Abstract
The mechanism of formation of an ozone–hydroxyl mixture and its properties have been investigated. The main channel for the consumption of hydroxyl radicals is the buildup of hydrogen peroxide. In the absence of ozone, almost all OH• radicals formed are converted to hydrogen peroxide. In the presence of ozone, the reaction O3 + H2O2 gives hydroxyl radicals again. The concentration of OH• radicals is maintained until ozone and hydrogen peroxide are consumed. This allows the transport of hydroxyl radicals up to 45 cm from the reactor. The results are confirmed by an experiment on the oxidation of oxalic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
An electric discharge is often used to produce plasma in which chemically active species are generated [1]. The composition of active species depends on the characteristics of the discharge and the composition of the gas in which the discharge burns. If oxygen is present in the gas phase, then ozone may form during discharge. Ozone is a long-lived species, but its chemical activity is relatively low, so ozone is a selective oxidizing agent [2]. Hydroxyl radicals are highly likely to decay in interactions with each other, forming more stable but less active species. Therefore, the action of hydroxyl radicals is manifested at small distances from the place of their formation.
The concentration of hydroxyl radicals at different distances from the site of their formation in an argon plasma with water vapor was studied in [3]. There was neither oxygen nor ozone in the discharge region. The diameter of the region with the maximum concentration of radicals was about 2 mm. Hydroxyl radicals appear mainly in the ground state and are not detectable by measuring the emission spectrum. Hydroxyl radicals were visualized via their excitation by a laser pulse. The concentration of hydroxyl radicals after passage of a laser pulse decreases to a background level within 150 ns. This figure allows us to judge the lifetime of hydroxyl radicals.
In [4], we explored the possibility of transport of hydroxyl radicals outside the reactor in which ozone was generated along with hydroxyl radicals. It was shown that OH• radicals can exist for up to 1 s under these conditions, but their concentration decreases several times.
In this work, we selected a solution of oxalic acid as the detector, which is oxidizable only by hydroxyl radicals [2]. The decomposition of oxalic acid by products formed during a spark corona discharge and transported through the tube to a distance of 45 cm was studied. The properties of the ozone–hydroxyl mixture formed in a pulsed corona discharge reactor in an atmosphere of air saturated with water vapor were analyzed in terms of a kinetic model of the process.
EXPERIMENTAL
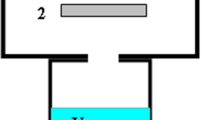
The block diagram of the experimental setup is shown in Fig. 1. A spark corona discharge, in which a wide spectrum of active species including ozone, hydrogen peroxide, and hydroxyl radicals was formed, occurred in discharge chamber 1 filled with air. The discharge chamber housing was made of aluminum, and the chamber lid was made of PTFE. Discharge electrodes 2 were introduced into the chamber through holes in the lid. The stainless steel electrodes were of 2 mm in diameter and 40 mm in length, and their number was 24. The distance between the electrodes was 25 mm. Discharge electrodes 2 were arranged at a height of 6.5 mm from ground electrode 3. A high voltage of negative polarity was applied from an 11-kV power supply through an RC matrix. Each electrode was connected to the power source through a ballast resistor of R = 20 MΩ and grounded through a capacitor of C = 32 pF. Given the voltage drop across the ballast resistor, the voltage across the electrode was 9.6 kV. The selected design and power circuit of the discharge electrodes ensured the formation of Trichel pulses on each electrode [5]. The Trichel pulse repetition rate was ~100 kHz, the pulse current was up to 2 mA, the average current from each electrode was 70 μA, and the total current at all 24 electrodes was 1.7 mA.
Liquid 4 to be processed was poured on the bottom of the discharge chamber below the level of the ground electrode. The total volume of processed liquid was 0.5 L. The bottom part of the discharge chamber containing the liquid was cooled with running water. The temperature of the liquid during its treatment was maintained at 20–22°C. Active species formed in the discharge chamber were sucked out through tube L by ejector 5. The length of tube L varied from 1 to 45 cm. The inner diameter of the tube was 4 mm. The liquid was pumped from the discharge chamber to the ejector by pump M. The pressure created by the pump at the inlet of the ejector was 3 atm. The flow rate of the liquid through the ejector was 160 L/h. The ratio of liquid and suction gas flow rates was 1 : 1. The velocity of the gas sucked through tube L from the discharge chamber was 353 cm/s, and the time of its traveling through the tube of a 45 cm length was 0.125 s. Water returned from the ejector to chamber 1. Thus, the liquid was processed in a recirculation mode. In the gas phase of the discharge chamber, a steady-state concentration of active species was established. To sample the liquid during its treatment, there was a sampling tap installed on the tube connected to the suction pump.
