Abstract—
Сu1 – xAl0.25In0.75Se2 copper-deficient solid solutions with the chalcopyrite structure have been prepared by elemental synthesis from Cu, In, Al, and Se, and their unit-cell parameters have been determined for the first time as functions of x in the range 0 < x ≤ 0.20. It has been shown that the band peaking at 1.320 eV in the 78-K cathodoluminescence spectra of the Сu1 – xAl0.25In0.75Se2 solid solutions is most likely due to CuM and MCu (M = Al, In) antisite defects resulting from exchange of atoms between the copper and indium–aluminum sublattices in the chalcopyrite structure and that the band peaking at 1.245 eV arises from Cu2+ · VCu associates, which are predominant defects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, intensive research effort has been concentrated on alternative power generation technologies, in particular on the fabrication of solar cells [1]. Promising inorganic materials for such devices include Cu1 – xGayIn1 – y(S,Se)2 (CIGS) solid solutions [1]. Chalcopyrite CIGS materials are used in the fabrication of a new generation of thin-film solar cells [1]. In the best laboratory prototypes of CIGS-based solar cells, solar energy conversion efficiency has reached 21.7% [2]. This value is substantially lower than the theoretically possible 30% [3]. Replacing gallium by aluminum to give a CIASe material has recently been viewed as a way to produce more efficient solar cell materials [4]. The CuAlSe2 compound crystallizes in the chalcopyrite structure [5] and has a broad band gap, Eg = 2.68 eV, whereas CuGaSe2 has Eg = 1.70 eV [1]. Therefore, aluminum substitution for gallium should lead to a broader band gap of CIASe materials, which is of considerable interest. Also important is that expensive gallium is replaced by cheap and readily available aluminum, which is, in addition, nontoxic.
Even the best CIASe-based laboratory prototypes of solar cells still have a comparatively low efficiency, near 16.9% [4]. This is attributable to the presence of deep levels in their band gap, which act as traps for photogenerated current carriers. Note that high-quality films are difficult to grow because the CuInSe2–CuAlSe2 system, containing CIASe, has been little studied: there are no phase diagram data in the literature and essentially no data on the nature of defects in CIASe. Paulson et al. [7] obtained films of CIASe solid solutions, but they did not determine their unit-cell parameters. Sugiyama et al. [8] determined the unit-cell parameters of films of CIASe solid solutions with a relatively low accuracy, to the second decimal place, which makes any comparison impossible. Gebicki et al. [9] studied only two samples in the range of CIASe compositions important for practical application. Their results showed that the samples consisted of solid solutions with the chalcopyrite structure. The materials were prepared by means of chemical vapor transport. Note that the composition of the solid solution crystals thus grown was assumed by Gebicki et al. [9] to be identical to the composition of the precursor mixture used in synthesis, which might be not so. Odin et al. [10] synthesized CuInSe2-based solid solutions in the CuAlSe2–CuInSe2 system with the chalcopyrite crystal structure, determined their unit-cell parameters as functions of composition, and studied their cathodoluminescence (CL) spectra.
The efficiency of CIASe can be improved by using copper-deficient materials. It is known that the best solar cell performance so far has been offered by Cu1 – xGayIn1 – ySe2 copper-deficient materials with y = 0.25–0.30 and x = 0.1–0.3 [11]. Odin et al. [12] determined the unit-cell parameters of the Cu1 – x(Ga0.3In0.7)Se2 (0 < x ≤ 0.32) copper-deficient solid solutions and demonstrated the presence of copper in the oxidation states Cu2+ and Cu+ in their crystal lattice. In addition, copper deficiency was shown to have a significant effect on the lifetime of photogenerated current carriers in Cu1 – x(Ga0.3In0.7)Se2 [13].
The reduced copper content in the copper-deficient solid solutions can ensure required p-type conductivity and increase the electrical conductivity of the material. Reports on studies of copper-deficient CIASe solid solutions, including Сu1 – xAl0.25In0.75Se2, are still missing in the literature. Clearly, potential practical applications of the CIASe materials are limited by the absence of data on structural characteristics of the Сu1 – xAl0.25In0.75Se2 copper-deficient solid solutions and the effect of their composition on the defect structure of the chalcopyrite phase.
The objectives of this work were to synthesize Сu1 – xAl0.25In0.75Se2 (0 < x ≤ 0.20) solid solutions, determine their phase composition, and investigate their structural and luminescence properties.
