Abstract—
We have studied the effect of ZrC and ZrO2 additions on the microstructure and properties of ceramic composites based on ZrB2–5% SiC, produced by pressure-sintering mechanically activated powders at a temperature of 1600°C. The results demonstrate that the addition of ZrO2 increases the density, hardness, and fracture toughness of ceramic composites based on ZrB2–5% SiC. In particular, the KIc of the ZrB2–5% SiC ceramic is 4.6 ± 0.3 MPa m1/2 and that of the composite containing 20% ZrO2 is 7.3 ± 0.4 MPa m1/2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Zirconium and hafnium borides, ZrB2 and HfB2, have high electrical and thermal conductivity, wear resistance, and chemical inertness, in particular with molten metals, which makes them attractive for use in machines and apparatuses operating at high temperatures, under friction contact conditions, and in contact with molten metals [1–7]. However, the high chemical bond covalence; small Zr, Hf, and B self-diffusion coefficients; and high melting point impair the sinterability of single-phase ZrB2 and HfB2 ceramics. One effective approach to the densification of ceramics based on zirconium and hafnium diborides is to add other components, that is, to produce composite structures.
Moreover, the fabrication of a composite structure in ZrB2- and HfB2-based ceramics enables one to obtain novel service characteristics. For example, the high-temperature stability of ZrB2–ZrC composites in an oxygen-containing atmosphere significantly surpasses that of single-phase ZrB2 ceramics. ZrB2- and HfB2-based ceramic composites containing MoSi2, SiC, TiC, and ZrO2 additions have an appreciably higher fracture toughness than do single-phase ceramics [8–13].
Ceramic composites containing ZrB2, ZrC, ZrO2, and SiC are of interest from the viewpoint of practical application in engineering structures and friction pairs. Studies concerned with properties of such ceramic composites are usually limited to a narrow range of component concentrations, which makes it impossible to carry out a complete analysis of the effect of composition on the structure and properties of composites.
The purpose of this work was to study the effect of composition on the microstructure and properties of ZrB2–SiC–(ZrC/ZrO2) ceramic composites.
EXPERIMENTAL
We studied ceramic composites based on ZrB2–5% SiC with ZrO2 and ZrC additions (5 to 20 vol %). The starting materials used to produce the ceramic composites were commercially available ZrB2, 6H-SiC, ZrC, and ZrO2 powders. Zirconia was stabilized with 3 mol % Y2O3 to give the tetragonal phase t-ZrO2. All of the powders consisted of irregularly shaped particles. The average particle size of the ZrB2 powder was 〈d〉 = 1.65 μm (〈d90〉 = 4.14 μm), that of the SiC powder was 〈d〉 = 2.2 μm (〈d90〉 = 5.84 μm), and that of the ZrC powder was 〈d〉 = 1.6 μm (〈d90〉 = 3.3 μm). The ZrO2 powder had the smallest average particle size: 〈d〉 = 0.35 μm (〈d90〉 = 0.7 μm).
Powder mixtures were prepared and simultaneously activated by grinding for 3 min in an AGO planetary activator mill under ethanol at a vial rotation rate of 1820 rpm. Ceramic composites were produced by sintering the powder mixtures at a temperature of 1600°C and pressure of 30 MPa for 15 min in an argon atmosphere.
The density of the ZrB2–6H-SiC–ZrO2 and ZrB2–6H-SiC–ZrC composites was determined by hydrostatic weighing after their open porosity was closed with varnish. The theoretical density of the composites was calculated using the rule of mixtures.
The phase composition of the composites was determined by X-ray diffraction on a Shimadzu XRD 7000 diffractometer with CuKα radiation (λ = 1.5405 Å).
Their hardness was determined on a polished sample surface by indenting a Vickers pyramid at a load of 98 N and dwell time of 10 s.
Fracture toughness was evaluated from the total length of cracks emanating from an indent using the relation KIc = 0.16HVa1/2(c/a)–3/2, where a is half the indent diagonal and c is the length of the cracks [14].
