Abstract
The contact properties, chemical structure and surface morphology of poly(ethylene terephthalate) films modified by direct-current discharge at the cathode and anode have been studied. A substantial improvement in wettability and an increase in total surface energy and its polar term, which are retained upon storage in air at ambient conditions, have been shown. The change in the chemical structure of the plasma-modified films has been studied by X-ray photoelectron spectroscopy, and the formation of a significant amount of oxygen-containing groups on the surface has been demonstrated. The investigation of the modified films by atomic force microscopy and scanning electron microscopy has provided revealed a change in morphology of the surface and an increase in its roughness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
It is known that poly(ethylene terephthalate) (PET) films are widely used for insulation of wires and cables, windings of electric motors, as well as in the composition of laminates for slot insulation of high-voltage electrical machines [1]. To promote the use of PET as a laminate component, we carried out works on the modification of PET films by low-frequency glow discharge treatment in order to increase their adhesion to impregnating compositions [2, 3]. In recent years, the polymer films have found application in solar cell elements, and the laminate on their basis serves as the back panel of the device [4]. The use of such a laminate in comparison with the alternative three-layer structure PVDF/PET/PVDF—PVDF is poly(vinylidene fluoride), makes it possible to improve electrical insulation properties, reduce moisture penetration, and significantly reduce the cost of the element [5]. Apparently, it is this kind of application that at present arouses interest in the study of the process of modifying the PET films surface.
It is known that the most practically feasible and environmentally friendly method that allows the contact properties of polymer films to be significantly improved by altering in the chemical structure and morphology of the surface without affecting the bulk properties is low-temperature plasma treatment [6–9].
For example, it was found that the treatment of 100 μm-thick PET films (M/S Garware Polyesters & Fibers Ltd., India) by O2 radiofrequency (13.56 MHz) discharge plasma resulted in an improvement in their contact properties [10]. The water contact angle for the initial sample was θw = 76°, and the glycerol contact angle was θg = 66°. Plasma treatment for 30 min resulted in a decrease in these values to 33° and 31°, respectively. The total surface energy (γ) increased from 32 to 61mJ/m2, and the value of the polar term (γp), from 11 to 40 mJ/m2. X-ray photoelectron spectroscopy showed an increase in the number of oxygen-containing groups on the surface of the modified film, and an increase in its roughness was revealed by atomic force microscopy. In addition, it was found that radiofrequency discharge treatment resulted in the destruction of the films, and the weight loss at the treatment time of 30 min was ~10%.
The use of dielectric barrier discharge (DBD) for modifying the surface of 2 mm-thick PET samples (Ensinger GmbH, Germany) caused a decrease in θw from 71° to 30°, but this effect proved to be unstable, and storage for 48 h in air under ambient conditions resulted in an increase in θw to 65° [11]. In the case of DBD processing PET films (20 μm, Eastman Chem Co., USA) on an APP system production unit (APJeT™, USA), which enables the film modification during rewinding at a speed from 15 to 200 cm/min, the θw value decreased from 84° to 25° (with N2-diluted He used as a plasma gas), to 35° with the addition of O2, and to 45° in an atmosphere of pure He [12].
Earlier, we studied the modification of PET films by an ac line-frequency glow discharge in air [13, 14]. It was found that this effect resulted in an improvement in the contact properties of the films: the water contact angle under optimal processing conditions decreased from 66° to 6° (almost complete spreading), and the γ and γp values increased by factors of ~1.8 and 3.5, respectively. However, a noticeable and rapid increase in θw with time was observed during the storage of the modified films.
In connection with the foregoing, it is of undoubted interest to study the process of modifying PET films by dc discharge and to reveal changes in their contact properties, chemical structure, and morphology.
EXPERIMENTAL
The object of study was a commercial biaxially oriented PETLAIN BT 1010 E PET film (Superfilm, Turkey) of 40 μm in thickness. Before use, the samples were degreased with ethyl alcohol and dried under ambient conditions. The structural formula of the repeat unit of the polymer is given below.

The process of modification by dc discharge was carried out using the procedure and the setup detailed in [15]. Samples of PET film with dimensions of 5 × 5 cm were mounted on the anode or cathode, filtered atmospheric air served as a plasma gas, the pressure in the system was p ~ 10 Pa, discharge current was I = 50 mA, and the treatment time varied from 10 to 180 s.
