-MS analysis of such solutions can lead to the destruction of quartz parts of the inlet system of a mass spectrometer. It was mentioned in studies [5, 22] that, after the first stage of digestion using HF, the obtained solutions were transferred into Teflon vessels and evaporated using a hot plate, the obtained residual was dissolved, and subsequent ICP-MS analysis was conducted to determine the elements. Autoclaves used in the MARS-5 microwave system do not have such drawbacks, and all digestion stages can be carried out in one vessel [5, 16].
Thus, each of the considered microwave systems has its features, and this work aims to estimate their efficiency in acid digestion of silicate rocks with subsequent ICP-MS analysis determining a wide range of elements (Sc, V, Cr, Co, Ni, Cu, Zn, Rb, Sr, Y, Zr, Nb, Cs, Ba, 14 REEs, Hf, Ta, Th, U).
EXPERIMENTAL
Analyzed samples. To practice the techniques of preparation of silicate rock samples, international reference materials were used: basalts BHVO-2 and BCR-2 (US Geological Survey, USA), serpentinite UB-N (Association Nationale de la Recherche Technique, France), and peridotite JP-1 (Geological Survey of Japan, Japan).
These reference materials are characterized by different numbers of elements. Basalts BHVO-2 and BCR-2 contain all 32 elements determined in this study [24]. Samples of ultrabasic rocks serpentinite UB-N and peridotite JP-1 are characterized to a lesser extent, and the content of determined elements obtained in different studies can differ by almost an order of magnitude [8, 25–29]. This is connected with a low level of content of analytes, especially Nb and Ta, in ultrabasic rocks.
Chemical reagents and facilities. The following reagents were used: deionized water (specific resistance of 18.2 MΩ cm) purified in a MilliQ filter system (Millipore, USA); nitric and hydrochloric acids (special purity grade) distilled twice using a DuoPure sub-boiling distillation system (Milestone, USA); hydrofluoric acid (special purity grade) distilled twice using a BSB-939-IR acid purification system (Berghof, Germany). Weighing was carried out using a BP211D high-precision balance (Sartorius, Germany). For dilution, polypropylene test tubes (15 mL volume) and constant-volume and adjustable dispensers were used; before dilution, all solutions were centrifugated using a 3-16L centrifuge (Sigma, Germany). Microwave digestion of samples was carried out using two microwave systems: MARS-5 (CEM Corporation, USA) and UltraWAVE (Milestone, Italy). For sample digestion, HP-500 autoclaves (100 mL volume) were used for the MARS-5 microwave system and polypropylene test tubes (15 mL volume) were used for the UltraWAVE microwave system. After each digestion cycle, HP-500 autoclaves were rinsed with aqua regia and water; test tubes for the UltraWAVE microwave system were rinsed with concentrated nitric acid according to the recommendations of the manufacturer.
ICP-MS analysis. The measurements were performed using an ELEMENT high-resolution mass spectrometer (Finnigan Mat, Germany) at the analitical center of the Sobolev Institute of Geology and Mineralogy of the Siberian Branch of the Russian Academy of Sciences. Instrumental parameters were optimized and the device was adjusted using standard solutions of indium and barium. Sampling gas flow was optimized before each measurement to provide the maximum intensity of the indium peak. The average sensitivity to indium was 50 000 cps per 1 μg/L at low resolution (LR). The formation of barium oxides in plasma was estimated at a level of 0.08%.
To calculate the concentration of determined elements, external calibration was used by multielement solutions CMS-1, IV-ICPMS-71B, CMS-5, and IV-STOCK-10 (Inorganic Ventures, USA) taking into account the composition of analyzed solutions [16] (Table 2); in addition, an internal standard (In) was used.
