Abstract
A CO oxidation catalyst based on plasma-chemical titanium carbonitride TiC0.2N0.8 and TiO2 with a palladium content of 10 wt % has been synthesized. The dependence of the reaction rate of CO oxidation at room temperature and low CO concentrations (<100 mg/m3) on the TiC0.2N0.8 content has been studied. It has been found that, at TiC0.2N0.8 concentrations from 8 to 10 wt %, the catalyst exhibited a maximum rate of the CO oxidation reaction, which was 2.5 times higher than the rate of reaction on a catalyst based on pure TiO2 that included palladium clusters. The catalysts are promising for use in catalytic and photocatalytic systems for air purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Titanium dioxide is used as an active material in photocatalytic air purifiers for the removal of toxic gases, viruses, and bacteria [1–3]. The development of combined catalysts for photocatalytic devices with the efficient removal of both organic impurities and carbon monoxide (CO) from air is required because the photocatalytic removal of CO from air on pure titanium dioxide in a continuous mode is ineffective. Vershinin with coauthors studied CO catalysts based on nanomaterials and found that it is possible to form the nanoclusters of a catalytic metal (Pd or Pt) on the surface of nanodiamond (ND) [4, 5], β-SiC [6], and TiN [7], which are structurally similar to palladium and platinum. In this case, the cluster thickness is close to several lattice parameters of a catalytic metal; this ensures a high reaction rate of CO oxidation at room temperature and at low CO concentrations. The aim of this work was to synthesize a catalyst based on TiO2 with the addition of plasma-chemical TiC0.2N0.8 nanoparticles including Pd clusters and to study its properties in the oxidation reaction of CO in order to assess the applicability of this catalyst to photocatalytic air purification systems.
EXPERIMENTAL
Titanium Carbonitride Synthesis Procedure
The TiC0.2N0.8 powder used in this work was obtained by the hydrogen reduction of titanium tetrachloride in a flow of microwave nitrogen plasma at atmospheric pressure. A mixture of titanium tetrachloride vapor with hydrogen and octane in a required ratio was introduced into a plasma nitrogen flow with an average bulk temperature of about 3000 K, which was obtained in a plasmatron using a microwave generator with a frequency of 2450 MHz and a maximum useful power of 5 kW. The volumetric flow rate of nitrogen was 2.5 m3/h. The TiCl4 feed rate was 3 g/min, and the flow rate of hydrogen was 0.4 m3/h. The chemical interaction of the reactants and the condensation of TiC0.2N0.8 nanoparticles occurred in a tubular reactor 50 mm in diameter and 250 mm in length, the inner walls of which were lined with quartz. The particles formed in the reactor were separated from the gas phase by filtration on a bag filter after cooling the flow.
Catalyst Synthesis Procedure
Hombicat UV-100 TiO2 (Sachtleben Chemie, Germany) and TiC0.2N0.8 obtained by the plasma-chemical method were used as a support for palladium. The average size of TiC0.2N0.8 particles was close to 32 ± 4 nm, and the specific surface area and the average crystallite size of TiO2 were about 380 ± 15 m2/g and 5–8 nm, respectively. To obtain catalysts, we used the formation of palladium clusters on the surface of support particles in an aqueous solution containing palladium chloride and a reducing agent, lithium formate LiCOOH, according to a previously patented method [8]. The catalyst was prepared as follows: an aqueous solution of PdCl2 (10−3–10−2 mol/L) was mixed with an aqueous solution of lithium formate (0.02–0.2 mol/L) at 20°C. Then, a required amount of the aqueous solution of PdCl2 and lithium formate was introduced into an aqueous suspension of TiC0.2N0.8 and titanium dioxide with a solid particle concentration of 2 g/L heated to 45–50°C. After an induction period (5–10 min), palladium clusters were deposited on the surface of TiC0.2N0.8 and titanium dioxide particles. Then, the solution was cooled to room temperature for 6 h, and the catalyst was washed with distilled water (five or six times) to remove reaction products and dried at 80°C for 24 h. An HD 3200 ultrasonic homogenizer was used to prepare an aqueous suspension of TiO2 and TiC0.2N0.8. After preparing the catalyst, we obtained an aqueous suspension of the catalyst once again, applied it to a porous plate (40 × 40 × 6 mm) sintered from glass beads 1 mm in diameter, and then dried it at 80°C for 24 h.
