Abstract
The effect of radiolysis and postradiation acid hydrolysis on the composition of the degradation products of tributyl phosphate (TBP, 30 wt %) in acidified Izopar-M has been studied. It has been found that the simultaneous presence of nitric acid and isoparaffins in the irradiated solution has a protective effect on TBP. The main radiolytic products are due to the nitration, nitroxylation, and alkylation of TBP. Upon the postradiation thermolysis, nitro derivatives and nitrates decomposed with partial regeneration of TBP. Heating above 110°C also provoked the dealkylation of radiolytic products with the formation of volatile compounds, which increased the fire and explosion hazard of using irradiated extraction mixtures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Radiation-chemical studies occupy an important place in the choice of extraction systems for the industrial separation of radioisotopes contained in spent nuclear fuel [1, 2]. Not only the radiation resistance of an extractant and a diluent. but also the thermal, chemical, and radiolytic stability of accumulated products is important [3]. Tri-n-butyl phosphate (TBP) is the main extractant in the PUREX, UREX, and COEX processes. Its degradation is caused by a combination of radiolysis, acid hydrolysis, and thermolysis processes [4]. Therefore, a wide set of data on radiation-chemical and postradiation transformations of TBP depending on the sequence and conditions of contact with nitric acid is needed to determine the extraction efficiency, the separation factors of radioisotopes, and the longevity of TBP recycling in extraction systems. Studies related to improving the fire and explosion safety of the extraction process [5] and the hydrodynamic and extraction performance of TBP solutions [6] are of particular importance.
Isopar-M, a mixture of isoparaffins with a boiling range of 208–257°C, can serve as a promising hydrocarbon solvent. This is a commercially available diluent, which has an increased dissolving power with respect to the solvates of tetravalent actinides with TBP [3]. In this present work, we studied the influence of the absorbed dose of ionizing radiation and the thermal-oxidative conditions of postradiation storage on the mechanism of transformations and the composition of an extraction solution of 30 vol % TBP in Isopar-M saturated with 3.4–4.0 M HNO3.
EXPERIMENTAL
Reagents and Irradiation
Tri-n-butyl phosphate (99%) from ACROS was purified by washing with an aqueous solution of potassium permanganate (10 g/dm3) and sodium hydroxide (15 g/dm3) to remove oxidizable synthesis products (the contact time was 30 min, and a volume ratio between organic and aqueous phases was 2 : 1). The resulting MnO2 precipitate was removed by washing with an aqueous solution of H2C2O4 (15 g/dm3) and 0.3 M HNO3 (the washing time was 1 h, and the organic and aqueous phases were in a volume ratio of 2 : 1). Next, the product was washed twice (for 30 min) with an equal volume of an aqueous solution of NaOH (15 g/dm3) and then with distilled water to a neutral reaction. The purified TBP was dissolved in Isopar-M (ExxonMobil) in a volume ratio of 3 : 7. Then, the samples were saturated with nitric acid (an aqueous 3.4 or 4.0 M HNO3 solution). Three repeated saturations were carried out for 20 min at equal volumes of the organic and aqueous phases. Irradiation was carried out at 20 ± 3°C in a cylindrical glass vessel with a water seal filled with an aqueous 4 M HNO3 solution.
An UELV-10-10-S-70 linear accelerator (electron energy, 8 MeV; pulse duration, 6 μs; pulse repetition rate, 300 Hz; and average beam current, 700 μA) or an RKhM-gamma-20 γ-radiation source (NPO RADON) served as irradiators. An electron beam was scanned along the vertical axis of the vessel at a frequency of 1 Hz. Intermittent irradiation was used: an interval of irradiation to a dose of 4.7 ± 0.1 kGy (average dose rate, 0.22 kGy/s) was alternated with an interval of sample cooling for 10 min. The dose rate of the γ-radiation source was 9.4 ± 0.4 kGy/h. A SO PD(F)R-5/50(GSO 7865-2000) copolymer (GSO 7865-2000) was used for dosimetry.
Hydrolysis
The irradiated samples were either subjected to high-temperature acid hydrolysis (at 90–170°C, in contact with 8 or 12 M HNO3) or stored for three years in the dark at +6°C in test tubes with ground stoppers. Thermolysis was carried out for 6 h in a 300-mL autoclave with temperature control. Precipitation, turbidity, or phase separation was not observed in the samples during postradiation storage. A HANNA HI 98127 PHEP 4 analyzer was used to control the pH of the aged solutions.