EXPERIMENTAL
Polycrystalline Сu1 – xAl0.25In0.75Se2 (0 < x ≤ 0.20) samples were synthesized in two steps. In the first step, appropriate amounts of high-purity copper, indium, aluminum, and selenium (5N grade) were fired at a temperature of 1100°C for 10 h in graphitized quartz ampules sealed off under vacuum (residual pressure pres = 2 × 10–3 mm Hg). To eliminate the risk of explosion during the synthesis process, the samples were heated to this temperature at a rate of 1°C/min. After the ampules were opened, the resultant materials were ground in an agate mortar. Next, the mixtures were again sealed in ampules under vacuum and homogenized by annealing 650°C for 600 h.
The phase composition of the samples thus prepared was determined by X-ray diffraction. Intensity data were collected on a DRON-4 diffractometer (\({\text{Cu}}{{K}_{{{{\alpha }_{1}}}}})\) radiation). X-ray diffraction patterns were analyzed using WinXPOW software.
CL spectra were measured at 78 K. The luminescence was excited by a pulsed electron beam with an energy of 40 keV. The CL spectra were recorded using a DFS-13 monochromator. The procedure used to measure CL spectra was described in greater detail previously [14].
RESULTS AND DISCUSSION
Structural Data
The unit-cell parameters and volume V of the CuInSe2-based chalcopyrite solid solutions in the CuAlSe2–CuInSe2 system were measured as functions of composition (Fig. 1). The observed decrease in these characteristics is due to the fact that the ionic radius of Al3+ is smaller than that of In3+. These data were considered in detail previously [10].
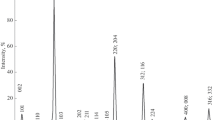
Cu1 – xAl0.25In0.75Se2 copper-deficient solid solutions. Сu1 – xAl0.25In0.75Se2 solid solutions were found to exist in the composition range 0 < x ≤ 0.20. All of the lines observed in the X-ray diffraction patterns of the Сu1 – xAl0.25In0.75Se2 samples could be indexed in a tetragonal chalcopyrite lattice (using data for 27 lines in each X-ray diffraction pattern), with space group \(I\bar {4}2d\). No lines of any impurity phases were detected. As an example, Fig. 2 shows the X-ray diffraction pattern of the Сu1 – xAl0.25In0.75Se2 sample with x = 0.20. No superstructure reflections were detected in the X‑ray diffraction patterns. The tetragonal unit-cell parameters of the Сu1 – xAl0.25In0.75Se2 solid solutions were determined with an accuracy of ±0.003 Å for the a cell parameter and ±0.005 Å for the c cell parameter and were shown to decrease with increasing x (Fig. 3). The unit-cell volume V (determined with an accuracy of ±0.06 Å3) was also found to decrease with increasing x (Fig. 3).
The observed behavior of the unit-cell parameters clearly indicates the formation of Сu1 – xAl0.25In0.75Se2 solid solutions in the composition range 0 < x ≤ 0.20. The copper sublattice in the crystal lattice of the Сu1 – xAl0.25In0.75Se2 solid solutions contains VCu vacancies (◻). To maintain electroneutrality of the materials, one copper atom per vacancy in the copper sublattice passes to the oxidation state 2+ (Table 1). Therefore, the general formula of the Сu1 – xAl0.25In0.75Se2 solid solutions should be represented in the form \({\text{Cu}}_{{1 - x}}^{ + }{\text{Cu}}_{{x/2\square x/2}}^{{2 + }}\)Al0.25In0.75Se2 (0 < х ≤ 0.20).
The above-mentioned decrease in unit-cell parameters can be accounted for by the fact that the ionic radius of Cu2+ is smaller than that of Cu+.