Instruments Used for Physicochemical Catalyst Characterization
Nitrogen adsorption/desorption isotherms and BET specific surface areas of titanium nitride samples were measured at liquid nitrogen temperature (77 K) using a Quantachrome Quadrasorb SI analyzer. The TiC0.2N0.8 samples were conditioned at 573 K in an atmosphere of helium using a FloVac Degasser for 3 h immediately before measurements. The X-ray diffraction spectra were recorded with a DRON ADP-2-02 diffractometer using Cu Kα radiation (λ = 0.154056 nm). A JEOL JEM 2100 transmission electron microscope and a SPECS PHOIBOS 150 MCD-9 electron analyzer with a magnesium anode X-ray source (Mg Kα radiation, 1253.6 eV) were used to study the structure and composition of the catalyst surface with Pd clusters. An Acculab ALC-80d4 electronic balance was used for weighing the reagents and samples.
Procedure for Studying Catalytic Properties
The kinetics of CO oxidation in air on the catalyst was studied according to a procedure described earlier [6]. The study of the catalytic properties was carried out as follows: a test chamber was blown with a gas mixture of carbon monoxide with air with a CO concentration of 150 mg/m3 for 600 s at a flow rate of 50 cm3/s. Then, the inlet and outlet valves of the test chamber were closed and a flow booster located in the test chamber was turned on to ensure gas mixture circulation through the sample with the catalyst at a rate of 30 cm3/s. After a decrease in the concentration of CO a level of 100 mg/m3 due to the catalytic reaction, a digital stopwatch was turned on and the readings of sensors were recorded. The test chamber with a volume of 0.3 ± 0.003 dm3 was equipped with CO, CO2, and humidity and temperature (RH/T) sensors. A microprocessor-based converter was used for the processing of sensor signals. The following sensors were used: a NAP-505 CO sensor (Nemoto), an MSH-P/CO2/NC/5/V/P optical CO2 sensor (Dynament), and an SHT75 humidity and temperature sensor (Sensirion).
Results of Studying the Physicochemical Properties of the Catalyst
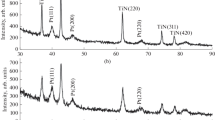
Figure 1 shows the results of X-ray diffraction analysis of the catalyst containing 81 TiO2 + 9 TiC0.2N0.8 + 10 wt % Pd. From the X-ray diffraction data, it was found that the coherent scattering region (CSR) of Pd clusters was close to 6 nm. Earlier, Vershinin et al. [6] found that the CSR of Pd clusters was close to 3.5 nm for a catalyst based on pure TiO2 containing 10 wt % Pd.
Figure 2 shows the results of XPS studies of the catalyst (81 TiO2 + 9 TiC0.2N0.8 + 10 wt % Pd) using the decomposition of the Pd3d photoelectron spectra into individual components as an example.
Three main peaks with Pd3d5/2 binding energies of 335.4, 336.5, and 337.8 eV can be distinguished in the Pd3d spectra. The signals in the spectra are in good agreement with published data, and they characterize palladium in a metallic state with a Pd0 binding energy of 335.4 eV (Pd3d5/2). The spin–orbital splitting between Pd3d5/2 and Pd3d3/2 sublevels is 5.26 eV. The peak of palladium with a binding energy of ~336.5 eV, which was observed in the spectra of a catalyst sample with titanium carbonitride, can be attributed to palladium(II) oxide, which was probably formed in the course of sample storage in air. The peak with a binding energy of 337.8 eV belongs to the state Pd4+, for example, in the composition of PdO2 or to clusters localized at defects on the support surface [9, 10].
