Abstract—
We have studied the phase composition and structure of titanium carbide with a nickel binder prepared by self-propagating high-temperature synthesis in a cocurrent inert or reactive gas stream using granulated mixtures containing different grades of titanium. The results demonstrate that, unlike in the case of powder mixtures with a loose bulk density, the products of combustion of a granulated Ti + C + 25% Ni mixture in flowing nitrogen or without it retain their structure and granule size and can readily be ground into powder. In the case of the powder mixture both in a flowing gas and without it and in the case of the granulated mixture in flowing argon, the combustion products have the form of unbreakable sinter cakes, independent of the grade of titanium. Microstructural analysis of the combustion products points to spontaneous dispersion of the titanium particles surrounded by the nickel binder, independent of the starting mixture (granules or powder with a loose bulk density). Moreover, the phase composition of the synthesis products depends on the size and morphology of the titanium particles. In the case of PTM titanium, the final synthesis product consists of titanium carbide and nickel phases. After the combustion of mixtures based on PTM-1 titanium powder or a 50% PTM + 50% PTM-1 mixture, the final product consists of TiC, Ni, and TixNiy intermetallic phases. Synthesis in flowing nitrogen has been shown to change the phase composition of the combustion products of the mixtures based on PTM-1 titanium powder and a 50% PTM + 50% PTM-1 mixture, causing the intermetallic phases to disappear. To account for the combustion behavior of the mixtures, we have proposed a two-step mechanism of interaction in the Ti + C + 25% Ni system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Owing to their high hardness and good wear resistance, titanium carbide-based materials have a wide range of applications: from abrasives and protective coatings to structural tribological alloys. To improve their plasticity, a metallic binder, for example, nickel, is commonly added to the starting mixture [1, 2]. At present, the main method for producing materials from starting powders is sintering [1, 3]. One possible alternative to sintering is self-propagating high-temperature synthesis (SHS) [4], a process in which heat necessary for obtaining final products results from reaction between starting reagents. An inherent feature of SHS is the melting of the lowest melting point components during the combustion process, which ensures melt spreading and self-dispersion of the starting reagents. At the same time, if a metallic binder is added to the reaction system, synthesis yields high-strength sinters, which are very difficult to grind, only with considerable energy consumption.
As shown in a study of the combustion behavior of granulated titanium carbide/carbon black mixtures [5], the granules retain their size after synthesis and do not sinter.
In this work, a granulated starting mixture was used instead of a powder mixture to synthesize titanium carbide with a metallic binder with the aim of obtaining not a high-strength sinter cake but granules of the order of 1 mm in size. We expected that grinding such granules into fine powder would require less energy.
EXPERIMENTAL
The starting chemicals used in this study and some of their characteristics are presented in Table 1.
Granules were produced as follows: A starting mixture of titanium and nickel powders and carbon black was mixed for 4 h in a tumble drum. Next, to the resultant mixture was added a 4% polyvinyl butyral solution in ethanol. The paste obtained by further mixing was rubbed through a sieve with a nominal aperture size of 1.25 mm. To impart a spherical shape to the resultant particles, they were rolled on a rotating horizontal surface. Next, the particles were dried in air for 10 h and classified into size ranges on a vibrating sieve. In this study, we used granules 0.63 to 1.6 mm in size.
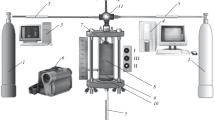
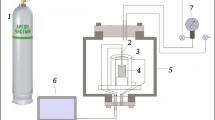
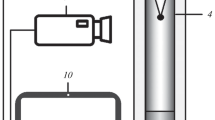
The combustion behavior of the granules was studied using an unconventional experimental setup. It allows for synthesis in a gas flow and without it, gas flow rate and pressure measurements during the combustion process, and video recording of the combustion process (Fig. 1). Frame analysis of videos makes it possible to assess the combustion front speed. All of the experimental data presented below were obtained in a cocurrent gas stream at a 1 atm pressure difference between the upper and lower levels of the granules (the gage pressure at the upper level was 1 atm). To preclude shrinkage of the unburned granules during the combustion process and obtain stable results, the sample was purged with argon at the same pressure difference before each experiment.
