Abstract—
This paper reports the synthesis of compounds with the pyrochlore and hexagonal tungsten bronze structures via thermal decomposition of heteropolyoxometalates. Using aqueous solutions, we have synthesized tungstophosphatometalates with the Keggin structure and the general formula Ct5[PW11O39(H2O)Z]⋅nH2O, where Ct = Rb+ or Cs+ and Z = Co2+, Ni2+, or Cu2+. We have studied the thermal decomposition of these compounds and identified their thermolysis products: phases with the pyrochlore and hexagonal tungsten bronze structures. Our results confirm that phosphorus, cobalt, nickel, and copper ions become incorporated into the pyrochlore and hexagonal tungsten bronze structures of the CtnxPxZxW2–2xO6 compounds. No phases with similar chemical compositions have been reported previously. Their synthesis temperature has been lowered by 200°C and the calcination time has been reduced by a factor of 2 in comparison with conventional synthesis methods. The proposed schemes of thermolysis of rubidium and cesium tungstophosphatometalates will be useful for predicting the thermal properties and phase composition of thermolysis products of analogous heteropolyoxometalates in designing new inorganic materials based on them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Investigation of the physicochemical properties of nonstoichiometric compounds of variable composition with the pyrochlore structure and the general formula А1+х\({\text{A}}_{{{\text{1}}-x}}^{'}\)Ву\({\text{B}}_{{{\text{2}}-y}}^{'}\)О6+хХ1–х (А2В2О6X), where A = Na, K, Ca, Sr, Ba, Ag, Pb, Sb, Mn, Fe, Zn, Сe, Y, U, Th, Sn, or Bi; B = Nb, Ta, Ti, Ru, Sb, or W; and X = O, F, OH–, or H2O, has shown that they have a number of valuable properties, namely, ferroelectric, semiconducting, optical, magnetic, ion-exchange, catalytic, and other properties, due to the specific features of the pyrochlore structure, which allows one to design novel composite materials based on such compounds [1–13].
In conventional methods for the synthesis of tungsten-containing compounds with the pyrochlore structure, starting mixtures of oxides, compounds readily decomposing into oxides, or substances prepared via coprecipitation from solution are calcined at high temperatures (800–1000°C) for 2 h or a longer time. However, such methods have a number of drawbacks inherent in thermal processes (ceramic processing) on the whole in comparison, for example, with synthesis in aqueous solutions. To prepare crystals with the pyrochlore structure, use is made of high-temperature solution growth. Congruently melting alkali metal polymolybdates are used as low-melting-point solvents for high-temperature solution growth of molybdate and other mixed oxide crystals [14–19].
A promising approach to the preparation of mixed oxides is the use of thermal decomposition of individual precursors, namely, heterometallic complexes containing metal ions in an appropriate ratio. This approach considerably reduces the synthesis time (by up to 2–3 h) in comparison with conventional ceramic processing route. Another advantage of the precursor approach is the possibility of extending the potential morphological diversity of mixed-oxide and sulfide synthesis products [20–23].
The preparation of many metal oxide nanomaterials is based on preliminary synthesis of metal complexes as precursors for subsequent thermolysis. The controlled thermolysis method is the simplest and most effective way of producing metal-containing nanoparticles and metal–polymer nanocomposites [24–26].
Thermolysis of some heteropolytungstometalates with 3d transition metals yields compounds with the pyrochlore and hexagonal tungsten bronze (HTB) structures [27, 28].
The purpose of this work was to synthesize compounds with the pyrochlore and HTB structures via thermal decomposition of tungstophosphatometalates.
EXPERIMENTAL
Tungstophosphatometalates were synthesized using techniques described previously [29, 30]. To synthesize cesium 11-tungstophosphatocobaltate, 7.0 g (2.2 mmol) of Na2H[PW12O40]∙15H2O was dissolved in 75 mL of distilled water. To the resultant solution was slowly (dropwise) added with constant stirring on a magnetic stirrer 5.1 mL (12.8 mmol) of a sodium hydroxide solution containing 0.1 g/mL NaOH. The pH of the resultant mixture of solutions was in the range 4.5–5.5. Next, we added 0.52 g (2.2 mmol) of CoCl2∙6H2O dissolved in 5 mL of water and 1.85 g (11.0 mmol) of CsCl dissolved in 5 mL of water. The resultant mixture of solutions was passed through filter paper and placed in a Petri dish. After several days, the crystals formed were separated from the mother liquor and recrystallized from water.