Results of Testing the Catalytic Properties of the Catalyst
Figure 3 shows the results of a study of the catalytic properties of the following catalysts: catalyst 1, 90 TiO2 + 10 wt % Pd and catalyst 2, 81 TiO2 + 9 TiC0.2N0.8 + 10 wt % Pd. The concentration of CO2 increased simultaneously with a decrease in the concentration of CO in the test chamber due to the irreversible reaction of CO oxidation by atmospheric oxygen (CO + 1/2 O2 = CO2) on the catalyst surface.
As can be seen in Fig. 3, the time dependence of the concentration of CO in the test chamber with a catalyst is described by the equation
where CCO(t) is the measured concentration of CO in the test chamber, CCO(0) is the concentration of CO at the initial (zero) point in time, k is the reaction rate constant, and t is time.
To study the effect of catalyst composition on the catalytic properties, we synthesized and tested ten catalyst samples based on titanium carbonitride and titanium dioxide with a palladium content of 10 wt %, and the concentration of titanium carbonitride was varied from 0 to 30 wt %. The weight of Pd in each of the test samples was close to 1.5 mg. The reaction rates of CO oxidation were determined for each sample after processing the experimental data based on Eq. (1). Then, the ratio between reaction rates Vx/V0 was determined, where V0 is the rate of CO oxidation at a concentration of 100 mg/m3 for a composition containing 0 wt % TiC0.2N0.8, and Vx is the rate of CO oxidation at a concentration of 100 mg/m3 for a catalyst containing x wt % TiC0.2N0.8. Figure 4 shows the results of the experimental studies.
To determine the adsorption properties of palladium in catalyst 1 and catalyst 2, the absorption of CO by a catalyst from the gas phase was measured. For this purpose, the sample with a catalyst was placed in a 50-cm3 stainless steel test chamber equipped with inlet and outlet valves for supplying a gas mixture. The test chamber was purged with dry nitrogen at a rate of 50 cm3/s for 100 s; then, it was purged with a mixture of CO and N2 for 5 s at a rate of 50 cm3/s. Then, the inlet and outlet valves were closed and the volume fraction of CO was determined after 10 min. It was experimentally found that a nearly equilibrium volume fraction of CO in the test chamber was established within 10 min. For the analysis of CO, 3-cm3 samples were taken from the test chamber after 10 min; then, the sample was injected into a 300-cm3 measuring chamber filled with air. The volume fraction of CO in the measuring chamber was analyzed with a NAP-505 CO sensor (Nemoto). The amount of CO molecules adsorbed by the surface of Pd per unit weight of Pd in the catalyst can be calculated using the following formula:
where Cic is the initial volume fraction of CO in %, Cfc is the final volume fraction of CO in %, Vo is the volume of the test chamber in dm3, NA is the Avogadro number, mPd is the weight of palladium in the catalyst in g, and VM is the molar volume of gas in dm3. The weight of the catalyst was chosen to provide the final concentration in a range from 0.45 to 0.55 of the initial volume fraction of 1% CO. After the processing of experimental results, it was found that the ratio NCO (Pd in catalyst 1)/NCO (Pd in catalyst 2) was close to 1.0 ± 0.15. The measurements were carried out at a temperature of 295 K and a gas mixture pressure of 101 kPa in the test chamber.
