Abstract
When reconstructing products obtained using additive technologies based on layer-by-layer melting of metal powder by concentrated energy flows, it is advisable to use methods that minimize melting of the initial powder and reduce structural heterogeneity of the material. Cold gas-dynamic spraying with laser-induced intensification of the process (CGDSL) is one of them. The multilayer coatings obtained by the CGDSL method have a homogeneous metal structure, though a significant surface roughness attributed to the particle size of the original powder is observed. The goal of the study is to develop a new method of post-processing of multilayer coatings obtained by CGDSL which can provide a hardened layer on their surface. A hardened layer is formed through introduction of boron carbide powder particles into the laser-molten region formed on the surface of the coating based on 316L stainless steel. An acoustic wave triggered by a “microexplosion” induced by a laser pulse above the surface pushes carbide particles in different directions. Some of them are embedded into the melt pool on the surface of the coating. Thus, the laser microexplosion cartooning of the surface of the CGDSL coating is implemented. Study of the hardened layer revealed a high content of B, C, Cr, Fe, and Ni. Moreover, it is shown that solid carbides of rhombic form are formed in the hardened layer. Chemical and elemental analyses showed that diamond-shaped carbides—carbides of the type (Fe, Cr)xBy—contain a high concentration of Cr and Fe and a relatively small percentage of C. Most likely, the formation of diamond-shaped carbides occurs owing to interaction of chromium which is a part of the initial hardened coating with boron that is released from the surface of BC particles under laser impact. The developed method provides hardening of the surface layer of the coating previously obtained by CGDSL by embedding BC powder particles into the surface. The technology of hardening CGDSL coatings can be implemented using other powder materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Direct laser spraying of metal (DLSM) is a method developed on the basis of laser cladding (LC), which is used for creating coatings with given physical and mechanical properties. In the DLSM method (as with LC), metal powder is melted by a focused laser. At the same time, partial melting of the substrate takes place, which provides the required contact between the coating and the substrate.
In recent years, cold gas-dynamic spraying (CGDS) [1] has been applied in additive manufacturing, in which powder is accelerated by a supersonic flux of gas in a Laval nozzle. Collision of metal particles with the substrate leads to their plastic deformation. Owing to an increase in kinetic energy, the particles are welded between each other and the substrate. In this way, adhesion of the particles to the substrate is achieved at a temperature lower than melting temperature of the initial materials [2, 3].
The use of laser with CGDS (CGDSL) extends the possibilities of spraying by means of melting of powder particles. This allows performing impregnation of the sample surface by particles of powder material [4]. Laser radiation, increasing the kinetic energy of the particles, promotes growth of plastic deformation at the moment of their collision with the substrate and correspondingly good adhesion [5, 6]. By softening the substrate and the particles, the laser provides conditions of formation of a dense surface at collision rates about two times less than that at classic CGDS [7]. Moreover, additional laser heating allows decreasing the temperature of the transporting gas [8], the efficiency of spraying increases, and the initial microstructure of CGDSL coatings is maintained [9–12].
The aim of this work is to develop a method of post-processing of multilayer CGDSL coatings making it possible to obtain a hardened layer on their surface.
MATERIALS, METHODOLOGY, EQUIPMENT
Hardening of CGDSL coating was implemented in an automatic mode at a facility for DLSM. A gas-powder mixture of boron carbide BC (size of particles of 20–250 μm, flow rate of 0.2 g/s) was supplied to the zone of contact of the laser with the surface of the coating from stainless steel 316L (initial size of particles of 45–100 μm) using a coaxial nozzle. Impregnation was performed using ytterbium fiber laser (wavelength of 1070 nm, pulsed mode, time betweem impulses of 50 ms). The powder material was transported using argon (flow rate of 3 L/min). The operating distance between the nozzle and the substrate was 5 mm. Figure 1 presents the initial powder materials.
The microhardness was determined using a Tukon 2500 automatic hardness meter; the roughness and profile of the surface was determined using an Abris-PM7 profilograph-profiler. Before analysis of micro- and macrostructure, the samples were sequentially ground by diamond grinding disks (120, 220, and 500 grit) and polished using diamond suspensions (9 and 3 μm). Etching was performed in a prepared reactive of nitric acid. The microstructure was analyzed with a Carl Zeiss Axio Observer D1m optical microscope using the Tizomet software package. Metallographic analysis was performed with a Carl Zeiss Axiovert-200M universal inverted microscope. The electron-microscopic (SEM) image of the surfaces of the samples and their elemental composition were obtained using Auriga CrossBeam.
The initial phase composition of metal of the coating was represented by γ-Fe [15]. The chemical composition of steel 316L (wt %): 18 Cr, 3 Mo, 14 Ni, 0.03 C, 2 Mn, 0.75 Si, 0.045 P, 0.03 S.
