Abstract—
The data on the content of the 14C cosmogenic isotope in the natural archives make it possible to study the solar activity (SA) in the past centuries and millennia. However, the 14C content in the natural archives is influenced not only by the intensity of incoming galactic cosmic rays (GCRs), which are modulated by the interplanetary magnetic field and vary according to SA variations; changes in the geomagnetic field and in the Earth’s climate also affect radiocarbon data. In particular, climate variation leads to the redistribution of radiocarbon between natural reservoirs. In the 19th century, increased anthropogenic activity was also reflected in radiocarbon data. This article presents the results of the reconstruction of the rate of 14C production under the influence of GCRs with allowance for the above factors. It is shown that the rate of radiocarbon release from the deep ocean into the surface layer and the atmosphere has increased since the second quarter of the 19th century. Apparently, this process is natural.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 INTRODUCTION
Radiocarbon dating method is one of the most informative methods for detailed studies of a number of natural processes on time scales covering last tens of thousands of years, because it can be used to determine the exact time scale of the considered events (Lingenfelter and Ramaty, 1970). Tree rings accumulate radiocarbon after its production in the Earth’s atmosphere due to cosmic rays and the drift in the global cycle, together with the ordinary carbon within CO2. The age of trees is determined within an accuracy of one year with dendrochronological methods. Therefore, tree rings provide the most accurate time scale for the studied natural events. The use of radiocarbon to solve environmental problems related to greenhouse gas emissions can hardly be overestimated. Radiocarbon data currently provide critical information on global natural processes associated with solar activity and climate change (e.g., Konstantinov and Kocharov, 1965; Sonett and Suess, 1984; Wigley and Kelly, 1990; Muscheler et al., 2007; Dergachev, 2016 et al.). Detailed information on climate-change patterns and the processes affecting climate change, as well as the trend in temperature variability over the last millennia, are needed in order to determine the end of the modern interglacial period which began about 11 000 years ago, and, therefore, to predict, roughly, the onset of the subsequent ice age (IA), because the duration of interglacial periods is usually estimated at 11 000–12 000 years.
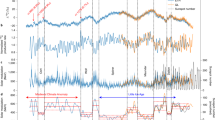
Kudryavtsev et al. (2016) and Kuleshova et al. (2018a, b) reconstructed the rate of 14C production in the Earth’s atmosphere, the heliospheric modulating potential (HMP), and the Wolf numbers from the beginning of the second millennium A.D. to the middle of the 19th century with allowance for climate change; the latter was a key point in our work. It is known that the global minima of solar activity (SA) were observed during this period: the Spörer (≈1400–1510), Maunder (≈1645–1715), and Dalton (≈1790–1830) minima. There were changes in the Earth’s climate in the second millennium A.D. There was the Little Ice Age (LIA), during which the global temperature and CO2 concentration in the atmosphere changed (Fig. 1). Both the annual data on ring-width changes and the 10-year resolution data on lake and ocean sediments, as well as multiproxy data from the pioneering work of Crowley and Lowery (2000), were used to reconstruct the global temperature (Moberg et al., 2005). Historical data on the CO2 concentrations were also obtained from Antarctic ice cores (Etheridge et al., 1998).
It was shown (Kuleshova et al., 2015, Kudryavtsev et al., 2016) that an accounting for climate changes affects the results of the reconstruction of the rate of 14C production in the atmosphere and, consequently, the HMP and Wolf numbers. In the cited works, it was assumed that the decrease in the atmospheric CO2 concentration is caused by the increased absorption of this gas by the ocean during the temperature decrease of the latter. The changes in the radiocarbon transfer rate from the upper (active) ocean layer to the atmosphere during global temperature changes (Craig, 1957) were taken into account. As a result, it unexpectedly turned out that the longer Maunder minimum could be comparable in depth with the Dalton minimum. It should be noted here that the Maunder minimum was lower than the Dalton minimum when the change in global temperature during the LIA interval disregarded (e.g., Ogurtsov, 2018).
2 RATE OF 14C PRODUCTION IN THE EARTH’S ATMOSPHERE
In this article we focus on the time interval between the end of the 18th century and the middle of the 20th century. It is known that the radiocarbon data in the 19th century begin to reflect not only variation in cosmic rays (CRs), solar activity and climate change but also anthropogenic activity. As a result of the combustion of coal, oil, and natural gas, carbon dioxide free from 14C is released into the atmosphere. That is, there is a decrease in the relative 14C content (Δ14C) (Fig. 2a), which is known as the Suess effect, because Δ14C describes the ratio of the concentrations of 14C and 12C isotopes, which changes under anthropogenic impact.