DISCUSSION
From the experimental results, it was found that the catalysts were similar in the adsorption properties of palladium. From the data on the kinetics of CO oxidation, which are shown in Figs. 3 and 4 (per unit weight of Pd), it was found that the maximum rate of CO oxidation on palladium supported onto titanium dioxide with titanium carbonitride additives was higher by a factor of 2.5 than the rate of CO oxidation on palladium supported onto pure titanium dioxide. In this case, an increase in the reaction rate of CO oxidation can be associated with a decrease in the activation energy of the reaction rate. From the Arrhenius equation, it follows that the activation energy decreases by 2.2 ± 0.3 kJ/mol as the reaction rate increases by a factor of 2.5. Similar results were obtained. in a study of silicon carbide additives [6]. At an optimal concentration of titanium carbonitride (8–10 wt %), TiC0.2N0.8 acts as a center for the formation of palladium clusters with a low activation energy in the CO oxidation reaction and, hence, a maximum rate of the oxidation reaction. Preliminary tests of the catalyst in the photocatalytic oxidation of ethanol showed that the catalyst also efficiently oxidizes ethanol; therefore, the catalyst is also promising for use in catalytic and photocatalytic air purification systems for domestic applications.
CONCLUSIONS
A CO catalyst was synthesized on the basis of plasma-chemical TiC0.2N0.8 and TiO2. As a result of studying the reaction rate of CO oxidation as a function of the TiC0.2N0.8 content at room temperature, it was found that the catalyst exhibited a maximum rate of the CO oxidation reaction at a titanium carbonitride concentration of 8 to 10 wt %. At low CO concentrations (<100 mg/m3), the reaction rate of CO oxidation on the developed catalyst was higher by a factor of 2.5 than the reaction rate on the catalyst based on pure TiO2 including palladium clusters. The catalysts are promising for use in catalytic and photocatalytic air purification systems.
REFERENCES
Mo, J., et al., Atmos. Environ., 2009, vol. 43, no. 14, p. 2229.
Paz, Y., Appl. Catal., B, 2010, vol. 99, nos. 3–4, p. 448.
Kolarik, B., et al., Build. Environ., 2010, vol. 45, no. 6, p. 1434.
Vershinin, N.N., Efimov, O.N., Bakaev, V.A., Aleksenskii, A.E., Baidakova, M.V., Sitnikova, A.A., and Vul’, A.Ya., Fuller. Nanotub. Carbon Nanostructures, 2011, vol. 19, p. 63.
Vershinin, N.N., Balikhin, I.L., Bakaev, V.A., Berestenko, V.I., Efimov, O.N., Kurkin, E.N., and Kabachkov, E.N., Izv. Akad. Nauk. Ser. Khim., 2017, vol. 66, no. 4, p. 648.
Vershinin, N.N., Bakaev, V.A., Berestenko, V.I., Efimov, O.N., Kurkin, E.N., and Kabachkov, E.N., High Energy Chem., 2018, vol. 52, p. 90.
Vershinin, N.N., Berestenko, V.I., Efimov, O.N., Kurkin, E.N., and Kabachkov, E.N., High Energy Chem., 2019, vol. 53, p. 400.
Vershinin, N.N. and Efimov, O.N., RU Patent No. 2348090 (2009).
Bukhtiyarov, A.V., Prosvirin, I.P., and Bukhtiyarov, V.I., Appl. Surf. Sci., 2016, vol. 367, p. 214.
Yang, F., Zhang, B., Dong, S., Wang, C., Feng, A., Fan, X., and Li, Y., J. Energy Chem., 2019, vol. 29, p. 72.
Funding
This work was carried out in accordance with a state contract (no. AAAA-A19-119061890019-5) with the use of the equipment of the Shared-Use Analytical Center at the Institute of Problems of Chemical Physics, Russian Academy of Sciences and the Scientific Center in Chernogolovka, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Rights and permissions
About this article
Cite this article
Vershinin, N.N., Balikhin, I.L., Berestenko, V.I. et al. Synthesis and Properties of a Carbon Monoxide Oxidation Catalyst Based on Plasma-Chemical Titanium Carbonitride, Titanium Dioxide, and Palladium. High Energy Chem 55, 75–79 (2021). https://doi.org/10.1134/S0018143921010148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143921010148