Schematic of the experimental setup: (1) nitrogen cylinder, (2) argon cylinder, (3) computer for video signal recording, (4) computer for sensor signal recording through an ADC, (5) flow rate and pressure sensors, (6) digital video camera, (7) electrical coil, (8) charge, (9) mineral wool layer, (10) metallic grid, (11) gas switch (position I, nitrogen; II, argon; III, off).
We studied compositions containing 25 wt % nickel. According to Dunmead et al. [6], such compositions ensure strong adhesion of titanium carbide with articles in the case of gas-plasma spraying.
RESULTS AND DISCUSSION
Our results showed that the combustion of a granulated Ti + C + 25% Ni SHS mixture containing PTM titanium involved two steps. First, a combustion front no longer than 3 s traveled through the charge. It was followed by bright glow of the combustion products (glow buildup) for 6–8 s (Fig. 2).
Such an effect was observed for the first time during the combustion of a granulated mixture. Previously studied granulated Ti + xC (0.5 ≤ x ≤ 1) compositions showed no afterglow. Afterglow was observed during the combustion of pressed Ti + C + 20% Ni samples [7]. To verify the existence of this effect, we carried out experiments in which Ti + C + 25% Ni powder mixtures with a loose bulk density were burned. These mixtures also exhibited an afterglow effect. Such combustion behavior can be accounted for by the fact that, having a lower melting point (1726 K) than does titanium (1933 K), nickel melts in the combustion front and spreads over titanium particles, preventing them from reacting with carbon black. As shown earlier [8], a nickel layer on the surface of titanium particles has a hindering effect on the combustion of a Ti + C + 20% Ni mixture. However, because of the small volume fraction of nickel in the mixture under consideration (≤8 vol %), only some of the titanium particles are coated with molten nickel. The other titanium particles melt in the combustion wave and spread over carbon black, reacting with it to form titanium carbide. It is this reaction that prevailed in the combustion front, because no combustion wave is formed in a Ti + Ni mixture without preheating [9]. Behind the combustion front, carbon forces out nickel from its melt with titanium to form titanium carbide, which is accompanied by an additional heat release, producing an afterglow effect, which is visually perceived as glow buildup. According to X-ray diffraction data, the final products obtained from both granulated and powder mixtures contain titanium carbide and nickel carbide phases (Fig. 3), in agreement with thermodynamic calculation results.
Experimental data indicate that, during the combustion of granulated Ti + C + 25% Ni mixtures, the granules retain their size and do not sinter. At the same time, the combustion products of Ti + C + 25% Ni powder mixtures have the form of a high-strength sinter cake. This result is an argument in favor of the use of granulated mixtures, instead of powder mixtures, in the preparation of ceramic materials with a metallic binder, because this can considerably reduce energy consumption, the time needed for further grinding of the synthesis products, and contamination of the final product with the material of the grinding media.
The microstructure of the condensed products (granules) obtained was examined by scanning electron microscopy (SEM) on a Carl Zeiss Ultra Plus microscope. Figure 4 shows a micrograph of as-synthesized Ti + C + 25% Ni granules. It is seen that the average grain size of the titanium carbide is 2–4 μm, which is an order of magnitude smaller than the initial size of the titanium particles (70 μm); that is, the combustion process reduced the particle size of the titanium, and the nickel binder prevented titanium carbide grain growth after the synthesis. Attention should be paid to the fact that, despite of its small volume fraction in the mixture, nickel is evenly distributed over the granule after the synthesis.
A cocurrent gas stream is known to be an effective control factor for the combustion of SHS mixtures [10, 11], so a natural question is what effect a gas flow will have on combustion characteristics and the phase composition and structure of the synthesis products.
Video recording of the combustion of a granulated Ti + C + 25% Ni mixture in a cocurrent inert gas (argon) or reactive gas (nitrogen) stream showed that the afterglow effect due to the presence of nickel persisted. The combustion front speed in the gas stream increased relative to combustion with no flowing gas from 27 to 36 mm/s for argon and to 47 mm/s for nitrogen. (The uncertainty in our combustion front speed measurements was 10%.)
The combustion of a granulated Ti + C + 25% Ni mixture in flowing argon was accompanied by 30% shrinkage of the material. The combustion product had the form of a high-strength sinter cake, which could not be separated into individual granules or ground. Figure 5a shows a photograph of a transverse section of an as-synthesized sample.