As test liquids, a 100 mmol/L aqueous solution of oxalic acid and pure water were used. During processing, dissolved ozone and hydrogen peroxide accumulated in pure water. The treated water was periodically sampled, and the concentrations of ozone and hydrogen peroxide were determined in the samples. The ozone concentration was determined iodometrically. The volume of the sampled water was 30 mL; 30 mL of a KI solution of 5 g/L concentration was injected into it. The concentration of I2 produced by oxidation was determined by titrating with 0.02 N sodium thiosulfate in an acidic medium. The hydrogen peroxide concentration was determined by measuring the absorbance at 410 nm of the complex formed by adding freshly prepared TiCl4 to the treated water sample [6].
Oxalic acid was used as a detector of hydroxyl radicals. To estimate the lifetime of active species, a decrease in the concentration of oxalic acid after 24-h treatment was measured depending on the length of suction tube L. The concentration of oxalic acid was determined by titration with a 0.05 N potassium permanganate solution in an acidic medium at a temperature of 80°C. Ozone accumulated during the treatment was destroyed at a temperature of 80°C and did not affect the titration result. Hydrogen peroxide also did not affect the result, because its concentration was low. For the experiments, doubly distilled water at pH 6.5 and the reagent grade chemicals were used. The absorption spectra were measured with an Aquilon SF-102 spectrophotometer (Russia).
The kinetics of the reactions of all reactive oxygen species that could have formed during the electric discharge treatment were calculated. The calculation was based on a scheme of 30 reactions between active species. The system of 11 differential equations describing the formation and consumption of reactive oxygen species was solved using the software suite Mathcad-14.
RESULTS AND DISCUSSION
Ozone is produced in an electric discharge and diffuses into water. Its concentration in the gas cavity and in water increases with treatment time and reaches a steady-state concentration after 30 min. The steady-state concentration of hydrogen peroxide is established after 20 min. Since the oxalic acid treatment time was 24 h, it can be assumed that the gas mixture with active species aspirated from the reactor was always in a steady state. The steady-state concentration of ozone in water was (5.8 ± 0.3) × 10−5 mol/L. The Henry constant for ozone in water is 1.7 [2]; therefore, its concentration in the gas phase is [O3] = (9.9 ± 0.4) × 10−5 mol/L. The steady-state concentration of hydrogen peroxide is (1.8 ± 0.3) × 10−5 mol/L.
The dependence of the concentration of oxalic acid on the time of movement of the gas mixture through the suction tube is shown in Fig. 2. The travel time along the 45-cm long tube was 0.125 s. With a minimum tube length of 1 cm and a mixture travel time of 0.003 s, a strong decrease in the oxalic acid concentration from 100 to 40 ± 7 mmol/L is observed. Such a decrease corresponds to the concentration of hydroxyl radicals in the gas mixture of 1.4 × 10−7 mol/L. The total discharge current was 1.7 mA. Hence, the yield of hydroxyl radicals is (20 ± 3) moles of OH• radicals per mole of electrons passed through the discharge circuit. The high voltage applied to the electrodes is 9.6 kV. Hence, the energy expenditure for the formation of one hydroxyl radical is (480 ± 70) eV. Figure 2 shows that over a time of about 0.1 s, the mixture of active species extracted from the reactor almost completely loses its activity and the oxidation of oxalic acid ceases. This means that during the movement of the mixture for 0.1 s, the concentration of hydroxyl radicals decreases to the level when the change in the oxalic acid concentration as compared with the initial value lies within the measurement error.
Dependence of the concentration of oxalic acid [C], mmol/L for oxalic acid samples with an initial concentration of 100 mmol/L, treated for 24 h with an ozone–hydroxyl mixture on the drift time of the mixture from the discharge chamber t, s. The squares with error bars show experimental data, and the dashed curve represents calculation results.
Based on published data, a scheme of the interactions of reactive oxygen species that could have been formed during a spark corona discharge in air was compiled. Active forms of nitrogen were not taken into account, since their yield during an electric discharge of this type in air is small [7]. The reaction scheme is presented in Table 1. During the discharge, a steady state is established and all the active species are being produced.