According to the definition of Δ14C, the variation in the atmospheric 14C concentration Na(t) can be expressed as (e.g., Kudryavtsev et al., 2016; Kuleshova et al., 2018a):
where Δ14C is the relative 14C content in the atmosphere as a percent and t0 is an arbitrary time point.
Figure 2b shows the variation Na(t) calculated according to expression (1) from 1010 AD based on data on the CO2 content in the Earth’s atmosphere obtained from ice cores at Law Dome station in Antarctica (Etheridge et al., 1998). The figure shows that the local 14C maxima at 1330, 1530, 1715, and 1830, are SA minima: the Wolf, Spörer, Maunder and Dalton minima, respectively. Note that after 1840 the concentration of 14C in the atmosphere starts to increase regularly (see also Roth and Joos, 2013). It is important to note that this increase occurs synchronously with the increase in the CO2 concentration. The global temperature is also increasing during this period. How can this be explained? Obviously, CO2, which contains 14C isotopes from other natural reservoirs, entered the atmosphere. The deep ocean, in which most of the Earth’s 14C is stored, can be such a reservoir.
Let us reconstruct the rate of 14C production based on the five-reservoir model of the carbon exchange system. As in the earlier works (Koudriavtsev et al., 2014; Kuleshova et al., 2015), we will use for the 14C transfer rate from the upper ocean to the atmosphere λmOa the expression λmOa = (1 + k1ΔT)\(\lambda _{{mOa}}^{0}\), where k1 is the temperature coefficient and ΔT are anomalies of the global surface temperature. For the radiocarbon transfer rate from the deep ocean to the upper ocean, we will use the expression λdOmO = (1 + k(ΔT – ΔT(t *))\(\lambda _{{dOmO}}^{0}\), where the temperature coefficient is k = 0 for t < t * and k = k2 for t ≥ t *. Therefore, let us assume that climate change did not affect the deep ocean until some threshold time t *, but the temperature of the deep ocean started to increase at t * and CO2 began to be released from the deep ocean.
Here it should be noted that we use variations in surface air temperature rather than ocean water temperature in expressions for λmOa and λdOmO for the following reasons. Shevenel et al. (2011) reconstructed the surface ocean (SO) temperature near Antarctica over the past 12 000 years. The variations in this temperature coincided with global climate variations and reached several degrees of Celsius. In particular, the SO near Antarctica could drop dramatically, by 2–3°C, during the LIA in the middle of the second millennium A.D. Unfortunately, the temporal resolution and accuracy of reconstructions of the ocean-water temperature do not allow us to use these data for our calculations. Thus, we assume in the calculations that the variation in the ocean water temperature is proportional to the variation in the global surface temperature.
The temperature reconstructions used in the calculations were averaged over 30 years, which corresponds to the lower time threshold of climate change (Monin and Shishkov, 2000). In Figure 3a curve 1 presents the results of the reconstruction of the radiocarbon production rate Q(t) obtained with using the temperature series after Crowley and Lowery (2000) for k1 = 0.1 K–1and k2 = 0. The selection of the value of the coefficient k1 was described earlier in more detail (Koudriavtsev et al., 2014; Kuleshova et al., 2015). We will briefly address this problem. Koudriavtsev et al. (2014) showed that the value of the temperature coefficient k1 should be ca 0.1 K–1 to reconcile the reduction of the CO2 concentration during the LIA (Fig. 1b) (Etheridge et al., 1998), the Δ14C variation (Fig. 1a) and the 10Be content in Greenland ice (Berggren et al., 2009). The value of this coefficient can be explained as follows. According to the previous works (e.g. Byutner, 1986; Malinin and Obraztsova, 2011; Takahashi et al., 1993, 2009), the total CO2 flux through the ocean surface is proportional to the difference in partial pressures in the surface water and in the atmosphere, and an increase in the SO water temperature by one degree leads to an increase in the partial pressure of CO2 dissolved in water by about 4%. As noted above, when the SO temperature changed by (2–3)°C during the LIA, the change in CO2 flux through the ocean surface can change by 10%. Conversely, reconstructions of the global surface air temperature during this period show a change of up to ≈1 degree. In our calculations, we consider synchronous changes in these temperatures, taking into account the results