Analysis
The analysis of the group composition of products was carried out on an IR Prestige-21 spectrometer (Shimadzu) in the single-beam mode. The spectra were recorded using CaF2 glasses and a cuvette with a lead gasket. The measurements were carried out using calibration curves: –NO2 at 1556 cm−1 (2-nitrooctane), –ОNO2 at 1639 cm−1 (1-octyl nitrate), ‒СООН at 1730 cm−1 (myristic acid), –СО at 1721 cm−1 (4-methyl-2-pentanone), and –СООR at 1740 cm−1 (hexyl butyrate).
The component composition of the samples was analyzed using a Thermo Scientific Trace 1310 gas chromatograph with an ISQ 8000 mass spectrometric detector (electron impact ionization, 70 eV) and a Thermo column (TG-5MS, 15 m × 0.25 mm) with a polydiphenylsiloxane to polydimethylsiloxane ratio of 5 : 95. The injector temperature was 275°С: the column oven was heated from 50 to 320°С, and the sample volume was 0.5 μL. The samples were preliminarily neutralized with sodium bicarbonate. Products were identified based on mass spectra and retention indices using the NIST MS Search 2.3 (2017) and ThermoXcalibur 2.2 software. Table 1 summarizes the designations and structures of detected phosphorus-containing products, where Bu, R1, R2, and R3 refer to butyl and heavier alkyl groups, respectively.
RESULTS AND DISCUSSION
The radiolysis of a fresh extraction solution led to a change in the component composition (Fig. 1) and a slight increase in density (from 0.849 to 0.862 kg/dm3 at 2 MGy). Organic nitro derivatives were dominant products upon both electron-beam treatment (Fig. 1a) and γ-radiolysis (Fig. 1b). Moreover, their concentrations changed only slightly in postradiation processes (Fig. 1c). At doses to 0.5 MGy, the radiation-chemical yields of buildup of nitro derivatives (‒NO2) and nitrates (–NO3) were maximal (to 0.3 µmol/J). Higher doses decreased these yields (to 0.06–0.09 and 0.01 µmol/J for nitration and nitroxylation products, respectively). Carboxylic acids and carbonyl compounds also appeared. An increase in their concentrations was most significant (to 0.03–0.05 µmol/J) at doses above 0.5 MGy. Esters appeared in trace amounts. The concentration of carboxyl and carbonyl compounds continued to increase in secondary postradiation processes.
Long-term storage also affected the composition of samples, primarily the nitrate content (Fig. 1c). The postradiation accumulation of nitrates was facilitated by a high residual concentration of nitric acid and, accordingly, a low value of pH (Fig. 2). Nitrates predominantly appeared in irradiated solutions, and they were hardly detectable in unirradiated solutions. A low concentration of nitrates was also observed in samples irradiated at 1 MGy and subjected to subsequent thermolysis at >110°C (Table 2). A decreased concentration of nitro derivatives was observed in these samples. The key reason was the low thermal stability of organic nitrates and nitro derivatives formed by radiolysis, although these compounds are relatively stable at room and lower temperatures.
In particular, HNO3 was efficiently consumed in fast diffusion-controlled reactions with free electrons generated by the ionization of all components of the solution [7]. In this case, electrons were captured by both H+ and nitrate ions. In the reaction of an electron with H+, a less reactive ⋅H radical was formed. In turn, the reaction of an electron with nitrate gave ⋅NO2:


The consumption of H+ in the reaction with an electron and OH− led to an increase in pH and, accordingly, to weakening of postradiation acid hydrolysis. Electrons are the strongest reducing agents. The effective capture of electrons in the primary radiolytic processes with acid decreased the probability of their further participation in the degradation of TBP. However, nitrate was also consumed in the reactions with the radical cations (⋅R+) of TBP and Isopar [7]

Naturally, reactions (1)–(3) become less significant with an increase in the absorbed dose, that is, with a decrease in the concentration of the nitrate ion.