Table 3 presents isotopes of determined elements with more significant polyatomic interferences formed by macroelements and plasma elements, as well as the calculated required resolution for distinguishing these interferences which was used during the experiment. Other elements were determined at a low resolution; interferences with barium oxides were considered in the calculation of europium concentrations. The contribution of interferences of light REEs to heavy REEs (141PrO+/157Gd+, 147SmO+/163Dy+, 153EuO+/169Tm+, 156GdO+/172Yb+, and 159TbO+/175Lu+) was estimated to be negligible because the formation of oxides is ~0.1% and the difference in content of these elements is less than two orders of magnitude, unlike the Ba/Eu ratio.
Techniques of microwave acid digestion. In this work, two methods were chosen for the digestion of reference samples: using a MARS-5 microwave system (method 1) and an UltraWawe microwave system (method 2). Method 1 was developed and applied successfully for sample preparation of basic and ultrabasic rocks to determine REEs [16]. At the first stage, the samples weighing 0.1 g were treated with a mixture of concentrated acids HF : HNO3 = 4 : 1 (5 mL) in the MARS-5 device at the maximum parameters (T = 190°C, P = 20 bar) for 1 h. Then, after distillation of HF and SiF4 excess from the digestion vessels, the dry residual was treated with a mixture of concentrated acids HCl : HNO3 = 1 : 3 (15 mL) at the maximum parameters of the MARS-5 microwave system. After digestion, the obtained solutions were put in disposable polypropylene test tubes. Before measurement, the solutions were centrifugated and diluted with introduction of the internal standard (In); the total dilution was 1500 times.
The conditions of digestion in the UltraWAVE reactor system at the first stage were chosen on the basis of literature data [22] and the manufacturer’s recommendations regarding sample weight and acid combination. It should be noted that these recommendations contain no information about the set of elements and instrumental method which can be used for their determination after digestion. Moreover, it was proposed to conduct the experiment at temperatures of 260–280°C, although the test tube’s material melts at 250°C. Instead of hermetically sealed autoclaves, as in MARS-5, specific test tubes with loose lids were used in the UltraWAVE reactor system (Fig. 1). The HNO3 : HF : HCl = 4 : 1 : 1 mixture (5 mL) was added to the weighed samples (0.05–0.1 g) and placed in a reactor on a tripod. The reactor is a vessel made of stainless steel; inside the vessel, a polytetrafluoroethylene cup closely adjacent to the walls of the reactor was placed, and the cup was filled with the basic solution (150 mL water and 5 mL of concentrated nitric acid). Before digestion, the reactor was closed with a tight lid and fixed with a clamp; further, argon was pumped into it (initial pressure was 40 bar). At the first stage, samples were digested at a temperature of 240°C and a pressure of 80 bar (the samples were heated for 30 min and held at the maximum temperature for 30 min). Microwave radiation, which is firstly absorbed by the basic solution, provides uniform heating of all samples without rotation of the test tubes. The microwave system is controlled through the touchscreen terminal, so the digestion process is as automated as possible. After microwave heating, automatic pressure relief and test tube cooling take place.
The removal of HF excess from test tubes after digestion failed, so after the first digestion stage, the solutions were transferred to Teflon vessels and evaporated to dry salts on a hotplate. Then, the residual was dissolved in an HNO3 : HCl = 1 : 1 mixture (5 mL) according to the first stage mode using the UtraWAVE device. When the digestion was finished, transparent solutions without precipitations were put into disposable polypropylene test tubes, centrifugated, and diluted before measurements with introduction of the internal standard (In); the total dilution was 1500 times.
Thus, the digestion stages took 2 h in both microwave systems, while digestion parameters were higher for the UltraWAVE system (240°C and 80 bar) than for the MARS-5 system (190°C and 20 bar).
RESULTS AND DISCUSSION
Detection limits. The detection limits of analytes are affected by the sensitivity of a mass spectrometer, the contamination during sample preparation, and the memory effect. Table 4 presents the limits of detection of elements of the developed techniques of microwave acid digestion calculated as 3σ variations of the blank correction procedure. The detection limits of ICP-MS analysis achieved after microwave digestion are comparable. Reduction of the detection limits was expected by implementing the UltraWAVE system owing to a decrease in the volume of used acids. However, at this stage, the detection limits were not reduced because additional vessels were used for evaporation during sample preparation in addition to test tubes.
