Abstract
A method for determination of aluminum, hafnium, iron, yttrium, calcium, magnesium, and titanium by inductively coupled plasma atomic emission spectrometry (ICP-AES) is described. Conditions for the decomposition of two modifications of the analyzed material—unburnt and subjected to stabilizing firing—have been studied. It has been found that the unburnt zirconia dissolves in sulfuric acid, and the burnt sample can be converted to solution only by fusing with potassium pyrosulfate or potassium bifluoride. However, the application of these reagents leads to high values of the control experiment correction for trace impurities (at the level of tenths and hundredths of a percent). In this connection, we have studied the possibility of acid dissolution of the burnt sample under microwave decomposition, varying the qualitative and quantitative composition of the acid mixture, reaction temperature, and time to reach and maintain the required temperature. It has been found that the decomposition in the mixture of hydrofluoric and sulfuric acids (2 : 1) in the microwave system with stepwise heating of the reaction mixture ensures quantitative dissolution of the burnt sample and sufficiently low values of the control experiment correction for trace impurities. The analytical lines have been chosen taking into account their relative intensity, possible spectral overlaps, and the matrix effect in the analysis of model solutions containing 1.3 mg/cm3 Zr, 0.2 mg/cm3 Y, and from 0.2 to 20 mg/cm3 impurities. As a result, the following analytical lines have been chosen: Al II 167.079 nm and Al I 308.215 nm, Ca II 184.006 nm and 393.366 nm, Fe II 238.204 nm, Mg II 279.553 nm, Ti II 334.941 nm, Y II 371.030 nm, and Hf II 232.247 nm. The developed method for the analysis of yttria-stabilized zirconia by ICP-AES allows simultaneously determining aluminum, iron, magnesium, and titanium in the range of 0.01–1.0%; calcium, 0.02–1.0%; hafnium, 0.1–5.0%; and yttrium, 2.0–15% with a relative standard deviation of 6–30 rel % (for Al, Fe, Mg, Ti, and Ca), 2–7 rel % (Hg), and 2–4 rel % (Y). The correctness of the method is confirmed by the standard addition technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Yttria-stabilized zirconia (YSZ) is used for manufacturing structural materials and tools for metal forming and as heat-protective coatings during plasma spraying in the production of parts and assemblies of gas turbine engines.
The content of yttrium oxide in stabilized zirconium dioxide (from 4 to 17%) is regulated depending on the field of application. In addition, the content of hafnium oxide (from 0.1 to 6.0%) and impurity elements—aluminum, iron, calcium, magnesium, and titanium (0.01–0.3%)—is limited (TU 342-2000. Zirconium dioxide for plasma spraying of coatings; TU 344-2000. Yttria-stabilized zirconium dioxide). Existing methods for analyzing materials based on zirconium dioxide, in particular, zirconium-containing refractories, include complexometric, photometric, and atomic absorption methods for the individual determination of oxides of aluminum (from 0.5 to 30%), iron (from 0.01 to 2.5%), yttrium (from 5.0 to 25%), calcium (from 0.1 to 35%), magnesium (from 0.2 to 10%), and titanium (from 0.05 to 3.0%). Therefore, these impurities cannot be determined simultaneously [1–6]. In addition, the sensitivity of these methods is insufficient to determine the impurities of aluminum, calcium, magnesium, and titanium at the required level of their content—from 0.01 to 0.02%. The method of atomic emission analysis is based on the excitation of a spectrum in a condensed spark or DC or AC arc and its photographic recording [7]. This technique uses no available capabilities of the multielement spectral analysis method, since it applies only to one component—hafnium oxide (from 0.1 to 3.0%). The study [8] presents the results of the development of a method for the atomic emission determination of magnesium and yttrium in materials based on zirconium dioxide using a multichannel analyzer of emission spectra. To eliminate the matrix effect of zirconium on the analytical signals of impurities, buffer mixtures consisting of graphite powder and metal oxides have been used. It has been shown that the magnesium impurity is the most reliably detected in the presence of cadmium oxide, and the yttrium impurity is the most reliably detected in the presence of lead oxide. Therefore, in this case, the techniques are single-element. Thus, the application of the above methods makes the analysis of materials based on zirconium dioxide time-consuming and does not allow one to determine trace impurities at the required level.
