Abstract
Alloys between tin and tin telluride have been prepared and their thermal conductivity has been measured in the temperature range ~90–300 K. We have determined the lattice (χl) and electron (χel) contributions to the thermal conductivity of the alloys and their thermal resistivity due to structural defects (Sn vacancies and defects). We assume that, at low excess Sn concentrations, Sn atoms form electroneutral complexes with Sn vacancies, leading to a reduction in χl and χel. At high concentrations, Sn atoms fill vacancies, leading to an increase in χl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The electrical properties of tin telluride and its solid solutions have been the subject of extensive studies [1–6] because they are potentially attractive as materials for thermo- and photoelectric converters and their crystallization behavior and valence band structure have interesting features.
The Sn–Te system contains one compound, SnTe, which melts congruently at 790°C. The extent of the homogeneity range of tin telluride was studied previously by microstructural analysis and X-ray diffraction [8, 9]. After annealing at 700°C, an alloy with the stoichiometric composition contained the SnTe + Sn eutectic, whereas alloys containing 0.3 and 0.5 at % excess tellurium were single-phase. An alloy enriched in tellurium by 0.8 at % contained noticeable amounts of the SnTe + Te eutectic. After annealing at 600, 500, and 400°C, this alloy was single-phase, and the SnTe + Te eutectic was found in an alloy containing 51 at % Te. In addition, Sn vacancies were shown to be predominant defects in tin telluride. Lorenz and Jepsen [10] proposed as qualitative model accounting for the high concentration of Sn vacancies in tin telluride.
The homogeneity range of tin telluride at 400°C extends from 50.1 ± 0.1 to 50.9 ± 0.1 at % Te [8, 9]. Note that, with increasing tellurium content, the lattice parameter of tin telluride decreases in the range 6.324 to 6.302 Å, in good agreement with data reported by Mazelsky and Lubell [11]. The position of the boundaries of the homogeneity range of tin telluride was also studied by Brebrick [12] in the temperature range 550–797°C. According to Brebrick [12], the homogeneity range of SnTe at 600°C is limited by the compositions 50.1 and 51.1 at % Te. Later, the homogeneity range of tin monotelluride was studied by Rogacheva et al. [13]. The homogeneity range of tin telluride was shown to not include its stoichiometric composition, being shifted to the tellurium-rich side, with a high density of native defects (mainly, cation vacancies). From the composition dependence of the lattice parameter within the homogeneity range in the Sn–Te system [8, 9, 13], Dzyubenko et al. [14] estimated the effective radius of a cation vacancy. According to their results, the generation of cation vacancies as a result of a deviation from stoichiometry in SnTe leads to a substantially higher lattice strain than any cation substitution [15].
Sn vacancies in a Sn–SnTe alloy would be expected to influence phonon scattering, as well as the carrier concentration and mobility in the alloy, that is, the lattice and electron components of its thermal conductivity, respectively. However, reports dealing with research into the effect of structural vacancies on the thermal conductivity of alloys between tin and tin telluride are not available in the literature. Such research is of interest as well because materials based on Sn–SnTe alloys are intermediate-temperature thermoelectrics whose efficiency is determined by their thermal conductivity and because they can provide information about phonon and electron scattering by structural vacancies.
It is reasonable to expect that the Sn vacancy concentration in alloys between tin and tin telluride can be varied by adding excess Sn atoms to a 50 at % Sn–50 at % Te melt.
Taking this into account, to gain information about the effect of Sn vacancies on the thermal conductivity of alloys between tin and tin telluride we prepared samples from a 50 at % Sn–50 at % Te melt containing up to 1.0 at % excess Sn and measured their thermal conductivity in the temperature range 90–300 K.
EXPERIMENTAL
Sn–SnTe alloys containing 0.01, 0.05, 0.10, 0.50, and 1.0 at % excess Sn were prepared by directly melting appropriate amounts of their constituent components at a temperature of ~1135 K over a period of 6 h in silica ampules pumped down to ~10–2 Pa. The starting chemicals used were OVCh-000 tin and T-sCh (99.999%) tellurium. During the synthesis process, the melt was vibration-stirred. The inner surface of the silica ampules was graphitized. The synthesized ingots were 13–14 mm in diameter and ~25 mm in length.