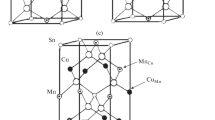
Defects in the copper and aluminum–indium sublattices of CIASe. The crystal lattice of CuGayIn1 – ySe2 solid solutions is known to be disordered [15, 16]: some of the copper atoms reside in the indium–gallium sublattice (in the general form M) instead of the copper sublattice, to form CuM defects (copper atoms on the M site), and some of the M atoms reside in the copper sublattice instead of the indium–gallium sublattice, to form MCu defects (M atoms on the copper site). Figure 4a shows the crystal structure of the CuGayIn1 – ySe2 chalcopyrite solid solutions free of antisite defects [11] (random gallium/indium distribution on the M site), and Fig. 4b shows a possible structure of CuAlyIn1 – ySe2 containing CuM and MCu (M = In, Al) antisite defects. We compared intensity of reflections in the X-ray diffraction patterns of the CuGayIn1 – ySe2 and CuAlyIn1 – ySe2 solid solutions with y = 0.25. The intensities of the weak lines characterizing the chalcopyrite structure were found to be roughly equal for these two phases (Table 2). A very similar situation was observed in the case of the Cu1 – хGa0.25In0.75Se2 and Cu1-xAl0.25In0.75Se2 solid solutions with x = 0.1 (Table 2). Most likely, the crystal lattices of CuAlxIn1 – хSe2 (0 < x < 0.30) and Сu1 – xAl0.25In0.75Se2 are also disordered: some of the Cu atoms reside in the indium–aluminum sublattice to form CuM defects (acceptors) and some of the aluminum and indium atoms reside in the copper sublattice to form MCu defects (donors).
(a) Crystal structure of CuGayIn1 – ySe2 solid solutions [11] and (b) possible structure of CuAlyIn1 – ySe2 solid solutions containing CuM and MCu antisite defects.
Cathodoluminescence Spectra
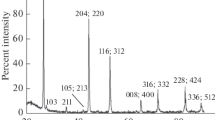
The 78-K CL spectra of the Сu1 – xAl0.25In0.75Se2 solid solutions were found to contain no excitonic lines suitable for accurately determining the band gap Eg of the samples. The spectrum of the x = 0 sample contained a band peaking at 1.320 eV (Fig. 5a). To find out the origin of this band, we turned to CuGayIn1 – ySe2, a better studied solid solution system. At gallium contents in the range y = 0.35–0.50, CL data indicate the presence of a level at 1.235 eV in the band gap. CuM and MCu defects produce similar energy levels in the band gap. As mentioned above, the crystal lattices of CuAlyIn1 – ySe2 (0 < y ≤ 0.30) and Сu1 – xAl0.25In0.75Se2 (0 < x ≤ 0.20) contain CuM and MCu defects, which produce similar levels in the band gap.
The 78-K CL spectrum of the Сu1 – xAl0.25In0.75Se2 sample with x = 0.10 contains a strong, broad band peaking at 1.245 eV (Fig. 5b), in addition to the 1.320-eV band considered above. Clearly, the 1.245-eV band is due to copper in the oxidation state 2+: the presence of Cu2+ instead of Cu+ gives a positively charged defect, which combines with a negatively charged copper vacancy (VCu) to form a Cu2+ · VCu defect associate. The VCu vacancy concentration in the copper sublattice is equal to the Cu2+ concentration (Table 1). These concentrations are high and, hence, the Cu2+ · VCu associates are predominant defects. It is of interest to note that, throughout the composition range x = 0.05–0.20, the peak position of the band remains unchanged (at 1.245 eV), even though the composition of the solid solutions varies in a wide range. The reason for this is that, throughout the composition range 0 < x ≤ 0.20, the structure of the Сu1 – xAl0.25In0.75Se2 solid solutions remains unchanged and contains such defect associates.
CONCLUSIONS
Сu1 – xAl0.25In0.75Se2 (0 < x ≤ 0.20) copper-deficient solid solutions with the chalcopyrite structure have been identified for the first time and their unit-cell parameters have been measured as functions of x. In the crystal lattice of Сu1 – xAl0.25In0.75Se2, some of the copper atoms reside in the indium–aluminum sublattice and some of the indium and aluminum atoms reside in the copper sublattice.
It has been shown for the first time that the band peaking at 1.320 eV in the 78-K CL spectra of the Сu1 – xAl0.25In0.75Se2 solid solutions is most likely due to CuM and MCu antisite defects resulting from exchange of atoms between the copper and indium–aluminum sublattices in the chalcopyrite structure and that the band peaking at 1.245 eV arises from Cu2+ · VCu associates, which are predominant defects. The formation of these defects should be taken into account in analyzing the electrical transport and optical properties of selenium-enriched CIASe materials.
REFERENCES
Novikov, G.F. and Gapanovich, M.V., Third generation solar cells based on Cu–In–Ga–(S,Se), Usp. Fiz. Nauk, 2017, vol. 187, pp. 173–191.