ICP-MS results for analytes in reference materials after decomposition in the MARS-5 microwave system. The method of microwave digestion in MARS-5 system (method 1) was tested in the analysis of reference materials of basic (BHVO-2, BCR-2) and ultrabasic (UB-N, JP-1) rocks. After digestion, transparent solutions without visible precipitation were obtained for BHVO-2, BCR-2, and UB-N samples, while a small amount of residual was observed for JP-1 samples after settling of the solution.
The results of the ICP-MS determination of 18 elements in reference materials are given in Tables 5 and 6; the results of the determination of 14 REEs were previously published [16]. To estimate the correctness of the obtained data, certified values of element contents in reference materials BHVO-2 and BCR-2 were used; these values were specified by the authors of [24] on the basis of the results collected for 20 years in the GeoReM database.
Since certified values of contents of elements are absent for UB-N and JP-1 samples, published data from different sources were used. Recent ICP-MS data are given for the UB-N sample; the data for this sample were obtained in seven laboratories using different digestion methods, including acid digestion, fusion, and laser ablation of fused glasses [25] and data obtained by various methods of acid digestion and fusion [8]. In the case of the JP-1 sample, the following results were used: for Zr, Nb, Hf, and Ta according to [27]; the results for other elements obtained by autoclave decomposition [26], and values for Sc, V, Cr, Co, Ni, and Zn after fusing with lithium metaborate and other elements after autoclave digestion [28]. For UB-N and JP-1 reference materials, ICP-MS results were obtained at preconcentration of trace elements (Zr, Nb, Hf, Ta, Th, U, REE) by coprecipitation after acid digestion [29]. It should be noted that, according to [7], the concentration of these elements is required owing to their low content in ultrabasic rocks, especially JP-1.
The results obtained in this work for all elements in BHVO-2 and BCR-2 samples agree well with the certified values within the confidence intervals. The relative error is from 2 to 9%. Thus, the developed method of microwave digestion using the MARS-5 device can be used to determine 32 set elements in basic rocks represented by the example of BHVO-2 and BCR-2 basalts.
In the case of the UB-N sample containing significantly lower amounts of REEs, Zr, Nb, Hf, Ta, Th, and U and higher amounts of Cr, Co, and Ni compared with BHVO-2 and BCR-2, the obtained results also agree with the literature data [25, 29]. Work [8] presents the results of Hf and Ta determination, which differ by 5–6 times depending on sample preparation conditions (Table 6). The values closest to the ones obtained in this study (0.1 μg Hf and 0.02 μg Ta) were obtained after fusion which ensures total decomposition of the sample.
The relative error was from 3 to 12%, except for Nb (16%) and Ta (25%) owing to approaching the detection limits.
As was mentioned above, the digestion of the JP-1 sample was incomplete when method 1 was applied: a small amount of precipitation was observed. According to the obtained results (Table 6), the degree of dissolution of chromium was ~40% due to incomplete decomposition of chromite in the JP-1 sample. However, incomplete digestion of this mineral had no pronounced effect on the results obtained for other analytes: they agree well with literature data within the measurement accuracy. The niobium concentration was determined with low accuracy, and the average value is higher than that obtained in [26] or after concentration [29]. The Ta concentration was not determined because of the insufficiently low detection limit (0.007 μg/g). The relative error of the ICP-MS results for the JP-1 sample is from 5 to 32%; error values above 20% are observed for the components whose concentration is less than 10 times higher than the detection limits.
Thus, the results obtained for UB-N confirm the possibility of dissolution of the given elements using the developed microwave digestion technique and determination of 32 elements by ICP-MS without additional concentration of trace elements. Upon analysis of sample JP-1, it was established that the developed approach to sample preparation allows the correct determination of 30 out of 32 target elements. Determination of Cr requires more “severe” conditions for chromite decomposition and chromium transfer into solution, and the detection limit should be reduced for determination of Ta.