The surface contact properties were characterized by the values of contact angles (θ) measured with an Easy Drop DSA100 (KRUSS, Germany) instrument and the Drop Shape Analysis V.1.90.0.14 software for two test liquids, deionized water (θw) and glycerol (θg) (error ± 1°). The measurements were carried out both immediately after treatment of the films and after their storage for 14 days in air under ambient conditions. The values of total surface energy and its polar and dispersion terms were calculated from experimentally obtained θ values according to the procedure described in [16].
The change in the chemical structure of the films was studied by X-ray photoelectron spectroscopy (XPS). The XPS spectra were obtained using a PHI 5500 VersaProbeII spectrometer. The experiments were carried out in an ultrahigh vacuum of 5 × 10–8 Pa using monochromatic AlKα radiation (hν = 1486.6 eV, power 50 W), the diameter of the analysis area was 200 μm. The C1s and O1s binding energies were determined from the high-resolution spectra measured at a pass energy of 11.75 eV and a step size of 0.1 eV, the approximation of the spectra was performed by a nonlinear least-squares method using the Gauss–Lorentz function. The calibration of the binding energy scale (E) was carried out using Au 4f 84.0 eV and Cu 2p3 932.6 eV and adjusted using E = 284.7 eV of the C1s peak of aromatic CH groups in the spectrum [17, 18]. The error in E determining was ± 0.1 eV.
The surface morphology of the film samples was studied using a Solver HV atomic-force microscope (NT-MDT, Russia) in air under normal conditions using standard HA-NC cantilevers (NT-MDT, Russia) with a tip curvature radius of 10 nm. The measurements were carried out in the tapping mode with the acquisition of surface topography and phase contrast. Average (Ra) and rms roughness (Rms) values were determined using the NOVA software version 1.1.0.1851 (NT-MDT, Russia).
Scanning electron microscopy (SEM) was also used to study the surface morphology of the PET films. The experiments were carried out using a Zeiss EVO 40 instrument with an X-Flash 1106 silicon drift detector. To ensure charge drain and an increase in thermal conductivity, a thin aluminum conductive layer (~20 nm) was deposited by high-vacuum thermal evaporation on the surface of the samples under study. The operating pressure in the microscope chamber was 6 × 10–4 Pa. The measurements were carried out in high resolution mode with an accelerating voltage of 15 kV, a minimum probing current from 15 to 50 pA, and a minimum working distance of 5–15 mm.
RESULTS AND DISCUSSION
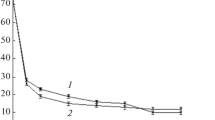
Figure 1 shows the experimental curves for the dependence of contact angle θw for the PET films modified at the (1) anode and (2) cathode on the treatment time (t) at a discharge current of 50 mA and a working gas (air) pressure of ~10 Pa. These values of the discharge current and pressure in the reaction chamber were chosen as optimal on the basis of the results of preliminary experiments to ensure obtaining the maximum experimental θ values. It can be seen that the main changes are observed in the case of treatment for 40–45 s: θw decreases rapidly with the increasing treatment time and reaches a plateau value after 50 s. Note that the difference in θw value for the films modified at the anode and the cathode is small and is observed mainly for short treatment times. Similar relationships were obtained for wetting the surface of the modified samples with glycerol (θg). Table 1 lists the θw and θg values for the initial PET film and the films treated at the anode and cathode by the discharge (50 mA, 10 Pa, 50 s). The calculated values of total surface energy (γ) and its polar (γp) and dispersion (γd) terms for the initial and anode- and cathode-modified films are presented, as well as similar data for discharge-treated samples after their 14-day storage in air under ambient conditions. It can be seen that the modification of the films leads to a significant decrease in θ and a significant surface hydrophilization, which is retained for a long time. The value of γ immediately after plasma treatment increases by a factor of ~2.7 times, and γp, by a factor of ~4.5. After storage for 14 days, the values of θ increase, but the modified films remain hydrophilic (θ < 60°) [19].
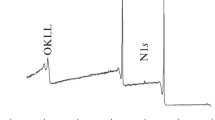
The chemical structure of the initial and modified PET films was studied using the XPS technique. Figure 2a depicts C1s spectrum of the original film. The spectrum consists of three main components: the first at a binding energy of 284.8 eV (aromatic ring carbon atoms), the second at E = 286.5 eV (C–O group carbon atoms), and the third at E = 288.9 eV (O=C–O group carbon atoms). The ratio of their intensities is 63 : 20 : 17%. In addition, there is a small wide peak at 290–292 eV due to the π–π* transition in the aromatic ring of the polymer. The spectrum and the ratio of the peaks agree with the relevant data known from the literature [17, 20]. The O1s spectrum of the original film (Fig. 3a) is represented by two peaks; the first corresponds to E = 531.6 eV (O=C–O), and the second, to E = 533.2 eV (C–O).