The ⋅Н radical reduces nitrate much more slowly than the electron [8]. However, it also makes a significant contribution to the conversion of nitrate into ⋅NO2 [9], thereby contributing to the subsequent dominance of nitration over nitroxylation. But most of the ⋅H radicals decayed in the reactions of hydrogen abstraction from the alkyl groups of TBP and Isopar components. The formation of a wide range of bulkier alkyl-type free radicals was a consequence of H abstraction. The mobility of ⋅NO2 and О–NO2 ensured their active participation in recombination with the alkyl-type radicals. In the case of the localization of radical centers on the butyl groups of TBP, the recombination products were Р3, Р4, Р6, and Р7. Accordingly, the light radicals ⋅NO2 and О–NO2 decreased the probability of recombination of alkyl radicals with each other. In particular, Table 2 shows that the radiolysis of preacidified solution yielded more P1, P2, and P8, as compared to the nonacidified sample, but the yield of alkylated products P10–P12 decreased by a factor of about 4.
Due to the accumulation of radiolytic products, the concentrations of TBP and Isopar in nonacidified solutions at a dose of 1 MGy decreased from 36 to 22 and from 64 to 56 wt %, respectively. In turn, the residual concentrations of TBP and Isopar upon the radiolysis of acidified solutions were about 29 and 54 wt %, respectively. Consequently, in the presence of HNO3, the radiolytic degradation of TBP was weakened, while the decomposition of Isopar was somewhat enhanced. This means that the reactions of NO2 and О–NO2 with alkyl radicals formed from Isopar were more competitive than those with radicals from TBP. Thus, in the presence of HNO3, Isopar components protected TBP from radiolytic degradation.
The TBP content changed relatively little upon aging and thermally stimulated hydrolysis. Even after storage for three years, an almost proportional dependence of the TBP concentration on dose was observed (Fig. 2). This fact indicates that radiolysis is the main factor of TBP degradation. Due to the high initial concentration of TBP, the formation of its excited molecules and radical cations can occur both by a mechanism of the direct action of radiation and as a result of excess energy and charge transfer from hydrocarbon diluent components. The positive charge and the unpaired electron in the TBP radical cation are uniformly distributed over three oxygen atoms connecting the phosphorus and carbon atoms with a small participation of C–C bonds and carbon atoms in the α-position [7]. Because of this, the P–O bond rupture can occur with the elimination of the butoxy radical

Neutralization of the residual cation with the OH– ion leads to the formation of stable Р1. The decomposition of excited TBP molecules can lead to the elimination of both butyl and butoxy radicals. The formation of the radical anion occurs as a result of electron capture by the TBP molecule, but the role of this process is small due to the predominant capture of electrons by nitric acid. The negative charge and the radical center in the radical anion are localized on oxygen and phosphorus atoms, respectively. The P–O and C–O bonds are significantly weakened to facilitate the removal of a butoxy or butyl radical, but the formation of the butoxy radical is more likely [7]

The neutralization of TBP radical cations and radical anions can serve as an important source of Р1:




The interaction of alkoxy radicals obtained upon the radiolytic debutylation of TBP with hydrocarbon molecules (R–H) is another route for the formation of P1:

Like other alkoxy radicals [10, 11], the butoxy radical (formed, for example, in reaction (4)) can abstract an H atom from alkyl groups. Accordingly, the butoxy radical serves as the main precursor of 1-butanol, which dominated among the light products of radiolysis. The fraction of 1-butanol in light alcohols was at least 90 wt %, and the average observed yield measured in the aged solution was 0.015 ± 0.005 µmol/J. Although the fractions of carbonyl compounds, alcohols, and hydrocarbons were comparable (Fig. 3), the fraction of 1-butanol was higher than that of any other light product by a factor of 3 to 16. Among relatively light alkanes, n-octane dominated in irradiated samples. Obviously, it is a dimerization product of butyl radicals, which appeared, in particular, in reaction (2). n-Octane and butanol were formed only at the stage of radiolysis, that is, in radical processes. In turn, postradiation hydrolysis afforded xylenes and ethylbenzene, the amount of which increased monotonically with temperature in a range of 110–170°C. Accordingly, n-butanol and n-octane can serve as indicators of the contribution of radiolysis, while xylenes and ethylbenzene can serve as indicators of the contribution of thermolysis to the total decomposition of the test solutions.