The modulus of elasticity of the ceramic composites was evaluated from the speed of ultrasound in the samples using a Tektronix TDS 220 oscilloscope (the United States) and a Panametrics 5800 pulser/receiver (Korea).
RESULTS AND DISCUSSION
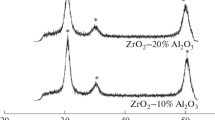
Figure 1 shows X-ray diffraction patterns of the ceramic composites produced by us. According to the X-ray diffraction results, the phase composition of the composites includes the starting materials ZrB2, 6H‑SiC, ZrO2, and ZrC. In the 95 vol % ZrB2 + 5 vol % SiC + x vol % ZrO2 composites, zirconia was present as the tetragonal phase t-ZrO2.
The unit-cell parameters of the components of the composites were similar to those presented in the JCPDS Powder Diffraction File: а = 3.167 ± 0.002 Å and с = 3.531 ± 0.001 Å for ZrB2 (JCPDS card no. 34-0423: а = 3.168 Å and с = 3.530 Å); а = 4.695 ± 0.002 Å for ZrC (JCPDS card no. 65-0332: а = 4.694 Å); а = 3.625 ± 0.002 Å and с = 5.198 ± 0.001 Å for t-ZrO2 (JCPDS card no. 89-7710: а = 3.629 Å and с = 5.197 Å). Because of the low intensity of the diffraction peaks of silicon carbide in the X-ray diffraction patterns of the composites, its unit-cell parameters were only determined for a powder 6H-SiC sample: а = 3.072 Å and с = 15.081 Å (JCPDS card no. 29-1131: а = 3.073 Å and с = 15.080 Å).
Figure 2 shows the relative density ρrel as a function of the volume percentage of ZrC for the ZrB2–SiC–ZrC ceramic composites and as a function of the volume percentage of ZrO2 for the ZrB2–SiC–ZrO2 composites. According to these data, the relative density of the ZrB2–SiC–ZrO2 composites increases in proportion to the increase in the volume fraction of zirconia. The ρrel of the ZrB2–5 vol % SiC ceramic containing no zirconia is 0.85 ± 0.05, whereas that of the composite containing 20 vol % ZrO2 is ρrel = 0.96 ± 0.2. Extrapolating ρrel as a function of the volume percentage of ZrO2 to a pore-free state, we find that the density of a ZrB2–SiC–ZrO2 composite prepared by sintering at a pressure of 30 MP and temperature of 1600°C for 15 min will be similar to its theoretical density at a ZrO2 content above 25 vol %. Zirconia is the lowest melting point component of the ZrB2–SiC–ZrO2 composites. Increasing the volume fraction of zirconia leads to a decrease in the melting point of ZrB2–SiC–ZrO2 mixtures (increase in the homologous temperature α = 1600°C/tm) and, as a consequence, an increase in the density of the composites.
The relative density of the ZrB2–SiC–ZrC samples exceeded that of the ZrB2–5% SiC, but only slightly. The relative density of the composites containing more than 5% ZrC was on average 0.88 ± 0.05.
Figure 3 illustrates the microstructure of the ZrB2–SiC based composites at different ZrC and ZrO2 contents. An increase in the volume fraction of ZrC or ZrO2 in the composites was accompanied by an increase in grain size. The average grain size was determined by the line intercept method using images of the microstructure of the composites [15]. In particular, the average grain size was 2.5 μm in the ZrB2–SiC–5% ZrO2 ceramic and 4.2 μm in the composite containing 15% ZrO2. In the ZrB2–SiC–ZrC composite with the lowest ZrC concentration, the average grain size was 2.57 μm; at 15% ZrC, the average grain size was 5.2 μm.
The modulus of elasticity E evaluated from the speed of ultrasound in the ZrB2–SiC ceramic was 373 ± 25 GPa. At the same time, the modulus of elasticity calculated for the ZrB2–SiC composite using the additivity rule is 458 GPa. The discrepancy between the experimentally determined and calculated moduli of elasticity is primarily due to the residual porosity in the ZrB2–SiC ceramic.