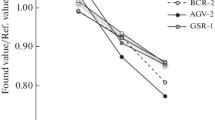
Results of ICP-MS determination of analytes in reference materials after digestion in the UltraWAVE microwave system. Because of the incomplete digestion of the JP-1 sample treated by method 1, the UltraWAVE microwave system was used to ensure the complete dissolution of analytes, including Cr. The technique of microwave digestion in the UltraWAVE microwave system (method 2) was also tested using the BHVO-2 sample. Table 7 presents the results of the ICP-MS determination of 32 elements in these reference materials after digestion according to method 2; certified data on BHVO-2 [24] and literature data on JP-1 [26, 27] are also provided. The ratio of certified (Ccert) or literature (Clit) values and average values obtained in this study (Cav) is used for the comparison.
Comparing the obtained results with certified values of concentrations of elements of BHVO-2, it can be concluded that the sample preparation by method 2 ensures complete dissolution of all elements, the deviation of values obtained in this work from certified ones does not exceed 5%. The error of determination of 32 elements by ICP-MS is from 2 to 10%.
Because of the application of method 2 to the JP-1 sample, it was completely dissolved, and Cr was transferred into solution (Table 7). The deviation of average values from the literature data is from 1 to 18% for different elements and it is not systematic; the error of determination is from 2 to 20%. The detection limit of Ta (0.007 μmg/g) is not low enough to determine this element using both methods 1 and 2.
CONCLUSIONS
The methods of sample preparation were developed including two-stage processing of geological samples with acid mixture in the MARS-5 and UltraWAVE microwave systems. These methods were proven to be effective for the digestion of BHVO-2, BCR-2, UB-N, and JP-1 reference materials followed by ICP-MS determination of up to 32 specified elements. The correctness of the obtained results was confirmed by their comparison with certified values (BHVO-2 and BCR-2) or literature data (UB-N and JP-1). The attained detection limits make it possible to determine most of the trace elements (REEs, Zr, Nb, Hf, Th, U) without an additional concentration stage. Both microwave systems can be used to digest basic and ultrabasic silicate rocks, and the sample preparation time can be significantly reduced to several hours, which is significantly lower than at open or autoclaved acid digestion, taking several days [8, 25, 27–29]. The UltraWAVE microwave system was proven to be more efficient than the MARS-5 system by the example of JP-1 peridotite containing chromite.
REFERENCES
Pinto, F., Escalfoni, R., and Saint’Pierre, T., Sample preparation for determination of rare earth elements in geological samples by ICP-MS: A critical review, Anal. Lett., 2012, vol. 45, no. 12, pp. 1537–1556. https://doi.org/10.1080/00032719.2012.677778
Balaram, V. and Subramanyam, K.S.V., Sample preparation for geochemical analysis: Strategies and significance, Adv. Sample Prep., 2022, vol. 1, p. 100010. https://doi.org/10.1016/j.sampre.2022.100010
Cotta, A. and Enzweiler, J., Classical and new procedures of whole rock dissolution for trace element determination by ICP-MS, Geostand. Geoanal. Res., 2012, vol. 36, no. 1, pp. 27–50. https://doi.org/10.1111/j.1751-908X.2011.00115.x
Navarro, M., Andrade, S., Ulbrich, H., et al., The direct determination of rare earth elements in basaltic and related rocks using ICP-MS: Testing the efficiency of microwave oven sample decomposition procedures, Geostand. Geoanal. Res., 2008, vol. 32, no. 2, pp. 167–180. https://doi.org/10.1111/j.1751-908X.2008
