Abstract
Data obtained on the distribution patterns of biomarker hydrocarbons (n-alkanes, isoprenanes, steranes, and terpanes) in oils, organic matter in rocks, prokaryotes, and thermolysis products of the insoluble part of prokaryotic biomass indicate that the relatively high concentration of diasteranes (rearranged steranes) in the organic matter of rocks and in oils generated by clay strata is caused by characteristics of the original organic matter but not by the process of isomerization of regular steranes, as has been previously thought.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Hydrocarbons of the oil series are a source of valuable information for resolving many problems of oil and gas exploration and reservoir geochemistry (Waples and Machihara, 1990, 1991; Peters et al., 2005; Gordadze, 2015). Repeatedly identified patterns in the distribution of hydrocarbons, first of foremost, hydrocarbon biomarkers provide an insight into the degree of organic matter (OM) or oil transformation, its age, lithological-facies conditions of its origin, the degree of its biodegradation, migration, etc.
One of the principal concepts of oil geochemistry is the maturity of OM (oil), which is used to draw correlations in systems oil–oil, oil–OM in rocks, etc. Biomarkers themselves and, particularly, their structures are the most sensitive indicators of dia- and catagenetic changes (maturation) of organic matter. It is currently believed that the characteristics of the distribution of sterioisomers of polycyclic HC biomarkers is the most informative when OM maturity has to be evaluated. It is known that ratios of HC concentrations in the original OM are principally different from the equilibrium ones. As OM matures, these ratios approach their equilibrium values (Petrov, 1984; Waples and Machihara, 1990, 1991; Peters et al., 2005; Gordadze, 2015; Peters and Moldowan, 2017). Ratios of concentrations of stereoisomers (enantiomers and epimers) usually reach thermodynamically equilibrium values much more rapidly than geometric isomers do and even more so than structural isomers, because the latter situation is characterized by the opening of C–C bonds, which is a process requiring much more energy (Petrov, 1971, 1984; Waples and Machihara, 1991). For example, it is known that equilibrium ratios are first reached by homohopanes of the composition C31–C35, which are characterized by fast epimerization reactions of the C22 chiral center in the aliphatic part of the molecule (C32 maturity coefficient). The maximum value of this coefficient C32 = 22S/(22S + 22R) is 60% (Petrov, 1984; Peters et al., 2005; Gordadze, 2015; Peters and Moldowan, 2017). This parameters is convenient to apply at initial catagenetic stages.
After the epimers of C31–C35 hopanes reach equilibrium ratios, equilibria ratios are reached by the epimeric forms (20R and 20S) of αα steranes C27–C29. As is well know, the high stereospecificity of biosynthesis results in that the structure of the original biosteranes is 5α(Н), 14α(Н), 17α(Н), and 20R, but an progress in the maturation is associated with an increase in the fraction of the 20S epimer because the 20R molecules change their configuration (Fig. 1). The ratio of these epimers is described by the coefficient \(K_{{{\text{mat}}}}^{{\text{1}}}\) = αЅ/(αS + αR), which can reach a maximum value of 0.55. After this, the original ααα20R and ααα20S steranes are transformed into αββ-isosteranes 20R and 20S. This process is described by the maturation coefficient \(K_{{{\text{mat}}}}^{{\text{2}}}\) = αββ/(αββ + αR), which reaches a maximum value of 0.78 (Seifert and Moldowan, 1981; Petrov, 1984; Peters et al., 2005; Gordadze, 2015).
Transformation of 20R and 20S steranes (epimers), 5α(Н),14α(H),17α(Н), and 5α(Н),14β(H),17β(Н) steranes (diastereomers). Dashed lines indicate the orientation of the hydrogen atom away from the observer relative to the page plane, and triangles show an orientation toward the observer relative to the page plane.