According to filtration combustion theory, this effect of an inert gas on characteristics of the combustion process and properties of the combustion products is due to the increased temperature in the combustion wave [5]. The increase in combustion temperature in turn leads to an increase in the fraction of liquid phase in the synthesis products, an increase in their flowability, and, as a consequence, consolidation of the granules under the effect of the pressure difference.
After the combustion of the granulated mixture in flowing nitrogen, the shrinkage of the sample was smaller, 15–20%, and the combustion speed was higher than in flowing argon. The combustion product had the form of a porous, low-strength material consisting of poorly sintered granules, which broke into small pieces when cut, so we failed to prepare a transverse section suitable for photographing. Figure 5b shows a photograph of a fracture surface of the burned sample.
According to thermodynamic calculations with the THERMO program [4], the only reaction products after the combustion of a Ti + C + 25% Ni mixture should be stoichiometric titanium carbide and nickel; that is, nitrogen should act as an inert gas. Therefore, like in flowing argon, a high-strength sinter cake should be obtained. However, the present experimental data lead us to conclude that the behavior of nitrogen differs from that of an inert gas. This finding is consistent with conclusions made in previous reports [5, 10], according to which flowing nitrogen ignites the surface of granules, producing a refractory titanium nitride crust on their surface. The crust prevents tight sintering of granules under the effect of the pressure difference and high temperature, despite the presence of a nickel binder.
Thus, we obtained further evidence that the combustion of granulated titanium-based mixtures in a cocurrent nitrogen stream follows a nonequilibrium mechanism: the sequence of chemical reactions in the combustion wave is determined by the kinetics of reactions of titanium with nitrogen and carbon [12].
Note that, independent of experimental conditions (in a flowing gas or without it), the combustion products of a Ti + C + 25% Ni powder mixture have the form of a sinter cake, which possesses plasticity and cannot be ground under laboratory conditions.
According to the chemical analysis data, the combustion products of the Ti + C + 25% Ni mixtures in flowing nitrogen contain 2.5–3 wt % nitrogen. At the same time, according to the X-ray diffraction results the synthesis products obtained by reacting a Ti + C + 25% Ni mixture in flowing nitrogen and without it consist of titanium carbide (major phase) and nickel (Fig. 3). One possible cause of this seeming contradiction is the presence of a TiCxN1 – x phase: the peaks in its X-ray diffraction pattern are close in angular position to those of titanium carbide [13]. Because of the presence of the TiCxN1 – x phase, the peaks of the major phase in the X-ray diffraction pattern of the reaction products (Fig. 3) obtained via combustion in flowing nitrogen are shifted to larger angles and broadened. We failed to determine the phase composition of the synthesis products obtained by reacting a Ti + C + 25% Ni mixture in flowing argon because the material could not be ground.
Thus, using combustion of a granulated PTM titanium-based Ti + C + 25% Ni mixture in a cocurrent nitrogen stream, we obtained nitrogen-doped titanium carbide with a nickel binder.
Studies of SHS processes for many years have shown that the grade of starting reagents can have a significant effect on the combustion process and the phase composition of the combustion products. Because of this, we studied general aspects of synthesis and the composition of combustion products obtained by reacting a Ti + C + 25% Ni mixture using another grade of titanium (PTM-1). The chemical compositions of titanium powders of these grades are essentially identical, so attention was paid to differences in the size and shape of the titanium particles. Figure 6 shows micrographs of different grades of titanium powders. It is seen that the particles of the different grades of titanium powders differ in shape and size. The PTM-1 titanium particles are about a factor of 1.5 larger (Table 1) and have a flat shape and a smaller specific surface area. The specific surface area of the PTM-1 titanium powder was 0.35 m2/g (as determined using a Sorbi-M instrument) and that of the PTM titanium powder was 0.59 m2/g. Particle size distributions were determined using a MicroSizer-201 analyzer.
First, it was checked whether the grade of titanium powder influenced the combustion front speed and the phase composition of combustion products of a stoichiometric Ti + C mixture. Our experiments showed that the combustion speed of the powder mixture based on PTM-1 titanium powder dropped by a factor of 2 in comparison with the mixture based on PTM titanium powder, to 8 mm/s. The combustion speed of the granulated mixture also dropped, from 75 to 22 mm/s. At the same time, according to the X-ray diffraction results the phase composition of the combustion products obtained using both the granulated and powder mixtures based on PTM-1 titanium included only stoichiometric titanium carbide, as in the case of PTM titanium.