obtained by Shevenel et al. (2011). Therefore, by associating the variation in λmOa not with water-temperature variation but with air-temperature variation, k1 should take a value up to ≈0.1 K–1. In this case, if the air temperature changes by ≈1 degree, the value of λmOa will also change by ≈10%. The calculation of Q(t) for k1 = 0.1 K–1 and k2 = 0 corresponds to the case in which only the change in the radiocarbon transfer rate from the upper ocean to the atmosphere during the LIA is taken into account; it was studied in detail by Kudryavtsev et al. (2016). We will now focus on the interval since the early 19th century. As can be seen from Fig. 3a (curve 1), the reconstructed values of Q(t) in this case (i.e., for k1 = 0.1 K–1 and k2 = 0) increase significantly after 1850 . This rise cannot be explained without an additional influx of radiocarbon into the atmosphere. As mentioned above, the deep ocean could be the source of this radiocarbon. Curve 2 shows the reconstructed values of Q(t) for k1 = k2 = 0.1 K–1, which describes an increase in the carbon-transfer rate not only from the upper ocean to the atmosphere but also from the deep ocean to the surface if the global temperature rises. Then, the calculated values of Q(t) in the first half of the 20th century fall below 2. Therefore, the reconstructed values of Q(t) at the end of the 19th century–the first half of the 20th century can coincide with conventional values (not exceeding 2 atoms/(cm2 s) (e.g., Roth and Joos, 2013) and not exceed values during the global minima of solar activity only if to assume a systematic increase in the radiocarbon transfer rate from the deep ocean in the corresponding period of time.
A similar situation occurs with the use of the temperature reconstruction by Moberg et al. (2005). Therefore, it can be concluded that changes in the deep ocean are beginning to manifest themselves in radiocarbon data as early as the second quarter of the 19th century.
The following conclusion can be drawn from this study. Radiocarbon data describe not only the variation in solar and anthropogenic activity in the Earth’s atmosphere and the surface ocean. At least since the second quarter of the 19th century, changes in the deep ocean have begun to manifest themselves, leading to a redistribution of carbon between the deep ocean and the atmosphere as the global temperature rises. This was not considered in earlier studies.
This increase in the rate of CO2 release from the deep ocean into the atmosphere (≈1825 AD), which is early relative to the industrial development of civilization, indicates that this effect is likely to be natural rather than anthropogenic.
It should be noted here that the CO2 content has increased repeatedly in the Earth’s climate history (mainly in the interglacial intervals). Changes in the CO2 content in the Earth’s atmosphere over the past 400 000 years were presented by Petit et al. (1999). The previous sharp increase in the CO2 concentration (over 280 ppm) occurred about ≈130 000 years ago. It was followed by a sharp decrease in global glaciation. This increase in atmospheric CO2 content also occurred in prior interglacial periods. The maximum of the last glaciation occurred about 20 000 years ago, and the CO2 concentration dropped to ≈185 ppm. As the glaciers melted (glaciation retreat), there was an increase in the CO2 concentration in the Earth’s atmosphere. Figure 4 shows the cyclical change in the CO2 concentration over the past 800 000 years (Bereiter et al., 2015). It should be noted that the time step (i.e., averaging) in the periods of the maximum CO2 concentrations (interglacial periods) in the past is hundreds of years, which makes it impossible to trace the annual (decadal) changes in CO2, while the systematic, modern increase in CO2 began in the middle of the 19th century.
3 CONCLUSIONS
In conclusion, let us note the following.
(1) The increase in the 14C concentration in the Earth’s atmosphere that began in the first half of the 19th century indicates that the beginning of the increase in the CO2 concentration at that time was not associated with the burning of fossil fuels. The source of this CO2 may be its accelerated release from the ocean to the atmosphere. At the same time, this oceanic CO2 contains radiocarbon. The subsequent increase in the 14C content in the Earth’s atmosphere, which is synchronous with the increase in the CO2 concentration, indicates that the increase in the CO2 concentration is caused not only by fuel-combustion products but also by an influx from the ocean.