Another important geochemical parameter that reflects the lithological-facies conditions under which OM is formed is the ratio of the regrouped steranes (diasteranes) to the regular ones (the dia/reg parameter). It has been thought until recently that regular steranes are structurally isomerized in nature into diasteranes under the effect of acid catalysts, such as clay rocks (Fig. 2) (Rubinstein et al., 1975; Connan et al., 1986; Van Kaam-Peters et al., 1998; Waples and Machihara, 1990, 1991). It is thus believed that the dia/reg ratio > 0.3 corresponds to sedimentation in clay sequences, whereas this ratio <0.2 corresponds to sedimentation in carbonate rocks, which do not act as catalysts. However, it should be pointed out that this can hardly explain why diasteranes occur (although in smaller amounts) in oils and OM in rocks generated by carbonate units (Gordadze, 2015).
EXPERIMENTAL
This study was carried out on sample of oils and rocks from various oil and gas provinces of different age across Russia, of marine (samples 1–8 in Table 1) and terrestrial (samples 9, 10, and 16 in Table 1) genesis, both mature and immature, and generated under different lithological-facies conditions.
In addition, we have selected individual strains of the bacterium Halomonas titanicae TAT1 (VKM B-3500D), which had been obtained from the Romashinskoe oil field, the bacterium Shewanella putrefaciens M-8m-1, which was obtained from the Condian hydrocarbon accumulation at the Dagan oil field in China, archaea Thermoplasma sp., cyanobacterium Spirulina platensis, relics of cyanobacterial mats (CBM) obtained from the Lower Cambrian reservoir (Osinskii horizon) of eastern Siberia, and procaryotic communities sampled in a spring at the Neftyanaya area of the Uzon caldera.
The biomass of the studied bacteria and prokaryotic communities lyophilize at a temperature of 25°C and pressure of 10 × 10–7 MPa for 1 day. The soluble part of the lyophilize biomass of the studied bacteria was extracted by rectified n-hexane at room temperature in a weighing bottle on a magnetic stirrer until the soluble part was completely removed (zero line in the chromatogram).
The insoluble part of the bacterium and archaea biomass, which can be considered to be an analogue of kerogen, and CBM relics were dried (to get rid of the solvent) and thermolyzed at 330°C for 6 h in sealed borosilicate-glass ampoules.
Hydrocarbons in the oils, OM in rocks, soluble part and thermolysis products of the insoluble part of the procaryote biomass and CBM relics were analyzed by methods of capillary gas–liquid chromatography (GLC) and chromatography–mass spectrometry on a Bruker 430-GC with a flame-ionization detector. The temperature was programmed for the range of 80 to 320°C, with an increase rate of 4°C/min. The carrier gas was hydrogen. Hydrocarbons were separated in capillary columns HP-1 25 m × 0.25 mm × 0.5 μm.
The chromatographic–mass spectroscopic studies in electron ionization regime were conducted on an Agilent 6890N/5975C. All spectra were recorded at an ionization energy of 70 eV and accelerating voltage of 3500 V. The temperature of the ionization chamber was 250°C. The selected ion monitoring (SIM) regime was applied, with the following characteristics of ions recorded: m/z 71 for n-alkanes and isoprenanes, m/z 217 and 218 for steranes, and m/z 191 and 177 for terpanes. The temperature was programmed for the range of 70 to 290°C at an increase rate of 4°C/min. Hydrocarbons were separated in capillary columns with a stationary phase HP-1MS (25 m × 0.25 mm × 0.5 μm). The carrier gas was He.
Compounds were identified by adding the surmised standard compounds to the studied samples, based on literature data, and using the NIST mass-spectrometric library.
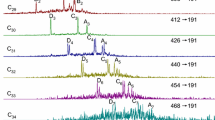
Figures 3 and 4 show, as an illustrating example, the typical mass chromatograms of oil steranes from the Romashinskoe and Untygei fields and the mass chromatograms of the thermolysis products of the insoluble part of the bacteria Halomonas titanicae and Shewanella putrefaciens. Table 1 presents the identification of the peaks of oil steranes marked in the mass chromatograms in Figs. 3 and 4.