The calculation of the stationary state according to the reaction scheme given in the table showed that the main stable product is hydrogen peroxide. The calculation included the hydroxyl and ozone generation rates in the discharge region of 3 × 10−4 and 5 × 10−4 mol L−1s−1, respectively, at which the concentrations of active species (hydroxyl radicals, hydrogen peroxide, and ozone) corresponding to the experimental values are reached in the steady state. The time to establish a steady-state concentration was 150 s. The steady-state concentrations of the active species were as follows: [OH•] = 1.4 × 10−7, [H\({\text{O}}_{2}^{\bullet }\)] = 1.4 × 10−8, [O•] = 5.8 × 10−9, [H] = 1.8 × 10−14, [H2] = 3 × 10−12, [H2O2] = 1.8 × 10−5, [\({\text{O}}_{2}^{\bullet }\)–] = 1.2 × 10−9, [H\({\text{O}}_{2}^{ - }\)] = 1 × 10−7, [O2S–] = 2.9 × 10−9, and [O3] = 9.9 × 10−5 mol/L. The obtained concentrations of hydroxyl radicals, hydrogen peroxide, and ozone corresponded to the experimentally measured values. Molecular oxygen was formed as the final product of a number of reactions, but it did not participate in further transformations of the active species, so its concentration was not set as a variable. Molecular oxygen and water vapor present in the discharge region determined the yield of hydroxyl radicals and ozone, but the mechanism of their formation was not considered; the yield of OH• and ozone was set as a parameter.
The calculated concentrations of hydroxyl radicals for hypothetical cases (with and without ozone) depending on the transport time up to 0.2 s are presented in Fig. 3. The figure shows that the concentration of hydroxyl radicals after a drift for 0.2 s decreases to 4.9 × 10−14 mol/L in the absence of ozone (Fig. 3, curve 1) and [OH•] = 1.5 × 10−8 mol/L in the presence of ozone (Fig. 3, curve 2). To maintain the concentration of hydroxyl radicals, hydrogen peroxide and ozone are consumed in reaction (30) (see Table 1).
Concentrations of active species [C], mol/L, transported from the generation region during time t, s: (1) OH• radicals in the absence of ozone in the system, (2) OH• radicals in the presence of ozone in the mixture, (3) hydrogen peroxide, and (4) ozone. The initial concentrations correspond to the steady state in the generation region, which was established after 150 s.
The values of the concentration of hydroxyl radicals (Fig. 3, curve 2) were used to calculate the concentration of oxalic acid after 24-h treatment depending on the time of drift in the tube. The results for comparison with experimental data are presented by the dashed curve in Fig. 2. It can be seen that the calculated values describe the experimental data, thereby confirming the proposed mechanism of formation of the ozone–hydroxyl mixture.
CONCLUSIONS
The results obtained show that an ozone–hydroxyl mixture is formed in a pulsed corona discharge reactor. The formation of this mixture ensures the transport of hydroxyl radicals from the reactor to a distance of 40 cm. The concentration of hydroxyl radicals is replenished via the reaction of accumulated hydrogen peroxide with ozone. This feature makes it possible to create devices for wastewater and drinking water treatment with hydroxyl radicals. The concentration of hydroxyl radicals extractable from the reactor and, thus, capable of oxidizing water impurities is close to the concentration in the reactor itself.
REFERENCES
Bruggeman, P.J., Kushner, M.J., Locke, B.R., et al., Plasma Sources Sci. Technol., 2016, vol. 25, no. 59, p. 053002.
Handbook of Chemistry and Physics, 97th ed., Haynes, W.M., Ed., Boca Raton: CRC, 2016–2017, p. 97.
Li, L., Nikiforov, A., Xiong, Q., Britun, N., Snyders, R., et al., Phys. Plasmas, 2013, vol. 20, p. 093502.
Aristova, N.A. and Piskarev, I.M., Russ. J. Phys. Chem., 2003, vol. 77, no. 5, p. 723.
Raizer, Yu.P., Gas Discharge Physics, Berlin: Springer, 1991.
Charlot, G., Les méthodes de la chimie analytique: analyse quantitative minérale, 5th Ed., Paris: Masson et Cie, 1966.
Piskarev, I.M., Russ. J. Phys. Chem., 2001, vol. 75, no. 11, p. 1832.
Pastina, B. and LaVerne, J.A., J. Phys. Chem. A, 2001, p. 9316.
Westley, F., Tables of Recommended Rate Constants for Chemical Reactions Occurring in Combustion, Washington, DC: National Bureau of Standards, 1980.
Hoigne, J., Bader, H., Haag, W.R., and Staehelin, J., Water Res., 1985, vol. 19, no. 8, p. 993.
Kulagin, Y.A., Shelepin, L.A., and Yarygina, V.N., Kinetics of Processes in the Gas Media Containing Metastable Oxygen, Moscow: Lebedev FIAN, 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Piskarev, I.M. The Role of Ozone in Chemical Processes in Electric Discharge Plasma. High Energy Chem 54, 205–209 (2020). https://doi.org/10.1134/S001814392003011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S001814392003011X