The purpose of this work is to develop a method for the simultaneous determination of aluminum, hafnium, iron, yttrium, calcium, magnesium, and titanium in zirconium dioxide stabilized by yttrium oxide. The quality of this material needs to be controlled for the content of several regulated elements, which determined the choice of inductively coupled plasma atomic emission spectrometry (ICP-AES) as a multielement and precision analysis method which requires no adequate standard samples.
For ICP-AES analysis, the object to be analyzed must be dissolved. The main component, i.e., zirconium dioxide, does not dissolve in most acids, except sulfuric acid, and fusion with various fluxes is mainly used for its decomposition [9]. The decomposition of zirconium dioxide is complicated by the fact that it can exist in two modifications, passing one into the other in the temperature range of 1000–1200°C. As a result, there is a transition from the monoclinic crystal lattice to the cubic lattice characterized by a higher chemical resistance. Therefore, in this work, special attention was paid to the development of a decomposition method which would ensure the complete dissolution of stabilized zirconium dioxide and the minimum value of correction of the control experiment for the determination of impurity elements. In addition, for subsequent ICP-AES analysis, it is desirable that the salt content in the sample solution be minimal, and this should be taken into account when choosing a method for transferring the analyzed sample into solution.
Two samples of zirconium dioxide stabilized by yttrium oxide were selected as the objects of this study. Sample no. 1 (unburnt) and sample no. 2 (subjected to stabilizing firing) of various modifications were used.
Sulfuric acid, hydrochloric acid (extrapure grade, Russian State Standard), and hydrofluoric acid (reagent grade, Russian State Standard), as well as potassium pyrosulfate and potassium bifluoride (pure grade, Russian State Standard), were used in this work.
The objects under study were analyzed using an iCAP 6300 spectrometer (Thermo Electron Corporation, USA) with radial plasma observation. The optimized design of the spectrometer provides simultaneous measurement of the intensity of any analytical lines in the range from 166 to 847 nm. The device is equipped with a modern semiconductor solid-state detector with high sensitivity and resolution (charge injection device, CID) and a powerful high-performance optical system (echelle optical system), which allows one to use higher spectral orders, owing to which the resolution at a wavelength of 200 nm is 7 pm while maintaining sufficient luminosity of the device. The operating parameters of the spectrometer are the following: generator power, 1200 W; argon spray flow, 0.7 L/min; plasma-forming (cooling) argon flow, 12 L/min; auxiliary argon flow, 0.5 L/min. The height of the spectrum observation is 12 mm above the upper turn of the inductor. The operation of the spectrometer is regulated by the ITEVA computer program.
The analytical lines were chosen taking into account their relative intensity and possible spectral overlaps of lines of matrix elements. In accordance with the requirements for the purity of stabilized zirconia, the content of oxides of aluminum, iron, calcium, magnesium, and titanium should not exceed 0.01–0.3%. Determination of these low contents by ICP-AES is possible only with a slight dilution of the sample, i.e., in the presence of high concentrations of matrix components. To select the analytical lines and study the matrix effect, we analyzed solutions containing 1.3 mg/cm3 of zirconium and 0.2 mg/cm3 of yttrium (which corresponds to 2 mg/cm3 of stabilized zirconium oxide), without impurity elements and in the presence of 0.2 to 20 μg/cm3 of impurities. For the preparation of model solutions, we used standard samples of the solutions of matrix and impurity elements (Russian State Standard). As a result, the following analytical lines free from spectral overlaps were chosen: Al II 167.079 nm and Al I 308.215 nm, Ca II 184.006 and 393.366 nm, Fe II 238.204 nm, Mg II 279.553 nm, and Ti II 334.941 nm. Our experiments showed that, in the presence of macrocomponents, the intensities of the analytical lines of iron, magnesium, and titanium are significantly suppressed (up to 8–15%); therefore, calibration solutions for the simultaneous determination of aluminum, iron, calcium, magnesium, and titanium were prepared against the background of 1.3 mg/cm3 zirconium and 0.2 mg/cm3 yttrium.