Along with the ⋅H radicals, other light radicals, in particular, ⋅СН3 and ⋅ОН, and alkoxy radicals can participate in the formation of alkyl radicals. The formation of ⋅H and ⋅СН3 is inevitable in the radiolysis of alkanes that are the constituents of Izopar-M [12]. In turn, ⋅OH radicals can arise upon the direct and indirect action of radiation on dissolved water or alcohols. H abstraction is possible at different positions of alkane molecules or the butyl groups of TBP [12, 13]. For example, in the decay of excited TBP molecules, the abstraction most likely occurs in the γ-position relative to the oxygen atom [14]. Accordingly, various structures of alkyl radicals led to a variety of their recombination products (Table 1, Fig. 5). Obviously, the radical products of H abstraction from the butyl groups of dibutyl phosphate molecules were the precursors of P3–P5, and the radical (⋅TBP) formed by H abstraction from TBP was a precursor of products P6–P12. The yield of ⋅NO2 radicals was significantly higher than that of ⋅NO3; therefore, the fraction of nitro derivatives was higher than the fraction of nitrates (Fig. 2a). In turn, due to the predominance of TBP over P1, the fraction of P6 and P7 in the nitration products was higher than the fraction of P3 and P4 by a factor of 4 to 7. The fractions of products Р3–Р5 in aged samples were relatively small: 0.2–0.4 wt % for Р3 + Р4 and 0.2% for Р5.
Obviously, product P9 (Figs. 4 and 5) resulted from the dimerization of ⋅TBP. Other high-molecular-weight products were formed in several ways. In particular, the radiolysis of Izopar-M served as a source of unsaturated hydrocarbons, which were involved in the formation of Р10–Р12 (Fig. 5). The addition of a short alkyl radical to an alkene gave a longer alkyl radical [8, 12], for example:

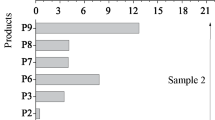
where the lengths of the radicals (q, x, y, and z) can be different primarily due to the variety of hydrocarbon components in Isopar-M. The hydrocarbon skeleton can also be elongated as a result of ion–molecule interaction: the addition of a primary carbocation to an alkene molecule followed by neutralization of the elongated carbocation. The formation of P10–P12 can be a long process due to the relatively low rate of radical enlargement and the difficult combination of bulky radicals with each other. Compounds in which the added alkyl group contained 14–15 C atoms dominated in the fraction of alkyl butyl phosphates P10 and P11 (m/z 379–564). Compounds with molecular weights of 740–780 predominated among dimers P12 (m/z 600–1000). Regardless of the absorbed dose, the total fraction of heavy phosphorus-containing products Р9–Р12 in aged samples was 31 ± 4% of the total of all phosphorus-containing products.
Of course, the radiolytic degradation of nitric acid had a key effect on the efficiency of postradiation hydrolytic processes. In particular, hydrolysis made a major contribution to the formation of P5 and P8. At a dose of 1 MGy or higher, when the pH of solution was many times higher than that in the nonirradiated sample, hydrolysis hardly changed the ratio between the products reached at the stage of radiolysis. However, hydrolysis was effective at lower doses (Fig. 1) and upon an increase in temperature and the addition of acid at the postradiation stage (Table 2). Moreover, high-temperature acid hydrolysis at >110°C led to efficient decomposition of radiolytic nitro derivatives and nitrates, including partial regeneration of TBP. About 1–1.5 wt % volatile products, including n-butane, n-octane, and n-butanol, were detected in irradiated samples without the subsequent stage of thermolysis. In turn, at temperatures above 110°C, postradiation thermolysis led to an increase in the yield of highly volatile products up to 16–17 wt % at 170°C. The dealkylation of the components of the irradiated solution played an important role in this effect.
CONCLUSIONS
The radiolytic degradation of TBP in its acidified mixtures with alkanes consists of many processes in which all components of the solution take an active part. The reactions of nitric acid with primary ionization products, electrons and radical cations, are of decisive importance. Thus, the probability of the reactions of these primary intermediates with TBP is decreased. In addition, the ⋅NO2 and ⋅О–NO2 radicals recombine with alkyl radicals formed from Izopar-M to weaken the effect on TBP. In this case, the reactions of the ⋅NO2 and ⋅О–NO2 radicals with TBP radicals lead to the formation of unstable nitro and nitroxy derivatives of TBP, which tend to decompose upon heating with the partial regeneration of TBP. In the absence of acid, the diluent participates more effectively in the formation of radiolytic degradation products of TBP; thus, it does not exhibit radiation protection functions. However, the combined presence of an acid and a diluent significantly decreases the radiolytic degradation of TBP, and the main contribution to the protective effect is due to the presence of the acid.