The samples were characterized before and after annealing at 773 K. Annealing was carried out in spectroscopically pure argon for 120 h. The samples thus annealed had stable electrical parameters [4].
X-ray diffraction patterns were collected on a Bruker XRD D8 Advance diffractometer. Homogeneity of the ingots was checked by measuring their electrical resistivity in a number of regions along their length. The ingot section uniform in electrical properties was up to 20–22 mm in length. For electrical measurements, cylindrical samples 13–14 mm in diameter and ~10 mm in thickness were cut by spark erosion from the uniform part of the ingots. The cutting-induced damage layers on the end faces of the samples were removed by electrochemical etching.
The thermal conductivity of the samples was measured by an absolute steady-state technique, as described by Okhotin et al. [16], along the ingot length. The uncertainty in our thermal conductivity measurements was less than 5% over the entire temperature range studied.
RESULTS AND DISCUSSION
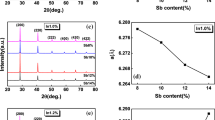
No lines of a second phase (Sn-based phase) were detected in X-ray diffraction patterns because of the insufficient sensitivity of the X-ray diffraction method used. The refined unit-cell parameter of the alloy between tin and tin telluride, a = 6.318 Å (sp. gr. Fm\(\bar {3}\)m) corresponds to the a of tin telluride in two-phase SnTe + Sn alloys.
Figure 1 shows a micrograph of the 50 at % Sn + 50 at % Te alloy. It is seen that the alloy contains a second phase.
Figure 2 shows temperature dependences of total thermal conductivity (χ) for our samples before annealing (Fig. 2a) and after annealing at 773 K for 120 h (Fig. 2b). It is seen that, in all cases, χ decreases with increasing temperature. Annealing changes the χ of the samples at a given temperature.
To gain insight into the mechanism underlying the influence of excess Sn on the thermal conductivity of the alloys, we assessed the components of their total thermal conductivity in the range 90–300 K.
In the general case, the thermal conductivity of a semiconductor is contributed by lattice vibrations (χl), conduction electrons (χel), ambipolar electron and hole diffusion in the intrinsic + mixed region (χi), magnons at low temperatures in magnetic semiconductors (χm), and photons at intermediate temperatures in sufficiently pure semiconductors (χph) [17, 18]. At the same time, if a semiconductor is opaque in the infrared spectral region, its total thermal conductivity in the extrinsic region can be represented as
In the case of metals, we have
where L is the Lorenz number and σ is the electrical conductivity of the metal. In the case of semiconductors with a parabolic conduction band, arbitrary degeneracy, and elastic scattering of current carriers, we have

where k is Boltzmann’s constant, e is the electron charge, and the parameter A depends on the scattering parameter. A was determined from experimental thermoelectric power (α) data using an A(α) curve [17, 18].
The measured χ and the 90- and 300-K χl and χel determined using the above relations are presented in Table 1, together with the σ and α of our samples at 90 and 300 K. It is seen that heat conduction in our samples is mainly due to lattice vibrations. The electronic contribution to the thermal conductivity of the samples does not exceed ~30% of their total thermal conductivity.
It also follows from the data in Table 1 that annealing produces significant changes in the thermal and electrical properties of the samples at a given temperature. In all cases, annealing is accompanied by an increase in lattice thermal conductivity.
Raising the excess Sn concentration to 0.05 at % reduces the ~90- and 300-K lattice thermal conductivity of both the unannealed and annealed samples, whereas above 0.05 at % Sn it rises.
Figure 3 shows temperature dependences of lattice thermal resistivity (Wl = 1/χl) for our samples. The linear temperature variation of Wl suggests that the thermal resistance is mainly due to phonon–phonon scattering.
Temperature dependences of lattice thermal resistivity for the samples (a) before and (b) after annealing: (1–6) see the caption to Fig. 2.