The determination of yttrium at the level of a percent and hafnium at the level of a few tenths of a percent in stabilized zirconium dioxide is possible when using more diluted solutions. According to the results of analysis of a zirconium solution with a concentration of 0.13 mg/cm3 and a solution containing 0.13 mg/cm3 of zirconium, 10 μg/cm3 of yttrium, and 2 μg/cm3 of hafnium, the lines Y II 371.030 nm and Hf II 232.247 nm were chosen, both free from spectral overlaps and from the matrix effect of zirconium. This fact made it possible to use clean solutions of these elements for the calibration of the spectrometer. The possibility of dissolving zirconium dioxide stabilized by yttrium oxide in sulfuric acid was previously studied. For this purpose, 0.1 g of the samples under test was placed in glassy carbon crucibles and heated with 2–3 cm3 of concentrated sulfuric acid. The experiments showed that sample no. 1 (unburnt) dissolved in sulfuric acid after 1.5–2 h of heating, while sample no. 2 (burnt) was practically undissolved after 7–8 h, even when hydrofluoric acid or bifluoride potassium was added. Therefore, subsequent experiments were performed with sample no. 2.
Fusion with potassium pyrosulfate or potassium bifluoride is usually used to decompose zirconium dioxide [9]. In the present work, these reagents were used to decompose yttria-stabilized zirconia. Fusion with potassium pyrosulfate was carried out at 900°C in crucibles made of quartz, and fusion with potassium bifluoride was performed in crucibles made of glassy carbon at 650°C or platinum at 800°C. A weighed sample (0.1 g) was placed in a crucible and mixed with 2 g of flux, then heated in a muffle furnace to the required temperature, and kept at this temperature for 5 min. After cooling, the melt was leached with 10 cm3 of hydrochloric acid diluted in a volume ratio of 1 : 1 (when fused with potassium pyrosulfate) or 5 cm3 of sulfuric acid diluted in a volume ratio of 1 : 1 (when fused with potassium bifluoride). The resulting solution was transferred into a polypropylene flask with a capacity of 50 cm3 and its volume was brought to the mark with deionized water. In the resulting initial solution, impurities of aluminum, iron, calcium, magnesium, and titanium were determined. To determine yttrium and hafnium, the initial solution was diluted 10 times with deionized water. When preparing solutions of the sample to be analyzed, solutions of the control experiment were prepared to take into account the content of the elements to be determined in the reagents: all the above operations were performed without adding the sample being analyzed. The solutions prepared were analyzed using the spectrometer under the above conditions, measuring the intensity of the selected analytical lines.
Our experiments have shown that the sample is completely dissolved because of fusion when using both potassium pyrosulfate and potassium bifluoride as a flux. However, the use of these reagents leads to high values of the control test amendment for aluminum, iron, calcium, magnesium, and titanium at the level of tenths and hundredths of a percent, which does not allow one to achieve the required minimum detectable limits of the contents of these elements. Thus, the decomposition of stabilized zirconia by fusion can be used to determine only yttrium and hafnium in it.
In order to achieve a quantitative dissolution of the analyzed sample and reduce the amendment of the control experiment for microimpurities, experiments were performed to dissolve sample no. 2 subjected to stabilizing roasting in acid under microwave decomposition conditions. For such a sample preparation, a MARS 6 laboratory microwave system (CEM Corporation, USA) was used equipped with an MTS-300 Plus temperature control sensor and an ESP-1500 Plus pressure control sensor. The samples were decomposed in sealed fluoroplastic EasyPrep Plus vessels with a capacity of 100 cm3, designed for a maximum temperature of 300°C and a maximum pressure of 54 atm.