RESULTS AND DISCUSSION
Figure 2 presents the schematic of hardening of a CGDSL coating. Powder particles of BC were supplied to the surface of the coating through circular channel of the coaxial nozzle [13, 14]. The focus of the laser was positioned above the surface of the hardened coating at distance of 1 mm. At the moment of peak power of the laser (7 kW), a “microexplosion” above the surface caused by fast local heating of the gas medium took place. One pulse was enough for melting a local region on the surface. The “microexplosion” initiated by the laser pulse created an acoustic wave, which pushed the particles of BC in various directions. Part of them were introduced into the “bath” of melt.
Figure 3 presents SEM images and elemental composition of the cross section of the CGDSL coating with the hardened layer. It can be seen that the upper layer on the surface of the coating is saturated with BC particles and the distributions of the main elements (Cr, Fe, Ni, Mo, Mn) are characterized by uniformity.
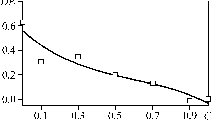
Figure 4 presents the dependence of microhardness of the hardened CGDSL coating on depth H. The hardness of BC particles introduced into the coating was 2300–2500 HV0.02; the hardness of the coating before hardening was 190 HV0.02.
It can be assumed that powerful laser pulse causes melting of the metal of the coating surface and fine powder particles of BC, and new phases are formed as a result of mixing in the melt.
The phase composition of the metal of the hardened layer was studied on the region of measurement of microhardness using EDS analysis (Fig. 5). It was established that the average value of intensities of Cr, Ni, and Fe during transition from the metal of the coating to the hardened layer remains almost identical. Separate peaks of Cr, Ni, and Fe are observed on local regions of the hardened layer.
It can be seen in the transition region (Fig. 5) that the boundary between the main metal of the coating and the hardened layer is indistinct, possibly because of formation of compounds like γ + (Fe, Ni)xC. EDS investigation of the hardened layer showed a high content of B, C, Cr, Fe, and Ni. At the same time, dark gray inclusions of rhombic and rectangular shapes can be distinguished in the metal of the layer. Presumably, dark gray inclusions of rhombic shape can be determined as compounds of type (Fe, Cr)xB [16].
The signal of boron in the transition region between the coating and the hardened layer is almost indistinguishable. However, the relative content of carbon in the matrix of the hardened metal is twice as high as in the transition region and in the metal of CGDSL coating. Apparently, particles of boron carbide are melted under the impact of a powerful laser pulse. The interaction with chromium, iron, and nickel in the matrix of the hardened layer leads to formation of compounds like CrxCy, γ-Fe, γ-NixB, and (Fe, Cr)xB [16, 17].
Figure 6 presents inclusions of rhombic shape (marked with a vertical line) discovered in the structure of the metal of the hardened layer. EDS analysis performed along scanning lines showed a significant increase in the intensity of Cr. In addition, the intensity of Cr is slightly higher on peripheral regions of the inclusion as compared with central zones. The rhombic shape of crystals is probably determined by the shape of the elementary crystallographic atomic lattice of the material used as a seeding agent [18]. Regions of metal adjacent to the inclusion are characterized by increased content of Ni and Fe, while the content of Cr on the contrary is decreased. It is necessary to note that concentrated sources of energy allow forming compounds of type (Fe, Ni)Cr [17].
Elemental analysis of the boundary domain of a separate particle of boron carbide in the hardened layer showed that a layer with thickness of 20 μm (Fig. 7) is formed by the boundaries of the particle. At the same time, bursts of Cr, C, Ni, and B with respect to the metal of the matrix are observed. It can be assumed that this layer contains nanosized particles of (Fe, Cr)xC and CrxBCx [16]. Stretched rectangular dark inclusions with size of 40–50 μm, presumably also compounds like (Fe, Cr)xC, are observed in the matrix of the metal, since peaks of Fe, Cr, and C correspondingly are present on the scanning line which intersects the inclusions.
CONCLUSIONS
Thus, the proposed method makes it possible to perform hardening of the surface of CGDSL coating by introduction of BC particles (their hardness is 10 times higher than that of the metal of the coating). Formation of secondary carbides in the hardened layer leads to a general increase in the hardness of the coating. Pulsed-laser impact and its focusing above the coating surface provide a “microexplosion” of the gas medium, and sharp local heating and cooling of the surface cause formation of carbides of rhombic shape. BC particles possess a transition layer on their surface with an increased content of Cr, Ni, and C, which allows assuming the presence of phases like NiC and CrxCy in it.
REFERENCES
Bagherifard, S., Monti, S., Zuccoli, M., et al., Cold spray deposition for additive manufacturing of freeform structural components compared to selective laser melting, Mater. Sci. Eng., A, 2018, vol. 721, pp. 339–350.