Zhang, W., Yang, F., Zhang, C., and Wang, W., An experimental method for effectively digesting geological samples, J. Geosci. Environ. Prot., 2019, vol. 7, pp. 83–89. https://doi.org/10.4236/gep.2019.76007
Bychkova, Y.V., Sinitsyn, M.Yu., Petrenko, D.B., et al., Methodical features of multielement analysis of rocks by inductively coupled plasma mass spectrometry, Vestn. Mosk. Univ., Ser. Geol., 2016, no. 6, pp. 56–63. https://doi.org/10.33623/0579-9406-2016-6-56-63
Magaldi, T., Navarro, M., and Enzweiler, J., Assessment of dissolution of silicate rock reference materials with ammonium bifluoride and nitric acid in a microwave oven, Geostand. Geoanal. Res., 2019, vol. 43, no. 1, pp. 189–208. https://doi.org/10.1111/ggr.12242
Roy, P., Balaram, V., Kumar, A., et al., New REE and trace element data on two kimberlitic reference materials by ICP-MS, Geostand. Geoanal. Res., 2007, vol. 31, no. 3, pp. 261–273. https://doi.org/10.1111/j.1751-908X.2007.00836.x
Nna-Mvondo, D., Martin-Redondo, M., and Martinez-Frias, J., New application of microwave digestion-inductively coupled plasma-mass spectrometry for multi-element analysis in komatiites, Anal. Chim. Acta, 2008, vol. 628, no. 2, pp. 133–142. https://doi.org/10.1016/j.aca.2008.09.008
Kubrakova, I.V. and Toropchenova, E.S., Microwave sample preparation for geochemical and ecological studies, J. Anal. Chem., 2013, vol. 68, no. 6, pp. 467–476. https://doi.org/10.1134/S1061934813060099
Totland, M., Jarvis, I., and Jarvis, K., An assessment of dissolution techniques for the analysis of geological samples by plasma spectrometry, Chem. Geol., 1992, vol. 95, nos. 1–2, pp. 35–62. https://doi.org/10.1016/0009-2541(92)90042-4
Yu, Z., Robinson, P., and McGoldrick, P., An evaluation of methods for the chemical decomposition of geological materials for trace element determination using ICP-MS, Geostand. Newslett., 2001, vol. 25, nos. 2–3, pp. 199–217. https://doi.org/10.1111/j.1751-908X.2001.tb00596.x
Fedyunina, N., Seregina, I., Bolshov, M., et al., Investigation of the efficiency of the sample pretreatment stage for the determination of the Rare Earth Elements in rock samples by inductively coupled plasma mass spectrometry technique, Anal. Chim. Acta, 2012, vol. 713, pp. 97–102. https://doi.org/10.1016/j.aca.2011.11.035
Gupta, J.G. and Bertrand, N.B., Direct ICP-MS determination of trace and ultratrace elements in geological materials after decomposition in a microwave oven. I. Quantitation of Y, Th, U and the lanthanides, Talanta, 1995, vol. 42, no. 11, pp. 1595–1607. https://doi.org/10.1016/0039-9140(95)01612-0
Yasnygina, T.A., Markova, M.E., Rasskazov, S.V., and Pakhomova, N.N., Determination of rare-earth elements Y, Zr, Nb, Hf, Ta, Th in reference specimens from DB series using inductuvely coupled plasma mass-spectromentry (ICP-MS), Zavod. Lab. Diagn. Mater., 2015, vol. 81, no. 2, pp. 10–20.
Kravchenko, A.A., Nikolaeva, I.V., and Palessky, S.V., Microwave acid digestion of mafic and ultramafic rocks in ICP-MS determination of the rare earth elements, Zavod. Lab. Diagn. Mater., 2020, vol. 86, no. 10, pp. 10–17. https://doi.org/10.26896/1028-6861-2020-86-10-10-17
UltraWAVE: the Game Changer in Microwave Sample Preparation. https://www. milestonesrl.com/products/microwave-digestion/ultrawave. Accessed November 18, 2022.
Michel, T., Breaking the sample preparation bottleneck with a new approach to microwave digestion, Am. Lab., 2010, vol. 42, no. 11, pp. 32–35.