(2) If we don’t consider increase in the rate of CO2 (and 14С) transfer from the deep ocean to the Earth’s atmosphere throughout 19–20 centuries, the reconstructed rate of 14С production becomes too high, exceeding even the values obtained for the Maunder minimum. In order for the reconstructed radiocarbon production rate in the first half of the 20th century to coincide with conventional values (not exceeding 2 atoms/(cm2 s), e.g., Roth and Joos, 2013) and not exceed values during global solar minima, it is necessary to assume a systematic increase in the radiocarbon transfer rate from the deep ocean to the atmosphere at corresponding time periods.
(3) In the history of the Earth’s climate, there were periods of extreme (sharp) increases of CO2 in the Earth’s atmosphere. These periods were followed by periods of decrease. The most recent period of such a low atmospheric CO2 concentration was observed during the last global glaciation.
The noted factors indicate that at least one of the reasons for the increase in the CO2 concentration in the Earth’s atmosphere since the 19th century is the CO2 release into the atmosphere from the deep ocean.
REFERENCES
Bereiter, B., Eggleston, S., Schmitt, J., Nehrbass-Ahles, C., Stocker, T.F., Fischer, H., Kipfstuhl, S., and Chappellaz, J., Revision of the EPICA Dome C CO2 record from 800 to 600 kyr before present, Geophys. Res. Lett., 2015, vol. 42, pp. 542–549. ftp://ftp.ncdc.noaa.gov/ pub/data/paleo/icecore/antarctica/antarctica2015co2composite.txt.
Berggren, A.-M., Beer, J., Possnert, G., Aldahan, A., Kubik, P., Christl, M., Johnsen, S.J., Abreu, J., and Vinther, B.M., A 600- year annual 10Be record from the NGRIP ice core, greenland, Geophys. Res. Lett., 2009, vol. 36, p. L11801.
Byutner, E.K., Planetarnyi gazoobmen O 2 i CO 2 (Planetary Gas Exchange of O2 and CO2), Leningrad: Gidrometeoizdat, 1986.
Craig, H., The natural distribution of radiocarbon and the exchange time of carbon oxide between atmosphere and see, Tellus, 1957, vol. 9, pp. 1–17.
Crowley, T.J. and Lowery, T.S., How warm was medieval warm period?, Ambio, 2000, vol. 29, pp. 51–54.
Dergachev, V.A., The impact of solar radiation and solar activity on climate variability after the end of the last glaciation, Geomagn. Aeron. (Engl. Transl.), 2016, vol. 56, no. 7, pp. 908–913.
Etheridge, D.M., Steele, L.P., Langenfelds, R.L., Francey, R.J., Barnola, J.-M.I., and Morgan, V.I., Historical CO2 record derived from a spline fit (75 year cutoff) of the Law Dome DSS, DE08, and DE08-2 ice cores. http://cdiac. ornl.gov/ftp/trends/co2/lawdome.smoothed.yr75.
Konstantinov, B.P. and Kocharov, G.E., Astrophysical phenomena and radiocarbon, Dokl. Akad. Nauk SSSR, 1965, vol. 165, pp. 63–64.
Koudriavtsev, I.V., Dergachev, V.A., Nagovitsyn, Yu.A., Ogurtsov, M.G., and Jungner, H., On the influence of climatic factors on the ratio between the cosmogenic isotope 14C and total carbon in the atmosphere in the past, Geochronometria, 2014, no. 3, pp. 216–222.
Kudryavtsev, I.V., Dergachev, V.A., Kuleshova, A.I., Nagovitsyn, Yu.A., and Ogurtsov, M.G., Reconstruction of the heliospheric modulation potential and Wolf numbers based on the content of the 14C isotope in tree rings during the Maunder and Spörer minimums, Geomagn. Aeron. (Engl. Transl.), 2016, vol. 56, no. 8, pp. 998–1005.
Kuleshova, A.I., Dergachev, V.A., Kudryavtsev, I.V., Nagovitsyn, Yu.A., and Ogurtsov, M.G., Possible influence of climate factors on the reconstruction of the cosmogenic isotope 14C production rate in the earth’s atmosphere and solar activity in past epochs, Geomagn. Aeron. (Engl. Transl.), 2015, vol. 55, no. 8, pp. 1071–1075.
Kuleshova, A.I., Dergachev, V.A., Kudryavtsev, I.V., Nagovitsyn, Yu.A., and Ogurtsov, M.G., Heliospheric modulation potential reconstructed by means of radiocarbon data from the beginning of 11th century ad till the middle of the 19th century ad, J. Phys.: Conf. Ser., 2018a, vol. 1038, no. 1, id 012005.