AL-Mangour, B., Vo, P., Mongrain, R., Irissou, E., and Yue, S., Effect of heat treatment on the microstructure and mechanical properties of stainless steel 316L coatings produced by cold spray for biomedical applications, J. Therm. Spray Technol., 2014, vol. 23, no. 4, p. 641.
Coddet, P., Verdy, C., Coddet, C., Debray, F., and Lecouturier, F., Mechanical properties of thick 304L stainless steel deposits processed by He cold spray, Surf. Coat. Technol., 2015, vol. 277, pp. 74–80.
Gorunov, A.I., Features of coatings obtained by supersonic laser deposition, J. Therm. Spray Technol., 2018, vol. 27, no. 7, pp. 1194–1203.
Yao, J., Li, Z., Li, B., Yang, L., and Yaoet, J., Characteristics and bonding behavior of Stellite 6 alloy coating processed with supersonic laser deposition, J. Alloys Compd., 2016, vol. 661, pp. 526–534.
Lupoi, R., Sparkes, M., Cockburn, A., and O’Neill, W., High speed titanium coatings by supersonic laser deposition, Mater. Lett., 2011, vol. 65, pp. 3205–3207.
Singh, R., Rauwald, K.-H., Wessel, E., et al., Effects of substrate roughness and spray-angle on deposition behavior of cold-sprayed Inconel 718, Surf. Coat. Technol., 2017, vol. 319, pp. 249–259.
Yuan, L.-J., Luo, F., Yao, J.-H., et al., Deposition behavior at different substrate temperatures by using supersonic laser deposition, J. Iron Steel Res. Int., 2013, vol. 20, no. 10, pp. 87–93.
Yao, J., Yang, L., Li, B., et al., Characteristics and performance of hard Ni60 alloy coating produced with supersonic laser deposition technique, Mater. Des., 2015, vol. 83, pp. 26–35.
Li, B., Jin, Y., Yao, J., et al., Solid-state fabrication of WCp-reinforced Stellite-6 composite coatings with supersonic laser deposition, Surf. Coat. Technol., 2017, vol. 321, pp. 386–396.
Li, B., Jin, Y., Yao, J., Li, Z., et al., Influence of laser irradiation on deposition characteristics of cold sprayed Stellite-6 coatings, Opt. Laser Technol., 2018, vol. 100, pp. 27–39.
Gorunov, A.I., Formation of wear-resistant coatings based on nickel by supersonic laser surfacing, Fiz. Khim. Obrab. Mater., 2016, no. 5, pp. 59–64.
Sova, A., Grigoriev, S., Okunkova, A., and Smurov, I., Cold spray deposition of 316L stainless steel coatings on an aluminum surface with the following laser post-treatment, Surf. Coat. Technol., 2013, vol. 235, pp. 283–289.
Ivannikov, A.Yu., Kalita, V.I., Komlev, D.I., et al., Investigation into improving microstructure and properties of plasma sprayed Ni coating via electromechanical treatment, J. Mater. Process. Technol., 2019, vol. 266, pp. 442–449.
Gorunov, A.I., The structure and mechanical properties of corrosion-resistant steel coating formed by gas-dynamic sputtering method with process activation by laser emission, Deform. Razrushenie Mater., 2016, no. 9, pp. 2–7.
Makarov, A.V., Sobolev, N.N., Malygina, I.Yu., and Osintseva, A.L., Formation of wear-resistant chromium-nickel coating with extra high thermal stability by combined laser-and-heat treatment, Met. Sci. Heat Treat., 2015, vol. 57, nos. 3–4, pp. 161–168.
Kalita, V.I., Yarkin, V.V., Bagmutov, V.P., et al., Formation of coatings with nanostructures and amorphous structures, Russ. Metall. (Engl. Transl.), 2007, vol. 2007, no. 6, pp. 534–539.
Gorunov, A.I., Investigation microstructure of carbon fibers reinforced composite on Fe and Ni-based obtained by laser metal deposition, Surf. Coat. Technol., 2019, vol. 364, pp. 279–288.
Funding
This work was supported by the Russian Science Foundation, project no. 19-79-00039 (development of the method of hardening of a multilayer CGDSL coating by means of acoustic effects created by a laser, investigation of the structure and chemical composition of the hardened layer) and the grant of the President of the Russian Federation, no. MK-3745.2019.8 (obtaining of multilayer coatings by CGDSL method).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by K. Gumerov
Rights and permissions
About this article
Cite this article
Gorunov, A.I. Study of a Hardened Multilayer Coating Obtained by Cold Gas-Dynamic Spraying with Laser Intensification. Inorg Mater 57, 1463–1467 (2021). https://doi.org/10.1134/S0020168521150085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521150085