Sn vacancies in the samples produce defects scattering phonons. The thermal resistivity of a material containing point defects can be represented in the form [17]
where W0 is the thermal resistivity of a defect-free material, and D and c are constants. It is seen that point defects make a temperature-independent contribution to thermal resistivity. This was demonstrated experimentally for iodine-doped PbTe [19] and Pb1 –хMnхTe [20] and Sn1 –хMnхTe [21] crystals. In connection with this, the additional thermal resistivity ΔW0 due to defects can be found by extrapolating the linear portion of the temperature dependence of lattice thermal resistivity Wl at low temperatures. The intercept on the thermal resistivity axis (at T = 0) will then be ΔW0. The ΔW0 values obtained are also presented in Table 1.
A joint analysis of the present data on the thermal and electrical properties of the alloys between tin and tin telluride leads us to assume the following: The donor centers produced by Sn atoms in our samples compensate doubly charged vacancies to form electrically neutral complexes. The compensation of doubly charged vacancies leads to a decrease in hole concentration and, accordingly, to a decrease in electrical conductivity σ and an increase in thermoelectric power α. The electrically neutral complexes scatter phonons, thus reducing the lattice thermal conductivity. At an excess Sn concentration of 0.05 at %, this process reaches completion and σ, χl, and χel have a minimum. As the amount of excess Sn is increased further, the donor centers produced by the Sn lead to an increase in electron concentration in the samples, that is, to an increase in σ and χel. At high concentrations of excess Sn, some of the Sn atoms fill Sn vacancies, thereby reducing the density of structural defects (vacancies), which entails an increase in χl. This is also evidenced by the fact that, at high concentrations of excess Sn (above 0.5 at % Sn), the thermoelectric power drops sharply. The above assumption is well consistent with the dependence of the additional thermal resistivity ΔW0 on the concentration of excess Sn. In particular, with increasing Sn content, the ΔW0 of both the unannealed and annealed samples first rises and then falls off. It follows from the data in Table 1 that, in all cases, the thermal resistivity due to defects is lower in the annealed samples in comparison with the unannealed samples. The reason for this is that annealing of crystals eliminates defects produced during the preparation of alloys and samples for measurements. Comparison of the ΔW0 data for the unannealed and annealed samples demonstrates that the contribution of structural defects to the total thermal resistivity reaches ~30%.
CONCLUSIONS
Alloys between tin and tin telluride have been prepared and their thermal conductivity has been measured in the temperature range 90–300 K. We have calculated the electron and lattice contributions to the thermal conductivity of the alloys and their thermal resistivity due to structural defects. The results demonstrate that heat conduction in the alloys is mainly due to lattice vibrations and that the electronic contribution to their thermal conductivity does not exceed ~30% of their total thermal conductivity. Structural defects related to Sn vacancies play a significant role in the thermal resistivity of the alloys. At a concentration of 0.05 at % or lower, excess Sn atoms act as donor centers, forming electroneutral complexes with vacancies and scattering phonons, thereby reducing χl. Above 0.05 at %, excess Sn atoms fill vacancies, leading to an increase in χl. Annealing eliminates defects produced during the preparation of the alloys and samples, leading to a reduction in additional thermal resistivity by 25–30%. The temperature dependence of the lattice thermal conductivity is determined by phonon–phonon scattering.
REFERENCES
Brebrik, R.F. and Strauss, A.J., Anomalous thermoelectric power as evidence for two valence bands in SnTe, Phys. Rev., 1963, vol. 131, no. 1, pp. 104–110.
Efimova, B.A., Kaidanov, V.I., Moizhes, B.Ya., and Chernik, I.A., A model for the energy band structure of SnTe, Fiz. Tverd. Tela (Leningrad), 1965, vol. 7, no. 8, pp. 2524–2527.
Kaidanov, V.I., Chernik, I.A., and Efimova, B.A., Energy band structure and carrier scattering mechanism in lead telluride, Fiz. Tekh. Poluprovodn. (Leningrad), 1967, vol. 1, no. 6, pp. 869–879.
Bagieva, G.Z., Abdinova, G.D., Mustafaev, N.B., and Abdinov, D.Sh., Effect of annealing on the electrical properties of SnTe crystals, Inorg. Mater., 2017, vol. 53, no. 4, pp. 358–360. https://doi.org/10.1134/S002016851704001X
Okhotin, A.S., Efremov, A.A., Okhotin, V.S., and Pushkarskii, A.S., Termoelektricheskie generatory (Thermoelectric Generators), Moscow: Atomizdat, 1976.