RESULTS AND DISCUSSION
Table 2 presents our data on numerous samples of oils and OM of rocks from various oil and gas provinces across Russia. These data display inconsistencies between the values of the aforementioned geochemical parameters. For example, low sterane parameters of maturity (i.e., ratios of sterane epimers significantly different from equilibrium values, with the equilibrium values being \(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.54, \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.84) are fairly often associated with high ratios of diasterane to regular steranes (samples 9–11, 13, 14, and 16 in Table 1), although, as has been pointed out above, epimerization proceeds faster than structural isomerization (Petrov, 1971, 1984; Waples, 1991).
As illustrative examples, Fig. 5 displays the aforementioned parameters of oils from the Anastasievsko–Troitskoe (horizon IV), Salym, and Romashinskoe fields. The oil from the Anastasievsko–Troitskoe field shows a very low C29 sterane maturity parameters: \(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.33 and \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.52, as compared to the equilibrium values of 0.55 and 0.78, respectively. However, it is readily seen that the dia/reg sterane ratio is 0.33. Oil from the Salym field is mature according to the C29 sterane index: \(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.55 and \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.84. This oil is a typical one of the Bazhenovo Formation, which is generated by clay rocks, and its dia/reg = 0.65 and is consistent with classic understanding of mechanisms forming diasteranes in clay rocks. At the same time, oil from the Romashinskoe field has high maturity indexes (\(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.55, \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.86), but its dia/reg is relatively as low as 0.20. It is known that the oil of the Romashinskoe field was generated by carbonate rocks, which do not act as catalysts. Nevertheless, this oil also contains diasteranes, although in lower concentrations.
Table 2 presents geochemical parameters (corresponding to hydrocarbon biomarkers) of OM in rocks of the Western Siberian and Volga–Ural oil and gas provinces. As is evident from this table, a high catagenic maturity according to maturity coefficients, \(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.49–0.53 and \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.77–0.80, is associated in this oil with ratios of diasteranes to regular steranes of 0.06 to 0.52. Therewith, in the situation with clay rocks and low-maturity OM with \(K_{{{\text{mat}}}}^{{\text{1}}}\) = 0.12–0.31 and \(K_{{{\text{mat}}}}^{{\text{2}}}\) = 0.22–0.45, the dia/reg ratio is elevated and varies from 0.33 to 1.02. It should be mentioned that maturity evaluated from data on regular steranes is confirmed by another commonly utilized maturity indicator: the moretane/hopane (M30/H30) ratio (Table 2). As known, the biological 17β(Н), 21β(Н)-configuration of hopanoid compounds is highly instable and is atypical of oils, and ββ-hopanes are readily transformed into βα-moretanes and αβ-hopanes at diagenesis. At catagenesis, relative contents of more labile moretanes decreases more rapidly than those of αβ-hopanes, and hence, the moretane/hopane ratio decreases with increasing thermal maturity from 0.8 in immature OM to >0.15 in mature source rocks and oils and reaches a minimum of 0.05 (MacKenzie et al., 1983; Seifert et al., 1979).
Thus, similar to oils, the OM of the same rock sample may sometimes simultaneously contain proportions of hydrocarbons typical very insignificantly and very significantly mature organic matter. Inasmuch as catalysts accelerate all isomerization reactions, it may hardly be probable that structural isomerization proceeds and stereo isomerization does not (Petrov, 1984).
To test the hypothesis about the role of the original OM in the origin of diasteranes, we have studied the soluble constituent and the thermolysis products of the insoluble components of various prokaryotic organisms. We have previously demonstrated that the soluble parts of prokaryotes and the thermolysis products of the insoluble constituent of their biomass (kerogen) are sources of oil hydrocarbon biomarkers (Gordadze et al., 2018, 2018a; Yusupova et al., 2020, 2021, 2022).