A similar procedure was used to synthesize rubidium 11-tungstophosphatocobaltate, with cesium chloride replaced by an equivalent amount of rubidium chloride. Cesium and rubidium salts of 11-tungstophosphatonickelate and 11-tungstophosphatocuprate were synthesized in a similar manner, using an equivalent amount of nickel chloride or copper chloride instead of cobalt chloride.
The chemical composition of the synthesized compounds was determined by inductively coupled plasma atomic emission spectroscopy (IRIS Intrepid II XSP Duo atomic emission spectrometer, Thermo Electron Corporation, the United States; error of determination, 5%), scanning electron microscopy (JEOL JSM-6490 LV electron microscope equipped with an INCA energy dispersive X-ray spectrometer system; error of determination, 5%), and gravimetric analysis for determination of hydrogen in the form of water (error of determination, 0.04%).
The tungstophosphatometalates and their thermolysis products were identified using their electronic and IR absorption spectra and X-ray diffraction. We used a Helios Gamma spectrophotometer (Thermo Electron Corporation) and Vertex 70 infrared spectrometer. X-ray diffraction patterns of polycrystalline samples were collected on a DRON-2.0 diffractometer (CuKα radiation) in the angular range 2θ = 10°–60° at a scan rate of 1°/min. Unit-cell parameters were determined by the Le Bail method using FULLPROF.2k (version 5.30) with a WinPLOTR graphic interface. Thermogravimetric (TG) scans were performed with an STA 449 F1 Jupiter analyzer in combination with differential scanning calorimetry (DSC) at a heating rate of 10°C/min in an argon atmosphere, using samples weighing 15–20 g.
RESULTS AND DISCUSSION
Using aqueous solutions, we synthesized compounds with the general formula Ct5[PW11O39Z(H2O)]∙mH2O, where Ct = Rb+ or Cs+ and Z = Co2+, Ni2+, or Cu2+. The tungstophosphatometalates were synthesized at room temperature according to the following reaction schemes:
The chemical compositions of the synthesized tungstophosphatometalates are given in Table 1.
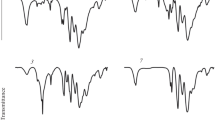
The electronic absorption spectra of these compounds in aqueous solutions suggest that the 3d transition metals present in the coordination sphere of these complexes are in octahedral coordination, in agreement with the structure of Keggin anions (Fig. 1). In particular, the absorption spectra of the tungstophosphatonickelate solutions contain absorption bands characteristic of octahedral nickel complexes, peaking near 422, 708, and 778 nm, and those of the tungstophosphatocobaltates and tungstophosphatocuprates contain characteristic bands at 539 and 874 nm, respectively. Moreover, in going from the [Z(H2O)6]2+ aqua complexes to the [PW11O39Z(H2O)]5– tungstophosphatometalate complexes the peak position of the absorption bands of the complexes shifts to longer wavelengths, which is due to changes in ligand field force.
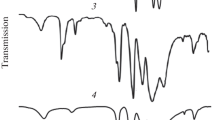
The IR spectra of the synthesized compounds in the stretching region of the metal–oxygen framework are similar to those of known Keggin compounds in which one tungsten atom is replaced by an atom of another metal (Fig. 2), for example, the compounds reported by Kato et al. [31]. Single-crystal X-ray structure analysis of cesium salts of 11-tungstophosphatonickelates and 11-tungstophosphatocobaltates was reported by Weakley [32].
Thus, the present results suggest that the synthesized compounds are tungstophosphatometalates with the Keggin structure in which one tungsten atom is replaced by a 3d transition metal atom.