Akhundova, N.M., Electrical and thermal conductivity of Sn1 –xMnxTe (0 ≤ x ≤ 0.04) solid solutions, Izv. Vyssh. Uchebn. Zaved.,Fiz., 2017, vol. 60, no. 9, pp. 114–117.
Hansen, M. and Anderko, K., Constitution of Binary Alloys, New York: McGraw-Hill, 1958, 2nd ed.
Shelimova, L.E. and Abrikosov, N.Kh., Sn–Te system the SnTe-rich region, Zh. Neorg. Khim., 1964, vol. 9, no. 8, pp. 1979–1882.
Abrikosov, N.Kh. and Shelimova, L.E., Poluprovodnikovye materialy na osnove soedinenii AIVBVI (IV–VI Semiconductor Materials), Moscow: Nauka, 1975.
Lorenz, M.R. and Jepsen, D.W., An explanation of high cation vacancy concentration and p-type conductivity in semiconductors containing a multivalent metal in its lowest valence state, J. Phys. Chem. Solids, 1965, vol. 26, pp. 1177–1179.
Mazelsky, R. and Lubell, S., Nonstoichiometry in some group IV tellurides, in Advances in Chemistry, Washington, DC: Am. Chem. Soc., 1963, pp. 210–217.
Brebrick, R.F., Deviations from stoichiometry and electrical properties in SnTe, J. Phys. Chem. Solids, 1963, vol. 24, no. 1, pp. 27–36. https://doi.org/doi.org/10.1016/0022-3697(63)90038-6
Rogacheva, E.I., Gorne, G.V., Zhigareva, P.K., and Ivanova, A.B., Homogeneity range of tin monotelluride, Izv. Akad. Nauk SSSR,Neorg. Mater., 1991, vol. 27, no. 2, pp. 267–276.
Dzyubenko, N.I., Rogacheva, E.I., and Kosevich, V.M., Influence of indium, gallium, antimony and bismuth on the properties of tin telluride, Izv. Akad. Nauk SSSR,Neorg. Mater., 1983, vol. 19, no. 9, pp. 1457–1461.
Rogacheva, E.I. and Nashchekina, O.N., Lattice strengthening due to cation substitutions in the compound semiconductor SnTe, Inorg. Mater., 1995, vol. 31, no. 6, pp. 723–726.
Okhotin, A.S., Pushkarskii, A.S., Borovikov, R.P., and Simonov, V.A., Metody izmereniya kharakteristik termoelektricheskikh materialov i preobrazovatelei (Characterization of Thermoelectric Materials and Converters), Moscow: Nauka, 1974.
Oskotskii, V.S. and Smirnov, I.A., Defekty v kristallakh i teploprovodnost’ (Defects in Crystals and Thermal Conductivity), Leningrad: Nauka, 1972.
Smirnov, I.A. and Tamarchenko, V.I., Elektronnaya teploprovodnost’ v metallakh i poluprovodnikakh (Electronic Thermal Conduction in Metals and Semiconductors), Leningrad: Nauka, 1977.
Devyatkova, E.D. and Smirnov, I.A., Effect of halogen doping on the thermal conductivity of lead telluride, Fiz. Tverd. Tela (Leningrad), 1961, vol. 3, no. 8, pp. 2298–2309.
Bagieva, G.Z., Abdinova, G.D., Mustafaev, N.B., and Abdinov, D.Sh., Thermal conductivity of tellurium-enriched Pb1 –xMnxTe single crystals, Inorg. Mater., 2013, vol. 49, no. 11, pp. 1078–1080. https://doi.org/10.1134/S0020168513110010
Bagieva, G.Z., Abdinova, G.D., Mustafaev, N.B., and Abdinov, D.Sh., Thermal conductivity of Sn1 –xMnxTe single crystals, Inorg. Mater., 2016, vol. 52, no. 12, pp. 1215–1219. https://doi.org/10.1134/S0020168516120013
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Bagieva, G.Z., Abdinova, G.D., Mustafaev, N.B. et al. Thermal Conductivity of Sn–SnTe Alloys. Inorg Mater 56, 690–694 (2020). https://doi.org/10.1134/S002016852007002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002016852007002X