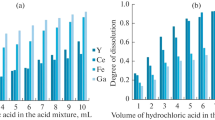
In developing the method of microwave decomposition of zirconium dioxide stabilized by yttrium oxide, the qualitative and quantitative composition of the mixture of acids for decomposition, temperature of the reaction, and time to achieve and maintain the desired temperature were varied. The weighed sample to be analyzed (0.1 g) was placed on the bottom of the EasyPrep Plus vessel and dissolved under microwave heating conditions or by decomposition in several stages, successively heating first in the presence of hydrofluoric acid, then when adding sulfuric acid, and at the last stage in the presence of hydrochloric acid or directly in a mixture of concentrated hydrofluoric and sulfuric acids.
The best results were achieved when using 3 cm3 of a mixture of hydrofluoric and sulfuric acids (2 : 1) with stepwise heating of the reaction mixture according to the temperature decomposition program shown in Table 1.
Under these conditions, the analyzed sample decomposes to form a precipitate of sparingly soluble aluminum, yttrium, calcium, and magnesium fluorides. At the end of the decomposition, the vessels were cooled to room temperature; the solutions with the precipitates were quantitatively transferred to crucibles made of glass carbon with 50 cm3 of hydrochloric acid (1 : 99) and 20 cm3 of deionized water. The contents of the crucible were heated until complete removal of hydrofluoric acid vapor and the appearance of sulfuric acid vapor, since the presence of fluoride ions is undesirable during subsequent spraying of the analyzed solutions into the plasma of the spectrometer. To convert sparingly soluble aluminum, yttrium, calcium, and magnesium fluorides, 2 cm3 of concentrated hydrochloric acid was added to soluble chlorides in crucibles after cooling, and the contents were heated to dissolve the precipitates. The solutions were transferred to polypropylene flasks with a capacity of 50 cm3; the volume of the solution was brought to the mark with deionized water and diluted 10 times with hydrochloric acid (1 : 19). Simultaneously, control experiments were carried out to take into account the content of the determined elements in the reagents.
The undiluted (initial) solutions were analyzed for aluminum, iron, calcium, magnesium, and titanium contents using solutions containing 2.10 and 20 μg/cm3 of each element to be determined in the presence of 1.3 mg/cm3 of zirconium and 0.2 mg/cm3 of yttrium to calibrate the spectrometer. A solution containing 1.3 mg/cm3 of zirconium and 0.2 mg/cm3 of yttrium without impurity elements was used as the “zero” solution. Yttrium and hafnium were determined in dilute solutions, using for calibration, respectively, pure solutions of yttrium with a concentration of 10, 20, and 30 μg/cm3 and pure solutions of hafnium with a concentration of 1.5 and 10 μg/cm3, and hydrochloric acid diluted in a volume ratio of 1 : 19 as the “zero solution.”
The experiments carried out have shown that the treatment of burnt yttria-stabilized zirconia with a mixture of acids using microwave heating allows the sample to be completely transferred into solution. At the same time, the values of the amendment of the control experiment were significantly decreased to thousandths of a percent, which made it possible to achieve the required lower limits of the specific content of trace impurities: 0.01 wt % for aluminum, iron, magnesium, and titanium and 0.02 wt % for calcium. The lower limits of the determined contents were calculated by the 6S criterion, where S is the standard deviation of the amendment of the control experiment.