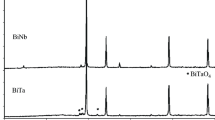
Thermal analysis data indicate that the tungstophosphatometalates are thermally unstable: heating to 200°C leads to their dehydration; in the temperature range 550–600°C, their thermolysis products—phases with the pyrochlore and HTB structures—crystallize (Fig. 3, Table 2). The X-ray diffraction pattern of the rubidium tungstophosphatonickelate calcined at a temperature of 600°C for 1 h showed reflections from a single phase with the pyrochlore structure (ICDD PDF no. 00-050-1861). Its X-ray diffraction pattern could be indexed in cubic symmetry with a lattice parameter a = 10.27 Å. The X-ray diffraction patterns of the Rb5[PW11O39Co(H2O)]∙8H2O and Rb5[PW11O39Cu(H2O)]∙9H2O compounds calcined at a temperature of 600°C contained reflections from two phases, with the pyrochlore and HTB (ICDD PDF no. 01-070-0803) structures. The X-ray diffraction patterns of the cesium tungstophosphatometalates calcined at 600°C for 1 h showed reflections of two phases, a pyrochlore phase (major phase, ICDD PDF no. 00-047-0566) and Cs3[PW12O40] (impurity phase, ICDD PDF no. 00-050-1857). After calcination at 850°C, there were two phases, with the pyrochlore and HTB (ICDD PDF no. 01-081-1244) structures (Fig. 4).
Tungsten(VI) oxide compounds with a pyrochlore face-centered cubic structure and the general formula A2B2O6, or CtnxMxW2–xO6, where Ct = K, Rb, or Cs and M = Fe, Со, Ni, Zn, Cr, or other elements, are nonstoichiometric compounds of variable composition. In our case, M stands for phosphorus(V) and a 3d transition metal, and the thermolysis products of the Ct5[PW11O39Z] rubidium and cesium tungstophosphatometalates can be represented by the formula Ct10/13P2/13W22/13Z2/13O6. Their compositions are consistent with the general formula CtnxPxZxW2–2xO6.
Electron micrographs of the thermolysis products of the tungstophosphatonickelates calcined at 600°C demonstrate that there are no regions differing in surface morphology and that the Rb, P, Ni, W, and O distributions over the surface of the Rb5[PW11O39Ni] powder are uniform, as evidenced by the Rb Kα1, P Kα1, Ni Kα1, W Lα1, and O Kα1 X-ray maps. Similar results were obtained for the thermolysis products of the rubidium and cesium tungstophosphatometalates (Fig. 5).
Particle morphology of thermolysis products of the (a) Rb5[PW11NiO39] rubidium 11-tungstophosphate and (b) Cs5[PW11NiO39] cesium 11-tungstophosphate after calcination at 600°C (scanning electron microscopy); (a, b) secondary electron and (c) backscattered electron imaging; (d) Rb Kα1, (e) P Kα1, (f) Ni Kα1, (g) W Lα1, and (h) O Kα1 X-ray maps.
The present results confirm that phosphorus, cobalt, nickel, and copper ions become incorporated into the pyrochlore and HTB structures of the Cs10/13P2/13W22/13Z2/13O6 compounds. We have found no phases with similar chemical compositions in the literature. The thermolysis of the rubidium and cesium tungstophosphatometalates with the Keggin structure can be represented by the following schemes:
It seems likely that the schemes found for the thermolysis of the rubidium and cesium tungstophosphatometalates with the Keggin structure have a more general character and can be used to predict thermolysis products of tungstophosphatometalates with other heteropolyanion structures, for example, with the Dowson structure ([P2W18O62]6–, [P2W17O61Z]m–, and others), as well as for other Z elements present in their structure.
Data on the catalytic activity of phases with the pyrochlore and HTB structures prepared via thermolysis of heteropolytungstometalates indicate that such phases are potentially attractive for the preparation of catalysts for the oxidation of organic compounds: they have been shown to have high activity for and selectivity in the oxidation of isopropanol to acetone with atmospheric air [8].