The addition of 25% nickel to a Ti + C mixture containing PTM-1 titanium powder was found to change the combustion behavior of the mixture and the composition of the reaction products in comparison with the mixture based on PTM titanium powder. The combustion of the granulated mixture was not accompanied by afterglow, and the combustion speed dropped from 27 mm/s in the case of the mixture containing PTM titanium powder to 11 mm/s. According to the X-ray diffraction data, the combustion products had a more complex composition and included TixNiy intermetallic phases. Figure 7 presents X-ray diffraction results for the combustion products of the granulated Ti + C + 25% Ni mixtures based on the two grades of titanium. The Ti + C + 25% Ni granules based on PTM-1 titanium powder retain their size after synthesis and do not sinter.
To verify whether replacing some of the PTM-1 titanium powder can lead to a reduction in the percentage of intermetallic phases in combustion products, we carried out experiments in which we burned Ti + C + 25% Ni granulated and powder mixtures in which 50% of the PTM-1 titanium powder was replaced by PTM titanium powder. The results showed that the combustion of this mixture was accompanied by afterglow. The combustion speed of such a granulated mixture (PTM/PTM-1 titanium), 20 mm/s, was intermediate between the combustion rates of the mixtures containing individual grades of titanium powders (27 mm/s for the PTM-containing mixture and 11 mm/s for the PTM-1-containing mixture). X-ray diffraction data for the combustion products indicated the presence of intermetallic phases, but the amount of these was smaller than in the case of the mixture based on PTM-1 titanium powder.
Next, we examined the influence of inert and reactive gas streams on the combustion behavior and phase composition of synthesis products in the case of mixtures based on PTM-1 titanium powder and a 1 : 1 mixture of PTM and PTM-1 powders. The combustion front speed of the granulated mixture in a gas stream (at a pressure difference of 1 atm) increased relative to combustion without flowing gas: in flowing argon, from 11 to 23 mm/s for the mixture containing PTM-1 powder and from 20 to 30 mm/s for the PTM + PTM-1 powder mixture; in flowing nitrogen, from 11 to 30 mm/s for the mixture containing PTM-1 powder and from 20 to 42 mm/s for the PTM + PTM-1 powder mixture.
The combustion of the granules based on PTM-1 powder and a PTM + PTM-1 powder mixture was observed to be accompanied by afterglow and the reaction products had a small shrinkage. The synthesis products had the form of a porous, low-strength material consisting of poorly sintered granules, which broke into small pieces when cut. Of special note is that, by purging the system with nitrogen, we were able to change the composition of the combustion products obtained using charges based on PTM-1 powder and a PTM + PTM-1 powder mixture, which contained no intermetallic phases according to X-ray diffraction data (Fig. 8). Thus, the present results demonstrate that the granulation of a mixture, followed by burning in flowing nitrogen, makes it possible to level out the effect of the grade of titanium on the phase composition of the final synthesis products.
The products of the combustion of the charges based on PTM-1 powder and a PTM + PTM-1 powder mixture in flowing argon had the form of a high-strength sinter cake with a noticeable shrinkage. The cake could not be ground (so no X-ray diffraction data were obtained for the materials synthesized in flowing argon).
Table 2 summarizes our results on the effect of gas flow on the combustion wave front propagation speed in the granulated mixtures based on different grades of titanium and the phase composition of the synthesis products.
CONCLUSIONS
We have studied the phase composition and structure of titanium carbide with a nickel binder prepared by SHS using a granulated Ti + C + 25% Ni mixture. The results demonstrate that, unlike in the case of powder mixtures, during the combustion of the granulated Ti + C + 25% Ni mixture in flowing nitrogen or without it the granules retain their size, without sintering to each other, and can be ground into powder. SEM results for the combustion products of such mixtures indicate that the synthesis process is accompanied by so-called spontaneous dispersion of the titanium particles and that the nickel binder prevents titanium carbide grain growth.
The phase composition of the combustion products has been shown to depend on the grade of titanium powder. If PTM titanium is used, the final synthesis product consists of titanium carbide and nickel. The final product of the combustion of Ti + C + 25% Ni mixtures based on PTM-1 titanium powder or a 50% PTM + 50% PTM-1 mixture consists of TiC, Ni, and a TixNiy intermetallic phase. Synthesis in flowing nitrogen changes the phase composition of the combustion products of the mixtures based on PTM-1 titanium powder and a 50% PTM + 50% PTM-1 mixture, causing the intermetallic phase to disappear.