The debutylation of TBP with the formation of acid phosphates P1 and P2 occurs as a result of both radiolysis and hydrolysis. Moreover, as the dose increases, postradiation hydrolytic processes become of little importance in comparison with radiolytic processes. Postradiation heating of the samples promotes the hydrolytic formation of butyl phosphates P1 and P2, while the formation of N-containing products and oxygenates is mainly determined by the acid concentration. Moreover, the products of the radiolytic conversion of TBP are more sensitive to heat than the initial TBP. The formation of dibutyl ether, xylenes, and ethylbenzene can serve as a specific indicator of the contribution of thermolysis to the decomposition of the solution. n-Butane, n-butanol, n-octane, and phosphorus-containing products P9–P12 can be indicators of the relative contribution of radiolysis in both acidified and neutral TBP solutions.
REFERENCES
Pearson, J. and Nilsson, M., Solvent Extra. Ion Exch., 2014, vol. 32, p. 584. https://doi.org/10.1080/07366299.2014.924305
Aneheim, E., Ekberg, C., Fermvik, A., Foreman, M., Grüner, B., Hájková, Z., and Kvičalová, M., Solvent Extr. Ion Exch., 2011, vol. 29, p. 157. https://doi.org/10.1080/07366299.2011.539462
Dzhivanova, Z., Kadyko, M., Smirnov, A., and Belova, E., J. Radioanal. Nucl. Chem., 2019, vol. 321, p. 439. https://doi.org/10.1080/07366299.2011.539462
Peterman, D.R., Mincher, B.J., Riddle, C.L., and Tillotson, R.D., Summary Report on Gamma Radiolysis of TBP/n-dodecane in the Presence of Nitric Acid Using the Radiolysis/Hydrolysis Test Loop (INL/TXT–10-19866), 2010. https://www.osti.gov/servlets/purl/ 993164-I2Pm13/. https://doi.org/10.2172/993164
Belova, E., Egorov, G., and Nazin, E., Radiochemistry, 2000, vol. 42, p. 238.
Mincher, B., Modolo, G., and Mezyk, S., Solvent Extr. Ion Exch., 2009, vol. 27, p. 1. https://doi.org/10.1080/07366290802544767
Serenko, Yu.V., Yudin, N.V., Gritcenko, R.T., Rodin, A.V., Belova, E.V., and Ponomarev, A.V., Radiat. Phys. Chem., 2021, vol. 185, p. 109495. https://doi.org/10.1016/j.radphyschem.2021.109495
Woods, R. and Pikaev, A., Applied Radiation Chemistry: Radiation Processing, New York: Wiley, 1994.
Ponomarev, A.V., Bludenko, A.V., and Makarov, I.E., Mendeleev Commun., 2002, vol. 12, p. 92. https://doi.org/10.1070/MC2002v012n03ABEH001583
Ponomarev, A.V., Vlasov, S.I., and Kholodkova, E.M., High Energy Chem., 2019, vol. 53, p. 314. https://doi.org/10.1134/S0018143919040106
Ponomarev, A.V., Vlasov, S.I., Kholodkova, E.M., Chulkov, V.N., and Bludenko, A.V., Radiat. Phys. Chem., 2019, vol. 165, p. 108405. https://doi.org/10.1016/j.radphyschem.2019.108405
Metreveli, A.K. and Ponomarev, A.V., High Energy Chem., 2016, vol. 50, p. 254. https://doi.org/10.1134/S0018143916040135
Lesage, D., Virelizier, H., and Jankowski, C.K., Spectroscopy, 1997, vol. 13, p. 275. https://doi.org/10.1155/1997/565194
Wang, F., Horne, G.P., Pernot, P., Archirel, P., and Mostafavi, M., J. Phys. Chem. B, 2018, vol. 122, p. 7134. https://doi.org/10.1021/acs.jpcb.8b03715
ACKNOWLEDGMENTS
We are grateful to the Center for Shared Use of Instrumental Research Methods at the Frumkin Institute of Physical Chemistry and Electrochemistry, Russian Academy of Sciences for the equipment provided.
Funding
This work was supported by the Russian Academy of Sciences, project no. AAAA-A18-118011190130-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Serenko, Y.V., Belova, E.V. & Ponomarev, A.V. Protective Effects in the Radiolysis of Acidified Hydrocarbon Solutions of Tributyl Phosphate. High Energy Chem 56, 190–196 (2022). https://doi.org/10.1134/S001814392203002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S001814392203002X