Heteropolytungstometalates with imperfect anion structures (lacunary heteropolyanions) are capable of completing the anion structure by attaching various cations and groups to form, for example, [XW11O39Z(H2O)]m– anions with X = B, Si, Ge, P, As, Sb, Fe, Co, Zn, or Cu and Z = Fe, Co, Ni, Mn, Cr, Zn, Cu, Mg, V, etc. Among the X and Z combinations possible for the Ctm[XW11O39Z(H2O)] compounds, only a small fraction of their thermolysis products have been studied to date. Substitution of other elements for tungsten atoms in the structure of heteropolyoxometalate anions can be used for the targeted synthesis of heteropolyoxometalates of tailored chemical composition and their thermolysis with the aim of obtaining new compounds with the pyrochlore and HTB structures. The composition and properties of such compounds can be tailored and controlled at a level of individual atoms in their structure. The high degree of their homogeneity is due to the fact that the chemical elements in the parent heteropolyoxometalates are evenly distributed at a molecular level.
CONCLUSIONS
New compounds with the pyrochlore and HTB structures and the general formula CtnxPxZxW2–2xO6, where Ct = Rb+ or Cs+ and Z = Co2+, Ni2+, or Cu2+, have been prepared via thermal decomposition of Ct5[PW11O39(H2O)Z]∙nH2O tungstophosphatometalates. The synthesis temperature has been lowered by 200°C and the calcination time was reduced by a factor of 2 (to 1 h) in comparison with conventional methods for the synthesis of such compounds.
The thermal decomposition processes studied here and the proposed schemes of thermolysis of rubidium and cesium tungstophosphatometalates will be useful for predicting the thermal properties and phase composition of thermolysis products of analogous heteropolyoxometalates in designing new inorganic materials based on them.
REFERENCES
Redozubov, S.S., Nenasheva, E.A., Gaidamaka, I.M., and Zaitseva, N.V., Low-temperature ceramic materials based on pyrochlore compounds in the Bi2O3–ZnO–Nb2O5 system, Inorg. Mater., 2020, vol. 56, no. 1, pp. 77–82. https://doi.org/10.31857/S0002337X20010121
Shlyakhtina, A.V., Belov, D.A., Knotko, A.V., Kolbanev, I.V., Streletskii, A.N., Karyagina, O.K., and Shcherbakova, L.G., Oxygen interstitial and vacancy conduction in symmetric Ln2±xZr2±xO7±x/2 (Ln = Nd, Sm) solid solutions, Inorg. Mater., 2014, vol. 50, no. 10, pp. 1035–1049. https://doi.org/10.1134/S002016851410015X
Krasnov, A.G., Piir, I.V., Sekushin, N.A., Baklanova, Ya.V., and Denisova, T.A., Electrophysical properties of bismuth titanates with the pyrochlore structure Bi1.6MxTi2O7–δ (M = In, Li), Russ. J. Electrochem., 2017, vol. 53, no. 8, pp. 866–872. https://doi.org/10.1134/S1023193517080122
Ikeda, S., Itani, T., Nango, K., and Matsumura, M., Overall water splitting on tungsten-based photocatalysts with defect pyrochlore structure, Catal. Lett., 2004, vol. 98, no. 4, pp. 229–233. https://doi.org/10.1007/s10562-004-8685-y
Jitta, R.R., Gundeboina, R., Veldurthi, N.K., Guje, R., and Muga, V., Defect pyrochlore oxides: as photocatalyst materials for environmental and energy applications: a review, J. Chem. Technol. Biotechnol., 2015, vol. 90, o. 11, pp. 1937–1948. https://doi.org/10.1002/jctb.4745
Spiridonov, F.M., Petrova, E.B., and Belova, I.D., Sintez, struktura i svoistva soedinenii semeistva pirokhlora (Synthesis, Structure, and Properties of Pyrochlore Compounds), Obzornaya informatsiya. Seriya auchno-tekhnicheskie prognozy v oblasti kataliza, korrozii i sinteza segnetomaterialov (Survey Information: Science and Technology Predictions in the Field of Catalysis, Corrosion, and Synthesis of Ferroelectric Materials), Moscow: NIITEKhIM, 1976.