In studying the combustion of Ti + C + 25% Ni granulated and powder mixtures with a loose bulk density, we observed for the first time two distinct steps of the synthesis process (except in the case of the mixture based on PTM-1 titanium powder): combustion front propagation was followed by afterglow.
REFERENCES
Kiparisov, S.S., Levinskii, Yu.V., and Petrov, A.P., Karbid titana: poluchenie, svoistva, primenenie (Titanium Carbide: Preparation, Properties, and Application), Moscow: Metallurgiya, 1987.
Zhang, X.-H., Han, J.-C., He, X.-D., and Kvanin, V.L., Combustion synthesis and thermal stress analysis of TiC–Ni functionally graded materials, J. Mater. Synth. Process., 2000, vol. 8, no. 1, pp. 29–34. https://doi.org/10.1023/A:1009469610918
Khimicheskaya tekhnologiya keramiki. Uchebnoe posobie dlya vuzov (Chemical Technology of Ceramics: A Learning Guide for Higher Education Institutions), Guzman, I.Ya.,Ed., Moscow: Stroimaterialy, 2003.
Merzhanov, A.G. and Mukas’yan, A.S., Tverdoplamennoe gorenie (Solid Flame Combustion), Moscow: Torus, 2007.
Seplyarskii, B.S., Kochetkov, R.A., and Vadchenko, S.G., Burning of the Ti + xC (1 > x > 0.5) powder and granulated mixtures, Combustion, Explosion Shock Waves, 2016, vol. 52, no. 6, pp. 665–672. https://doi.org/10.1134/S001050821606006X
Dunmead, S.D., Readey, D.W., Semler, C.E., and Hol, J.B., Kinetics of combustion synthesis in the Ti–C and Ti–C–Ni systems, J. Am. Ceram. Soc., 1989, vol. 72, no. 12, pp. 2318–2324. https://doi.org/10.1111/j.11512916.1989.tb06083.x
Rogachev, A.S., Shkiro, V.M., Chausskaya, I.D., and Shvetsov, M.V., Gasless combustion in the system titanium–carbon–nickel, Combustion, Explosion Shock Waves, 1988, vol. 24, no. 6, pp. 720–726. https://doi.org/10.1007/BF00740417
Kochetov, N.A., Rogachev, A.S., and Pogozhev, Yu.S., The effect of mechanical activation of a reaction mixture on the velocity of the wave propagation of SHS reactions and microstructure of the TiC–Ni hard alloy, Russ. J. Non-Ferrous Met., 2010, vol. 51, no. 2, pp. 177–181.
Seplyarskii, B.S., The nature of the anomalous dependence of the velocity of combustion of “gasless” systems on the sample diameter, Dokl. Phys. Chem., 2004, vol. 396, nos. 4–6, pp. 130–133. https://doi.org/10.1023/B:DOPC.0000033505.34075.0a
Seplyarskii, B.S., Tarasov, A.G., and Kochetkov, R.A., Experimental study of the combustion of a “gasless” granulated Ti + 0.5C composition in cocurrent streams of argon and nitrogen, Fiz. Goreniya Vzryva, 2013, no. 5, pp. 55–63.
Itin, V.I., Monasevich, T.V., and Bratchikov, A.D., Effect of mechanical activation on general relationships of self-propagating high-temperature synthesis in the titanium–nickel system, Fiz. Goreniya Vzryva, 1997, no. 5, pp. 48–51.
Seplyarskii, B.S. and Kochetkov, R.A., Combustion behavior of Ti + xC (x > 0.5) powders and granules in a cocurrent gas stream, Khim. Fiz., 2017, vol. 36, no. 9, pp. 21–31.
Seplyarskii, B.S., Tarasov, A.G., Kochetkov, R.A., and Kovalev, I.D., Combustion behavior of Ti + TiC mixtures in a cocurrent nitrogen stream, Fiz. Goreniya Vzryva, 2014, no. 3, pp. 61–67.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seplyarskii, B.S., Kochetkov, R.A., Lisina, T.G. et al. Phase Composition and Structure of Titanium Carbide/Nickel Binder Synthesis Products. Inorg Mater 55, 1104–1110 (2019). https://doi.org/10.1134/S0020168519110116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168519110116