On the basis of the research conducted, a technique of atomic emission analysis with inductively coupled plasma of zirconium dioxide stabilized by yttrium oxide was developed which allows one to determine simultaneously aluminum, iron, magnesium, and titanium in the range of contents of 0.01–1.0%, calcium at the level of 0.02–1.0%, hafnium at 0.1–5.0%, and yttrium at 2.0–15%. The ranges of the determined contents of hafnium and yttrium correspond to the requirements of the specifications for stabilized zirconium dioxide. According to the method developed, samples of unburnt and burnt yttria-stabilized zirconia were analyzed. The results of the analysis are given in Table 2.
The correctness of the results obtained was tested by the standard addition method. The results of the analysis of samples with additives are presented in Table 3.
The significance of the assessment of the systematic error of the analytical method was tested by the t‑criterion according to RMG 61-2010 [10]. It was determined that, for all the elements being determined, the estimates of the mathematical expectation of the systematic error θ are insignificant against the background of random variation, since tcalc < ttable, which indicates the correctness of the analysis technique.
The relative standard deviation in the determination of aluminum, iron, magnesium, titanium, and calcium microimpurities is 6–30 rel %; for hafnium, 2–8% rel %; and for yttrium, 2–4 rel %.
Thus, as a result of the research performed, the problem of decomposing chemically stable yttria-stabilized zirconia regardless of its modification has been solved. The proposed decomposition method ensures the complete transfer of the sample into solution and the minimum value of the amendment of the control experiment in determining impurity elements. Subsequent analysis of the sample solution by the ICP-AES method allows simultaneous determination of macrocomponents and microcomponents in the analyzed material, which significantly reduces the time to perform the analysis and the consumption of reagents.
REFERENCES
GOST (State Standard) 13997.7-84: Zirconium Containing Refractory Materials and Products. Methods for Determination of Aluminum Oxide, Moscow: Izd. Standartov, 2004.
GOST (State Standard) 13997.5-84: Zirconium Containing Refractory Materials and Products. Methods for Determination of Iron Oxide, Moscow: Izd. Standartov, 2004.
GOST (State Standard) 13997.10-84: Zirconium Containing Refractory Materials and Products. Methods for Determination of Yttrium Oxide, Moscow: Izd. Standartov, 2004.
GOST (State Standard) 13997.8-84: Refractory Containing Refractory Materials and Products. Methods for Determination of Calcium Oxide, Moscow: Izd. Standartov, 2004.
GOST (State Standard) 13997.9-84: Zirconium Containing Refractory Materials and Products. Methods for Determination of Magnesium Oxide, Moscow: Izd. Standartov, 2004.
GOST (State Standard) 13997.6-84: Zirconium Containing Refractory Materials and Products. Methods for Determination of Titanium Dioxide, Moscow: Izd. Standartov, 2004.
GOST(State Standard)13997-68: Zirconium Dioxide (Technical), Zirconium Concentrate and Zirconium Fireproof Materials, Methods of Analysis, Moscow: Izd. standartov, 1975.
Otmakhov, V.I., Adamova, E.P., and Kul’kov, S.N., Atomic emission analysis of zirconium nanoceramics, Ogneupory Tekh. Keram., 2006, no. 3, pp. 9–13.
Bock, R., A Handbook of Decomposition Methods in Analytical Chemistry, London: Wiley, 1979.
RMG 61-2010: Gosudarstvennaya sistema obespecheniya edinstva izmerenii. Pokazateli tochnosti, pravil’nosti, pretsizionnosti metodik kolichestvannogo khimicheskogo analiza. Metody otsenki (RMG 61-2010: State System for Ensuring the Uniformity of Measurements. Accuracy, Trueness and Precision Measures of the Procedures for Quantitative Chemical Analysis. Methods of Evaluation), Moscow: Izd. Standartov, 2012.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Glinskaya, I.V., Teselkina, A.E., Alekseeva, T.Y. et al. Analysis of Yttria-Stabilized Zirconia by Inductively Coupled Plasma Atomic Emission Spectrometry. Inorg Mater 55, 1359–1364 (2019). https://doi.org/10.1134/S0020168519140048
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519140048