Karasinski, J., Bulska, E., Wojciechowski, M., et al., On-line separation of strontium from a matrix and determination of the 87Sr/86Sr ratio by ion chromatography/multicollector-ICPMS, J. Anal. At. Spectrom., 2016, vol. 31, no. 7, pp. 1459–1463. https://doi.org/10.1039/C6JA00109B
Karasinski, J., Bulska, E., Wojciechowski, M., et al., High precision direct analysis of magnesium isotope ratio by ion chromatography/multicollector-ICPMS using wet and dry plasma conditions, Talanta, 2017, vol. 165, pp. 64–68. https://doi.org/10.1016/j.talanta2016.12.033
Pontér, S., Pallavicini, N., Engström, E., et al., Chromium isotope ratio measurements in environmental matrices by MC-ICP-MS, J. Anal. At. Spectrom., 2016, vol. 31, pp. 1464–1471. https://doi.org/10.1039/c6ja00145a
Cruz-Hernández, Y., Ruiz-García, M., Villalobos, M., et al., Fractionation and mobility of thallium in areas impacted by mining-metallurgical activities: Identification of a water-soluble Tl(I) fraction, Environ. Pollut., 2018, vol. 237, pp. 154–165. https://doi.org/10.1016/j.envpol.2018.02.031
Shafiee, N.S., Achmad Bahar, A.M., and Ali Khan, M.M., Potential of rare earth elements (REEs) in Gua Musang granites, Gua Musang, Kelantan, IOP Conf. Ser.: Earth Environ. Sci., 2020, vol. 549, no. 1, p. 012027. https://doi.org/10.1088/1755-1315/549/1/012027
Jochum, K., Weis, U., Schwager, B., et al., Reference values following ISO guidelines for frequently requested rock reference materials, Geostand. Geoanal. Res., 2016, vol. 40, no. 3, pp. 333–350. https://doi.org/10.1111/j.1751-908X.2015.00392.x
Godard, M., Awaji, S., Hansen, H., et al., Geochemistry of a long in-situ section of intrusive slow-spread oceanic lithosphere: Results from IODP Site U1309 (Atlantis massif, 30 degrees N mid-atlantic-ridge), Earth Planet. Sci. Lett., 2009, vol. 279, nos. 1–2, pp. 110–122. https://doi.org/10.1016/j.epsl.2008.12.034
Makishima, A. and Nakamura, E., Determination of major, minor and trace elements in silicate samples by ICP-QMS and ICPSFMS applying isotope dilution-internal standardization (ID-IS) and multi-stage internal standardisation, Geostand. Geoanal. Res., 2006, vol. 30, no. 3, pp. 245–271. https://doi.org/10.1111/j.1751-908X.2006.tb01066.x
Makishima, A., Nakamura, E., and Nakano, T., Determination of zirconium, niobium, hafnium and tantalum at ng g-1 levels in geological materials by direct nebulisation of sample HF solution into FL-ICP-MS, Geostand. Newslett., 1999, vol. 23, no. 1, pp. 7–20. https://doi.org/10.1111/j.1751-908X.1999.tb00555.x
Debret, B., Albers, E., Walter, B., et al., Shallow forearc mantle dynamics and geochemistry: New insights from IODP Expedition 366, Lithos, 2019, vol. 326, pp. 230–245. https://doi.org/10.1016/j.lithos.2018.10.038
Rospabe, M., Benoit, M., and Candaudap, F., Determination of trace element mass fractions in ultramafic rocks by HRICPMS: A combined approach using a direct digestion/dilution method and preconcentration by coprecipitation, Geostand. Geoanal. Res., 2018, vol. 42, no. 1, pp. 115–129. https://doi.org/10.1111/ggr.12181
Funding
This work was carried out in the framework of the government-financed program FWZN-2022-0032, registration number 122041400171-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by N. Saetova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikolaeva, I.V., Palesskiy, S.V. Application of MARS-5 and UltraWAVE Microwave Systems to the Digestion of Silicate Rocks Followed by ICP-MS Analysis. Inorg Mater (2024). https://doi.org/10.1134/S0020168524700031
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0020168524700031