The addition of ZrC to a ZrB2–SiC ceramic matrix had no significant effect on the modulus of elasticity of the composites (Fig. 4). The modulus of elasticity of the ZrB2–SiC–ZrO2 composites was found to increase with increasing zirconia content. In particular, the composite containing 5% ZrO2 had Е ≈ 387 ± 5 GPa. Since the modulus of elasticity of ZrO2 is lower than those of ZrB2 and SiC, it is clear that the main cause of the observed increase in the modulus of elasticity of the ZrB2–SiC–ZrO2 composites is the reduction in porosity [16–18].
Table 1 presents the hardness (HV) and fracture toughness (KIc) data for the composites. According to these data, increasing the percentage of zirconium carbide in the ZrB2–SiC–ZrC composites had no significant effect on their hardness. For example, the HV of the ZrB2–SiC ceramic was 12.7 ± 0.2 GPa and that of the ZrB2–SiC–20% ZrC was 13.5 ± 0.4 GPa. On the whole, the hardness of the ZrB2–SiC–ZrC composites obtained in this study was lower than would be expected, which was due to the residual porosity.
The hardness of the ZrB2–SiC–ZrO2 composites was found to increase with increasing ZrO2 content. Zirconia has lower hardness than do the other components of the composites—silicon carbide and zirconium boride—so the observed increase in hardness is attributable to the densification of the composites with increasing ZrO2 content.
Fracture toughness KIc evaluated for the ZrB2–5% SiC ceramic from the total length of cracks emanating from a Vickers indent is 4.6 ± 0.3 MPa m1/2, which is comparable to the fracture toughness of the composites richer in silicon carbide (the KIc of ZrB2–20% SiC is ≈4–6 MPa m1/2) and exceeds the KIc of monolithic ZrB2 ceramics [19–21]. The main mechanism behind the increase in the fracture toughness of the ceramic composites containing finely dispersed inclusions is the change in crack propagation trajectory at interfaces [22, 23]. It is reasonable to assume that the high KIc of the ZrB2–5% SiC ceramic (with a low concentration of silicon carbide inclusions) is due to the residual porosity: pores hinder crack propagation.
The ZrB2–SiC–ZrC composites differing in ZrC content differed rather little in KIc. For example, the fracture toughness of the composite containing 5% zirconium carbide was 4.5 ± 0.3 MPa m1/2, and that of the composite containing 20% zirconium carbide was 4.7 ± 0.3 MPa m1/2.
Increasing the percentage of zirconia in the ZrB2–SiC–ZrO2 composites led to an increase in their KIc. For example, the fracture toughness of the composite containing 20% ZrO2 was 7.3 ± 0.4 MPa m1/2. Owing to their lower porosity, these composites had higher fracture toughness. The increased fracture toughness of the composites containing finely dispersed inclusions of tetragonal zirconia, which undergoes a martensitic transformation under the effect of a mechanical load, can be due to not only crack deflection at interfaces but also crack energy dissipation through the tetragonal-to-monoclinic phase transition of the t‑ZrO2 particles [24–26].
CONCLUSIONS
The addition of ZrC to a ZrB2–5% SiC ceramic composite prepared by pressure sintering at a temperature of 1600°C has been shown to cause no appreciable increase in the density of the material and have no significant effect on its properties. In contrast, the addition of ZrO2 helps increase the density, hardness, and fracture toughness of ceramic composites based on ZrB2–5% SiC. In particular, the KIc of the ZrB2–SiC ceramic is 4.6 ± 0.3 MPa m1/2 and that of the composite containing 20% ZrO2 is 7.3 ± 0.4 MPa m1/2.