Lopanov, A.N., Lozinskii, N.S., and Moroz, Ya.A., Chemical processes accompanying the formation of modified ruthenium resistors and their functional properties, Russ. Chem. Bull., 2020, vol. 69, no. 9, pp. 1719–1723. https://doi.org/10.1007/s11172-020-2955-8
Cherednichenko, L.A. and Moroz, Ya.A., Catalytic properties of heteropolytungstates with 3d elements and their thermolysis products, Kinet. Catal., 2018, vol. 59, no. 5, pp. 572–577. https://doi.org/10.1134/S0453881118050039
Okuhara, T., Watanabe, H., Nishimura, T., Inumaru, K., and Misono, M., Microstructure of cesium hydrogen salts of 12-tungstophosphoric acid relevant to novel acid catalysis, Chem. Mater., 2000, vol. 12, pp. 2230–2238. https://doi.org/10.1021/CM9907561
Nikul’shin, P.A., Mozhaev, A.V., Ishutenko, D.I., Minaev, P.P., Lyashenko, A.I., and Pimerzin, A.A., Influence of the composition and morphology of nanosized transition metal sulfides prepared using the Anderson-type heteropoly compounds [X(OH)6Mo6O18]n– (X = Co, Ni, Mn, Zn) and [Co2Mo10O38H4]6– on their catalytic properties, Kinet. Catal., 2012, vol. 53, no. 5, pp. 620–631.
Allmen, K., Moré, R., Müller, R., Soriano-López, J., Linden, A., and Patzke, G.R., Nickel-containing Keggin-type polyoxometallates as hydrogen evolution catalysts: photochemical structure–activity relationships, ChemPlusChem., 2015, vol. 80, pp. 1389–1398. https://doi.org/10.1002/cplu.201500074
Camarillo-Cisneros, J., Arzola-Álvarez, C., Cabral-Lares, R.M., Arzate-Quintana, C., and Arzola-Rubio, A., Optical properties of W1–xMoxO3⋅0.33H2O semiconductor oxides synthesized by hydrothermal and microwave techniques, Inorg. Chem. Commun., 2020, vol. 119, paper 107984. https://doi.org/10.3390/polym11111740
Solodovnikov, S.F., Ivannikova, N.V., Solodovnikova, Z.A., and Zolotova, S.S., Synthesis and X-ray diffraction study of potassium, rubidium, and cesium polytungstates with defect pyrochlore and hexagonal tungsten bronze structures, Inorg. Mater., 1998, vol. 34, no. 8, pp. 845–853.
Klevtsov, P.V. and Sinaiko, V.A., Double tungstates of potassium, rubidium, and cesium with the Al, Ge, Cr, and Fe trivalent metals, Zh. Neorg. Khim., 1975, vol. 20, no. 8, pp. 2104–2107.
Kapyshev, A.G., Stefanovich, S.Yu., Venevtsev, Yu.N., and Katsnel’son, L.M., Growth and properties of Pb2Li1/2Nb3/2O6 antiferroelectric single crystals with the pyrochlore structure, Kristallografiya, 1976, vol. 21, no. 4, pp. 838–840.
Timofeeva, V.A., Rost kristallov iz rastvorov-rasplavov (Crystal Growth from High-Temperature Solutions), Moscow: Nauka, 1978.
Mokhosoev, M.V. and Bazarova, Zh.G., Slozhnye oksidy molibdena i vol’frama s elementami I–IV grupp (Mixed Molybdenum and Tungsten Oxides with Group I–IV Elements), Moscow: Nauka, 1990.
Solodovnikov, S.F., Zolotova, E.S., Solodovnikova, Z.A., Korol’kov, I.V., Yudin, V.N., Uvarov, N.F., Plyusnin, P.E., and Saranchina, E.M., Structure and Properties of the α-Cs2Mo2–xWxO7 Solid Solution, J. Struct. Chem., 2019, vol. 60, no. 6, pp. 952–960. https://doi.org/10.26902/JSC_id40567
Drobasheva, T.I., Snezhkov, V.I., and Rastoropov, S.B., Alkali metal polytungstate molybdate ionic melts and their application in targeted crystal growth, Sovrem. Naukoemkie Tekhnol., 2011, no. 5, pp. 69–70.