Kuleshova, A.I., Dergachev, V.A., Kudryavtsev, I.V., Nagovitsyn, Yu.A., and Ogurtsov, M.G., Reconstruction of the Wolf numbers based on radiocarbon data from the early 11th century until the middle of the 19th century with respect to climate changes, Geomagn. Aeron. (Engl. Transl.), 2018b, vol. 58, no. 8, pp. 1097–1102.
Lingenfelter, R.E. and Ramaty, R., Astrophysical and geophysical variations in 14C production, Proc. of 12 Nobel Symposium “Radiocarbon Variations and Absolute Chronology”, Olson, I.U., Ed., Stockholm: Almqvist and Wiksell, 1970, pp. 513–537.
Malinin, V.N. and Obraztsova, A.A., Variability of exchange by carbon dioxide in the ocean–atmosphere system, Obshchestvo. Sreda. Razvitie, 2011, no. 4, pp. 220–226.
Moberg, A., Sonechkin, D.M., Holmgren, K., Datsenko, N.M., and Karlen, W., Highly variable Northern Hemisphere temperatures reconstructed from low- and high-resolution proxy data, Nature, 2005, vol. 433, pp. 613–617.
Monin, A.S. and Shishkov, Yu.A., Climate as a problem of physics, Phys.-Usp., 2000, vol. 43, no. 4, pp. 381–406.
Muscheler, R., Joos, F., Beer, J., Muller, S.A., Vonmoos, M., and Snowball, I., Solar activity during the last 1000 yr inferred from radionuclide records, Quat. Sci. Rev., 2007, vol. 26, pp. 82–97.
Ogurtsov, M.G., Solar activity during the Maunder Minimum: Comparison with the Dalton Minimum, Astron. Lett., 2018, vol. 44, no. 4, pp. 278–288.
Petit, J.R., Jouzel, J., Raynaud, D., et al., Climate and atmospheric history of the past 420000 years from the Vostok ice core, Antarctica, Nature, 1999, vol. 399, no. 6735, pp. 429–436.
Reimer, P.J., Baillie, M.G.L., Bard, E., et al., IntCal09 and Marine09 radiocarbon age calibration curves, 0–50 000 years cal BP, Radiocarbon, 2009, vol. 51, no. 4, pp. 1111–1150.
Roth, R. and Joos, F., A reconstruction of radiocarbon production and total solar irradiance from the Holocene 14C and CO2 records: Implications of data and model uncertainties, Clim. Past, 2013, vol. 9, pp. 1879–1909.
Shevenell, A.E., Ingalls, A.E., Domack, E.W., and Kelly, C., Holocene Southern Ocean surface temperature variability west of the Antarctic Peninsula, Nature, 2011, vol. 470, pp. 250–254.
Sonett, C.P. and Suess, H.E., Correlation of bristlecone pine ring widths with atmospheric 14C variations: A climate–Sun relation, Nature, 1984, vol. 307, pp. 141–143.
Takahashi, T., Olafson, J., Goddard, J.G., Chipman, D.W., and Sutherland, S.C., Seasonal variation of CO2 and nutrients in the high-latitude surface oceans: A comparative study, Global Biogeochem. Cycles, 1993, vol. 7, no. 4, pp. 843–878.
Takahashi, T., Sutherland, S.C., Wanninkhof, R., et al., Climatological mean and decadal change in surface ocean pCO2, and net sea–air CO2 flux over the global oceans, Deep Sea Res., Part II, 2009, vol. 56, nos. 8–10, pp. 554–577.
Wigley, T.M.L. and Kelly, P.M., Holocene climate change, 14C wiggles and variations in solar radiance, Philos. Trans. R. Soc., A, 1990, vol. 330, pp. 547–560.
Funding
This work was supported in part by the Russian Foundation for Basic Research, project nos. 18-02-00583 and 19-02-00088. Yu.A. Nagovitsyn thanks the Ministry of Science and Higher Education of the Russian Federation (grant no. 075-15-2020-780) also.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by O. Pismenov
Rights and permissions
About this article
Cite this article
Larionova, A.I., Dergachev, V.A., Kudryavtsev, I.V. et al. Radiocarbon Data from the Late 18th Century as a Reflection of Solar Activity Variation, Natural Climate Change, and Anthropogenic Activity. Geomagn. Aeron. 60, 840–845 (2020). https://doi.org/10.1134/S0016793220070166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016793220070166