REFERENCES
Rangaraj, L. et al., Processing of refractory metal borides, carbides and nitrides, Key Eng. Mater., 2009, vol. 395, pp. 69–88. https://doi.org/10.4028/www.scientific.net/KEM.395.69
Monteverde, F., Ultra-high temperature HfB2–SiC ceramics consolidated by hot-pressing and spark plasma sintering, J. Alloys Compd., 2007, vol. 428, nos. 1–2, pp. 197–205. https://doi.org/10.1016/j.jallcom.2006.01.107
Zimmermann, J.W., Hilmas, G.E., Fahrenholtz, W.G., Monteverde, F., and Bellosi, A., Fabrication and properties of reactively hot pressed ZrB2–SiC ceramics, J. Eur. Ceram. Soc., 2007, vol. 27, no. 7, pp. 2729–2736. https://doi.org/10.1016/j.jeurceramsoc.2006.11.074
Squire, T.H. and Marschall, J., Material property requirements for analysis and design of UHTC components in hypersonic applications, J. Eur. Ceram. Soc., 2010, vol. 30, pp. 2239–2251. https://doi.org/10.1016/j.jeurceramsoc.2010.01.026
Fahrenholtz, W.G., Hilmas, G.E., Chamberlain, A.L., Zimmermann, J.W., and Fahrenholtz, B., Processing and characterization of ZrB2 based ultra-high temperature monolithic and fibrous monolithic ceramics, J. Mater. Sci., 2004, vol. 39, pp. 5951–5957.
Melendez, J.J., Dominguez-Rodriguez, A., Monteverde, F., Melandri, C., and Portu, G., Characterisation and high temperature mechanical properties of zirconium boride-based materials, J. Eur. Ceram. Soc., 2002, vol. 22, pp. 2543–2549. https://doi.org/10.1016/S0955-2219(02)00114-0
Kalish, D., Clougherty, E.V., and Kreder, K., Strength, fracture mode and thermal stress resistance of HfB2 and ZrB2, J. Am. Ceram. Soc., 1969, vol. 52, pp. 30–36. https://doi.org/10.1111/j.1151-2916.1969.tb12655.x
Buyakov, A.S. and Kulkov, S.N., Porous ceramic composite ZrO2(MgO)–MgO for osteoimplantology, IOP Conf. Ser.: Mater. Sci. Eng., 2017, vol. 175, paper 012 025. https://doi.org/10.1088/1757-899X/175/1/012025
Fahrenholtz, W.G., Hilmas, G.E., Talmy, I.G., and Zaykoski, J.A., Refractory diborides of zirconium and hafnium, J. Am. Ceram. Soc., 2007, vol. 90, pp. 1347–1364. https://doi.org/10.1111/j.1551-2916.2007.01583.x
Guo, S.Q., Densification of ZrB2-based composites and their mechanical and physical properties: a review, J. Eur. Ceram. Soc., 2009, vol. 29, pp. 995–1011. https://doi.org/10.1016/j.jeurceramsoc.2008.11.008
Zhang, S.C., Hilmas, G.E., and Fahrenholtz, W.G., Mechanical properties of sintered ZrB2–SiC ceramics, J. Eur. Ceram. Soc., 2011, vol. 31, pp. 893–901. https://doi.org/10.1016/j.jeurceramsoc.2010.11.013
Guo, W.M., Yang, Z.G., and Zhang, G.J., Comparison of ZrB2–SiC ceramics with Yb2O3 additive prepared by hot pressing and spark plasma sintering, Int. J. Refract. Met. Hard Mater., 2011, vol. 29, pp. 452–455. https://doi.org/10.1016/j.ijrmhm.2011.02.001
Zhou, P., Hu, P., Zhang, X., and Han, W., Laminated ZrB2–SiC ceramic with improved strength and toughness, Scr. Mater., 2011, vol. 64, pp. 276–279. https://doi.org/10.1016/j.scriptamat.2010.10.005
Evans, A.G. and Charles, E.A., Fracture toughness determination by indentation, J. Am. Ceram. Soc., 1976 vol. 59, pp. 371–372. https://doi.org/10.1111/j.1151-2916.1976.tb10991.x
Saltykov, S.A., Stereometricheskaya metallografiya (Stereometric Metallography), Moscow: Metallurgiya, 1976.