Nesterov, A.A., Kogan, V.A., Borodkin, S.A., Krikov, V.V., Vasil’eva, G.I., and Vasil’ev, I.V., Low-temperature synthesis of the BaTiO3 and PbTiO3 phases with the perovskite structure using barium and lead complexes as precursors, Sovrem. Probl. Nauki Obraz., 2013, no. 4, pp. 353–361.
Semenov, S.A., Musatova, V.Yu., Drobot, D.V., and Dzhardimalieva, G.I., Thermal decomposition of acidic cobalt(II) carboxylates with unsaturated dicarboxylic anions, Russ. J. Inorg. Chem., 2020, vol. 65, no. 1, pp. 61–68. https://doi.org/10.31857/S0044457X20010146
Belova, E.V., Gavrikov, A.V., Ilyukhin, A.B., and Efimov, N.N., New cadmium cymantrene carboxylate complexes as precursors of CdMn2O4-based oxide systems, Spektroskopiya koordinatsionnykh soedinenii: sbornik nauchnykh trudov XVII mezhdunarodnoi konferentsii (Spectroscopy of Coordination Compounds: Proc. XVII Int. Conf.), Krasnodar: Kubansk. Gos. Univ., 2020, pp. 23–24.
Semenov, V.N., Samofalova, T.V., Naumov, A.V., and Ovechkina, N.M., Growth of cadmium sulfide and lead sulfide layers via deposition from thiosulfate–thiourea complexes and investigation of their properties, Kondens. Sredy Mezhfaznye Granitsy, 2019, vol. 21, no. 2, pp. 240–248. https://doi.org/10.17308/kcmf.2019.21/762
Jeon, B., Kim, T., Lee, D., Shin, T.J., Oh, K.W., and Park, J., Photothermal polymer nanocomposites of tungsten bronze nanorods with enhanced tensile elongation at low filler contents, Polymers, 2019, vol. 11, no. 11, paper 1740. https://doi.org/10.3390/polym11111740
Pyrochlore Oxide Nanoparticles: Electrical and Dielectric Properties, Farid, M.A., Ed., Lambert: Academic, 2015.
Pomogailo, A.D. and Dzhardimalieva, G.I., Metallopolimernye gibridnye nanokompozity (Metal–Polymer Hybrid Nanocomposites), Moscow: Nauka, 2015.
Moroz, Ya.A. and Cherednichenko, L.A., Concerning some general relationships in the thermolysis of heteropolyoxometalates with 3d elements, Zh. Vestn. Donetsk. Nats. Univ, Ser. A: Estestv. Nauki, 2018, no. 1, pp. 95–103.
Moroz, Ya.A., Lozinskii, N.S., Lopanov, A.N., Chebyshev, K.A., and Burkhovetskii, V.V., Investigation of cesium tungstophosphate thermolysis products, Zh. Vestn. Belarus. Gos. Tekh. Univ. im. V. G. Shukhova, 2020, no. 12, pp. 126–135. https://doi.org/10.34031/2071-7318-2020-5-12-126-125
Pope, M.T., Heteropoly and Isopoly Oxometallates, Berlin: Springer, 1983.
Moroz, Ya.A., General relationships in the synthesis of heteropoly compounds with 3d elements, Zh. Vestn. Donetsk. Nats. Univ, Ser. A: Estestv. Nauki, 2017, no. 1, pp. 92–110.
Kato, C.N., Ukai, N., Miyamae, D., Arata, S., Kashiwagi, T., Nagami, M., Mori, T., Kataoka, Y., Kitagawa, Y., and Uno, H., Syntheses and X-ray crystal structures of magnesium-substituted polyoxometalates, Advanced Topics in Crystallization, Mastai, Y., Ed., Norderstedt: Books on Demand, 2015. https://doi.org/10.5772/59598
Weakley, T.J.R., Crystal structure of cesiumaquanickelo(II)undecatungstophosphate dihydrate, J. Crystallogr. Spectrosc. Res., 1987, vol. 17, no. 3, pp. 383–391.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Moroz, Y.A., Lozinskii, N.S., Lopanov, A.N. et al. Low-Temperature Synthesis of Compounds with the Pyrochlore and Hexagonal Tungsten Bonze Structure. Inorg Mater 57, 835–842 (2021). https://doi.org/10.1134/S0020168521080069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521080069