In the situation with prokaryotes, the distribution patterns of regular and regrouped steranes are analogous to those found in oils and the OM of rocks (Table 3). In both the soluble part and the thermolysis products of the insoluble part of the bacteria biomass at a low OM maturity (the maturity coefficients \(K_{{{\text{mat}}}}^{{\text{1}}}\) and \(K_{{{\text{mat}}}}^{{\text{2}}}\) are 0.29–0.43 and 0.59–0.69, respectively), the dia/reg ratio also broadly varies: 0.20–0.58. The ratio of diasteranes to regular steranes also lies within roughly the same range (0.14–0.64) at a high degree of maturity inferred from regular steranes (\(K_{{{\text{mat}}}}^{{\text{1}}}\) and \(K_{{{\text{mat}}}}^{{\text{2}}}\) are 0.45–0.51 and 0.62–0.79, respectively).
We are not aware of any literature data that biologically synthesized matter contains regrouped stertols, but own our results allowed us to conclude that they do occur in this matter. The original OM, which contained elevated contents of diasteroles, was likely related to clay rocks. In other words, elevated values of the dia/reg geochemical indicator, which reflects the lithology of the rocks, was controlled not by the isomerization reaction of steranes into regrouped ones but by living organisms the produced oil hydrocarbons in the clay rocks.
With regard to the aforementioned discrepancies in the distribution patterns of epimers and structural isomers of the composition C27–C29 in oils, the OM of rocks, the soluble part and the thermolysis products of the insoluble constituents of prokaryotes, it is reasonable to suggest that no conditions favorable for the structural isomerization of hydrocarbons occur in the Earth’s interiors. The leading processes proceeding in nature seem to be those of geometric isomerization and thermal cracking of high-molecular compounds, which reflect the maturity of the organic matter.
CONCLUSIONS
The distribution patterns of hydrocarbon biomarkers (n-alkanes, isoprenanes, steranes, and terpanes) in oils, organic matter in rocks, prokaryotes, and the thermolysis products of the insoluble part of the prokaryote biomass lead to the conclusion that the relative contents of diasteranes in oils and organic matter in rocks depend on the original organic matter. With reference to steranes, this means that organic matter or oil generated by bacterial communities associated with clay rocks produce much more steranes that bacterial communities in carbonate rocks.
It is also reasonable to hypothesize that the leading processes occurring when oil hydrocarbons are generated in nature are geometric isomerization and thermal cracking of high-molecular compounds. No structural isomerization of hydrocarbons likely proceeds in the Earth’s interiors.
REFERENCES
J. Connan, J. Bouroullec, D. Dessort, and P. Albrecht, “The microbial input in carbonate–anhydrite facies of a sabkha palaeoenvironment from Guatemala: a molecular approach,” Org. Geochem. 10, 29–50 (1986).
G. N. Gordadze, Hydrocarbons in Oil Geochemistry. Theory and Practice (RGUNiG im. I.M. Gubkina, Moscow, 2015) [in Russian].
G. N. Gordadze, E. B. Grunis, and M. S. Zonn “Role of hydrocarbon study at the molecular level in oil geochemistry,” Geol., Geofiz. Razrab. Neft. Mestorozhd., No. 11, 19–23 (2001).
G. N. Gordadze, A. R. Poshibaeva, M. V. Giruts, A. A. Gayanova, E. M. Semenova, and V. N. Koshelev, “Formation of petroleum hydrocarbons from prokaryote biomass: 2. Formation of petroleum hydrocarbon biomarkers from biomass of Geobacillus jurassicus bacteria isolated from crude oil,” Petrol. Chem. 58 (12), 1005–1012 (2018a).
G. N. Gordadze, A. R. Poshibaeva, M. V. Giruts, A. A. Perevalova, and V. N. Koshelev, “Formation of Petroleum Hydrocarbons from Prokaryote Biomass: 1. Formation of Petroleum Biomarker Hydrocarbons from Thermoplasma sp Archaea Biomass,” 58 (3), 1005–1013(2018b).