Zhang, G.-J., Deng, Zh.-Y., Kondo, N., Yang, J.-F., and Ohji, T., Reactive hot pressing of ZrB2–SiC composites, J. Am. Ceram. Soc., 2000, vol. 83, no. 9, pp. 2330–2332. https://doi.org/10.1111/j.1151-2916.2000.tb01558.x
Zhu, T., Li, W., Zhang, X., Hu, P., Hong, Ch., and Weng, L., Damage tolerance and R-curve behavior of ZrB2–ZrO2 composites, Mater. Sci. Eng., A, 2009, vol. 516, pp. 297–301. https://doi.org/10.1016/j.msea.2009.03.023
Hussainova, I., Voltšihhin, N., Cura, E., and Hannula, S.-P., Densification and characterization of spark plasma sintered ZrC–ZrO2 composites, Mater. Sci. Eng., A, 2014, vol. 597, pp. 75–81. https://doi.org/10.1016/j.msea.2013.12.058
Zamora, V., Ortiz, A.L., Guiberteau, F., and Nygren, M., In situ formation of ZrB2–ZrO2 ultra-high-temperature ceramic composites from high-energy ball-milled ZrB2 powders, J. Alloys Compd., 2012, vol. 518, pp. 38–43. https://doi.org/10.1016/j.jallcom.2011.12.102
Li, W., Zhang, X., Hong, Ch., Han, W., and Han, J., Preparation, microstructure and mechanical properties of ZrB2–ZrO2 ceramics, J. Eur. Ceram. Soc., 2009, vol. 29, pp. 779–786. https://doi.org/10.1016/j.jeurceramsoc.2008.06.033
Zhang, X., Li, W., Hong, C., and Han, W., Microstructure and mechanical properties of ZrB2-based composites reinforced and toughened by zirconia, Int. J. Appl. Ceram. Tech., 2008, vol. 5, no. 5, pp. 499–504. https://doi.org/10.1111/j.1744-7402.2008.02199.x
Faber, K.T. and Evans, A.G., Crack deflection processes—1. Theory, Acta Metall., 1983, vol. 31, no. 4, pp. 565–576. https://doi.org/10.1016/0001-6160(83)90046-9
Faber, K.T. and Evans, A.G., Crack deflection processes—II. Experiment, Acta Metall., 1983, vol. 31, no. 4, pp. 577–584. https://doi.org/10.1016/0001-6160(83)90047-0
Porter, D.L., Evans, A.G., and Heuer, A.H., Transformation-toughening in partially-stabilized zirconia (PSZ), Acta Metall., 1979, vol. 27, no. 10, pp. 1649–1654. https://doi.org/10.1016/0001-6160(79)90046-4
Loganathan, A. and Gandhi, A.S., Effect of phase transformations on the fracture toughness of yttria stabilized zirconia, Mater. Sci. Eng., A, 2012, vol. 556, pp. 927–935. https://doi.org/10.1016/j.msea.2012.07.095
Mamivand, M., Zaeem, A.M., and Kadiri, E.H., Phase field modeling of stress-induced tetragonal-to-monoclinic transformation in zirconia and its effect on transformation toughening, Acta Metall., 2014, vol. 64, pp. 208–219. https://doi.org/10.1016/j.actamat.2013.10.031
Funding
This work was supported through the State Academies of Sciences Basic Research Program (2013–2020, program no. III.23.2.3) and by the National Research Tomsk Polytechnic University (project no. 223/2018: Nanomaterials and Nanotechnologies Science and Education Innovative Center, Leading Research University).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Gusev, A.Y., Voitsik, V.F., Dedova, E.S. et al. Effect of ZrC and ZrO2 Additions on the Microstructure and Properties of ZrB2–SiC Ceramics. Inorg Mater 56, 522–527 (2020). https://doi.org/10.1134/S0020168520050040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520050040