Jackson, P., Hariskos, D., Wuerz, R., et al., Properties of Cu(In,Ga)Se2 solar cells with new record efficiencies up to 21.7%, Phys. Status Solidi, 2015, vol. 9, pp. 28–31.
Baek, E.R., Astini, V., Tirta, A., and Kim, B., Phase evolution of CIGS alloyed compound synthesis by direct melting method, Curr. Appl. Phys., 2011, vol. 11, pp. S76–S80.
Lopez-Garcia, J., Placidi, M., Fontane, X., et al., CuIn1 – xAlxSe2 thin film solar cells with depth gradient composition prepared by selenization of evaporated metallic precursors, Sol. Energy Mater. Sol. Cells, 2015, vol. 132, pp. 245–253.
Shay, J.L. and Wernick, J.H., Ternary Chalcopyrite Semiconductors: Growth, Electronic Properties, and Applications, Oxford: Pergamon, 1975, 1st ed.
Barkat, L., Morsli, M., Amory, C., et al., Study on the fabrication of n-type CuAlSe2, Thin Solid Films, 2003, vols. 431–432, pp. 99–104.
Paulson, P.D., Haimbodi, M.W., Marsillac, S., et al., CuIn1 − xAlxSe2 thin films and solar cells, J. Appl. Phys., 2002, vol. 91, pp. 10153–10156.
Sugiyama, M., Umezawa, A., Yasuniwa, T., et al., Growth of single-phase Cu(In,Al)Se2 photoabsorbing films by selenization using diethylselenide, Thin Solid Films, 2009, vol. 517, pp. 2175–2177.
Gebicki, W., Igalson, M., Zajac, W., and Trykozko, R., Growth and characterization CuAlxIn1 – xSe2 mixed crystal, J. Phys. D: Appl. Phys., 1990, vol. 23, pp. 964–965.
Odin, I.N., Gapanovich, M.V., Chukichev, M.V., and Novikov, G.F., Structural and luminescence characteristics of CuAlxIn1 – xSe2 (0 < x ≤ 0.30) chalcopyrite solid solutions, Mendeleev Commun., 2020, vol. 30, no. 5, pp. 666–668.
Conibeer, G. and Willoughby, A., Solar Cell Materials: Developing Technologies, New Delhi: Wiley, 2014.
Odin, I.N., Chukichev, M.V., Gapanovich, M.V., et al., Magnetic and luminescent properties of copper-deficient Cu1 – x(In0.7Ga0.3)Se2 (0 < x ≤ 0.32) solid solutions with chalcopyrite structure, Mendeleev Commun., 2018, vol. 28, no. 3, pp. 248–250.
Gapanovich, M.V., Odin, I.N., Rabenok, E.V., et al., Defect structure and photogenerated carrier loss processes in Cu1 – x(In0.7Ga0.3)Se2 (0 ≤ x ≤ 0.3) chalcopyrite solid solutions, Inorg. Mater., 2019, vol. 55, no. 7, pp. 648–652.
Gapanovich, M.V., Odin, I.N., Chukichev, M.V., et al., Synthesis, structure, and luminescence properties of Cd1 – xMgxTe (0 < x < 0.20) solid solutions, Inorg. Mater., 2016, vol. 52, no. 1, pp. 53–56.
Baranowski, L.L., Zawadzki, P., Lany, S., et al., A review of defects and disorder in multinary tetrahedrally bonded semiconductors, Semicond. Sci. Technol., 2016, vol. 31, paper 123004.
Igalson, M. and Urbaniak, A., Defect states in the CIGS solar cells by photocapacitance and deep level optical spectroscopy, Bull. Pol. Acad. Sci.: Tech. Sci., 2005, vol. 53, no. 2, pp. 157–161.
Funding
This work was supported by the Russian Federation Ministry of Science and Higher Education, state research target no. AAAA-A19-119070790003-7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Gapanovich, M.V., Odin, I.N., Chukichev, M.V. et al. Structure and Luminescence Properties of Сu1 – xAl0.25In0.75Se2 (0 < x ≤ 0.20) Copper-Deficient Chalcopyrite Solid Solutions. Inorg Mater 57, 124–129 (2021). https://doi.org/10.1134/S0020168521010064
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168521010064