For the PET film sample modified at the cathode, the positions of the peaks in the C1s (Fig. 2b) and O1s (Fig. 3b) spectra do not change, but their appreciable broadening is observed, and the peak corresponding to the π–π* transition in the polymer aromatic ring shifts to lower energies. The spectrum shows an increase in intensity of the second peak, indicating an increase in the amount of oxygen-containing C–O groups in the polymer. The intensity ratio of the peaks in the C1s spectrum also changes, it makes 56 : 26 : 18%. Similar changes are observed in the O1s spectrum, namely, the intensity of the second peak due to C–O groups increases noticeably (Fig. 3b).
For the sample modified at the anode, the positions of the three peaks in the C1s spectrum are also unchanged, but they are even more broadened, and the peak corresponding to the π–π* transition in the aromatic ring of the polymer disappears (Fig. 2c). An increase in the first peak (C–C bonds) and a significant decrease in the third one are observed, and the ratio of peaks is 65 : 22 : 13%. A possible explanation for this pattern of the spectrum is the etching of the film during the discharge process, followed by the deposition of products on its surface. Probably, the amount of ether groups included in the polymer decreases and the amount of C–O groups corresponding to the oxidation of deposited products increases in this process. This assumption is confirmed by the shape of the O1s spectrum: the intensity of the peak at 531.6 eV (O=C–O) decreases, while the peak at 533.2 eV (C–O) is noticeably broadened, and its area increases (Fig. 3c). As a result, despite the similarity in elemental composition between such a film and the original one, the chemical structure of its surface is significantly different.
Thus, XPS data indicate that a significant amount of new oxygen-containing groups is formed on the surface of the PET films modified at the cathode or anode of dc discharge.
Note that an insignificant concentration (<2%) of nitrogen and aluminum was found on the surface of the modified PET samples. The presence of nitrogen, presumably in the form of nitrogen-containing functionalities such as N–COO or OC–N–CO, has been observed in a number of studies dealing with the process of PET modification by air discharges of various types, and it is due to the activity of nitrogen ions formed in air plasma [11, 21, 22]. The presence of aluminum, apparently, should be attributed to the formation of its compounds with oxygen [18] when the aluminum electrode is sputtered due to positive ion bombardment. Similar results were observed in our earlier study [23] of the process of polyester sulfone films modification by dc discharge.
The surface morphology of the films was studied using atomic force microscopy (AFM) and scanning electron microscopy (SEM).
Figure 4 depicts a three-dimensional AFM image of the surface of the (a) original PET film and films modified by dc discharge at (b) the cathode and (c) the anode at I = 50 mA for 50 s. Calculations of the average (Ra) and rms roughness (Rms) values showed that they are 0.51 and 0.89 nm, respectively, for the untreated film. After plasma processing, the film roughness increased in both cases: to Ra = 1.21 and Rms = 1.72 nm at the cathode and to Ra = 0.89 and Rms = 1.25 nm at the anode. Figure 5 presents SEM images for (a) the initial films and the films modified at (b) the cathode and (c) the anode. They show that the effect of plasma in both cases leads to an increase in the roughness of the samples in comparison with the initial ones. Note that the nature of change in roughness differs between the cathode and anode cases. After treatment of the films at the anode, the values of Ra and Rms are smaller than those after the cathode treatment, but bands appear that could have formed during orientation stretching of PET films and become visible as a result of surface etching during plasma treatment. It is possible that the better retention of the contact properties of the films modified at the anode is associated with the morphology features.
CONCLUSIONS
Based on the above results, it can be concluded that the modification of PET films by dc discharge treatment at the anode and the cathode results in significant surface hydrophilization, which is retained for a long time. This effect is explained by substantial changes in the chemical structure and morphology of the surface of the films investigated using the XPS, AFM, and SEM techniques. In both the cathode and anode cases, an increase in the number of oxygen-containing groups on the surface of the modified films is observed. However, the chemical structure of the surface treated at the anode differs, apparently, as a result of the plasma etching of the film followed by the deposition of products on its surface. According to the AFM and SEM data, the surface roughness of the films noticeably increases. Chemical and morphological changes in the surface layer are associated with the radiation dose absorbed in the plasma and localized in a thin near-surface layer of the polymer [24].