A. S. MacKenzie, U. Disko, and J. Rullkotter, “Determination of hydrocarbon distributions in oils and sediment extracts by gas chromatography–high resolution mass spectrometry,” Org. Geochem. 5 (2), 57–63 (1983).
K. E. Peters and J. M. Moldowan, “Biomarker: assessment of thermal maturity, Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth, Ed. by W. M. White, (Springer International Publishing, 2017).
K. E. Peters, C. C. Walters, and J. M. Moldowan, The Biomarker Guide, 2nd Edition. Vol. I. Biomarkers and Isotopes in the Environment and Human History. Vol. II. Biomarkers and Isotopes in Petroleum Exploration and Earth History (Cambridge University Press, 2005).
Al. A. Petrov, Naphthene Chemistry (Nauka, Moscow, 1971) [in Russian].
Al. A. Petrov, Oil Hydrocarbons (Nauka, Moscow, 1984) [in Russian].
I. Rubinstein, O. Sieskind, and P. Albreeht, “Rearranged sterenes in a shale: occurrence and simulated formation,” J. Chem. Soc., Perkin Trans. I, 1833–1836 (1975).
W. K. Seifert and J. M. Moldowan, “Paleoreconstruction by biological markers,” Geochim. Cosmochim. Acta 45, 783–794 (1981).
H. M. E. Van Kaam-Peters, J. Köstter, S. J. Van der Gaast, M. Dekker, J. W. De Leeuw, and Damste J. S. Sinninghe, “The effect of clay minerals on diasterane/sterane ratios,” Geochim. Cosmochim. Acta 62, 2923–2929 (1998).
D. W. Waples and T. Machihara, “Application of sterane and triterpane biomarkers in petroleum exploration,” Bull. Can. Petrol. Geol. 38, 357 (1990).
D. W. Waples and T. Machihara, “Biomarkers for geologist—A practical guide to the application of steranes and triterpanes in petroleum geology,” In AAPG Methods in Exploration Series 9 (American Association of Petroleum Geologists, Tulsa, 1991).
A. A. Yusupova, M. V. Giruts, E. M. Semenova, and G. N. Gordadze, “Formation of petroleum hydrocarbons from prokaryote biomass: 3. Formation of petroleum biomarker hydrocarbons from biomass of Shewanella putrefaciens bacteria and asphaltenes isolated from crude oil,” Petrol. Chem. 60 (11), 1216–1225 (2020).
A. A. Yusupova, M. V. Giruts, and G. N. Gordadze, “Prokaryotes as a source of petroleum hydrocarbons,” Dokl. Earth Sci. 497 (1), 211–216 (2021).
A. A. Yusupova, M. V. Giruts, D. S. Vylekzhanina, E. M. Semenova, and G. N. Gordadze, “Formation of petroleum hydrocarbons from prokaryote biomass: 4. Formation of petroleum biomarker hydrocarbons from the biomass of Halomonas titanicae bacteria Isolated from Romashkino crude oil,” Petrol. Chem. 62 (4), 405–410 (2022).
ACKNOWLEDGMENTS
The authors thank the scientific editor Dr. V.B. Polyakov and the reviewers who highly appreciated the materials presented in the manuscript and rendered invaluable assistance in improving it and making it better understandable to the wide readership of the journal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by E. Kurdyukov
The Editorial Board of Geochemistry International considers the publication of this paper as an invitation to discuss the interesting and important problems of organic geochemistry touched upon in this paper.
Rights and permissions
About this article
Cite this article
Yusupova, A.A., Giruts, M.V., Vasil’eva, A.V. et al. On the Formation of Diasteranes in Oil and Organic Matter of Rocks. Geochem. Int. 61, 703–710 (2023). https://doi.org/10.1134/S0016702923070054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702923070054