REFERENCES
Pak, V.M. and Trubachev, S.G., Novye materialy i sistemy izolyatsii vysokovol’tnykh elektricheskikh mashin (Novel Insulation Materials and Systems for High-Voltage Electrical Machines), Moscow: Energoatomizdat, 2007.
Drachev, A.I., Pak, V.M., Gilman, A.B., and Kuznetsov, A.A., Elektrotekhnika, 2002, no. 4, p. 19.
Drachev, A.I., Pak, V.M., Gilman, A.B., and Kuznetsov, A.A., Elektrotekhnika, 2003, no. 4, p. 35.
Endo, T., Reddy, L., Nishikawa, H., Kaneko, S., Nakamura, Y., and Endo, K., Procedia Eng. J, 2017, vol. 171, p. 88.
De Bergalis, M., J. Fluorine Chem., 2004, vol. 125, p. 1255.
Vesel, A. and Mozetic, M., J. Phys. D: Appl. Phys., 2017, vol. 50, no. 29, p. P.112536.
Polymer Surface Modification: Relevance to Adhesion, Mittal, K.L., Ed., Utrecht: VSP, 2009.
Friedrich, J., The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design, Weinheim: Wiley–VCH, 2012.
Pocius, A.V., Adhesion and Adhesive Technology, Munich: Carl Hanser, 2002, 2nd ed.
Deshmukh, R.R. and Bhat, N.V., Mater. Res. Innovat., 2003, vol. 7, no. 5, p. 283.
Al-Maliki, H., Zsidai, L., Samyn, P., Szakal, Z., Keresztes, R., and Kalacska, G., Polym. Eng. Sci., 2017, p. 1002.
Rezaei, F., Dickey, M.D., Bourham, M., and Hauser, P.J., Surf. Coat. Technol., 2017, vol. 309, p. 371.
Drachev, A.I., Gilman, A.B., Pak, V.M., and Kuznetsov, A.A., High Energy Chem., 2002, vol. 36, no. 2, p. 116.
Drachev, A.I., Gilman, A.B., Pak, V.M., and Kuznetsov, A.A., High Energy Chem., 2006, vol. 40, no. 6, p. 417.
Demina, T.S., Drozdova, M.G., Yablokov, M.Y., Gaidar, A.I., Gilman, A.B., Zaytseva-Zotova, D.S., Markvicheva, E.A., Akopova, T.A., and Zelenetskii, A.N., Plasma Process. Polym., 2015, vol. 12, no. 8, p. P. 710.
Wu, S., Polymer Interfaces and Adhesion, New York: Marcel Dekker, 1982, p. 152.
Beamson, G. and Briggs, D., High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, Chichester: Wiley, 1992.
Briggs, D., Surface Analysis of Polymers by XPS and Static SIMS, Cambridge: Cambrige Univ. Press, 1998.
Wade, W.L., Mammone, R.J., and Binder, M., J. Appl. Polym. Sci., 1991, vol. 43, no. 9, p. 1589.
Rezaei, F., Dickey, M.D., Bourham, M., and Hauser, P.J., Surf. Coat. Technol., 2017, vol. 309, p. 371.
Vesel, A., Mozetic, M., and Zalar, A., Vacuum, 2008, vol. 82, p. 248.
Deshmukh, R.R. and Bhat, N.V., Mater. Res. Innovat., 2003, vol. 7, p. 283.
Piskarev, M.S., Gilman, A.B., Ionov, A.M., and Kuznetsov, A.A., High Energy Chem., 2016, vol. 50, no. 2. p. 155.
Yablokov, M.Yu., Sokolov, I.V., Malinovskaya, O.S., Gilman, A.B., and Kuznetsov, A.A., High Energy Chem., 2013, vol. 47, no. 1, p. 32.
ACKNOWLEDGMENTS
The study was supported by the Russian Foundation for Basic Research, project no. 18-32-00901 (XPS, AFM, and SEM studies).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Makhaev
Rights and permissions
About this article
Cite this article
Piskarev, M.S., Gilman, A.B., Gatin, A.K. et al. The Effect of Modification by Direct-Current Discharge on the Surface Properties, Chemical Structure, and Morphology of Poly(ethylene terephtalate) Films. High Energy Chem 53, 76–81 (2019). https://doi.org/10.1134/S0018143919010119
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0018143919010119