Abstract
The geochemical dependences of the migration of natural radionuclides (U and Th) in different species are analyzed for the Semipalatinsk test site using methods of multivariate statistics. The region is distinguished by the wide diversity of geochemical environments and a large number of water bodies. A brief study of water geochemistry of the studied water bodies of the Semipalatinsk test site is reported, and the contents of natural radionuclides and proportions of their forms (suspended, colloidal and dissolved) are established. Differences in the migration of thorium and uranium in water bodies, streams, and minor water streams have been determined. It was found that the Ca/Mg, \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}},\) and Th/U ratios serve as markers of geochemical processes in the studied waters. Non-parametric correlation analysis indicates that the major component ratios are good to use as a geochemical descriptors of thorium and uranium speciation. According to the discriminant analysis data, the suspended and colloid thorium species are indicators of colloidal transport. Factor and cluster analyses have revealed the paragenetic associations of major and trace components related to the uranium and thorium speciation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Aquatic media is the main pathway for the far-field transport of pollutants, including radionuclides. Special attitude in this problem is given to transuranic elements, the half-life of which reaches millions of years. This causes their long residence time in biosphere.
It is widely known that the radionuclide migration in aquatic systems is mainly controlled by their speciations and landscape-geochemical conditions. Mineral exploration and consequences of nuclear technogenesis also increase strongly the migration of natural and anthropogenic radionuclides caused by the change of hydrological and geochemical conditions (Placzek et al., 2016, and others).
Numerous studies (Ilina et al., 2016; Toropov, 2017, 2018; Ure and Davidson, 2002; and others) showed that the determination of element speciation is a difficult issue. A choice of most suitable tools (fractionation protocols of given species, techniques, developing of hybrid methods, and others) will depend on the nature and character of samples and a set-up of measuring elements. The experimental separation of migration species is a problematic procedure and requires high skill, sensitivity of analytical equipment, verification of definite fractionation schemes, and application of essentially different methods of study (Alekhin et al., 2020; Environmental Colloids…, 2007, and others).
A wide but incomplete list of methods for study of the migration species of uranium, thorium, and other elements in natural surface waters is reported in (Markich, 2002, 2019; Environmental Colloids…, 2007). In general, it should be noted that methods based on the size fractionation of element species were/are prevailed.
The main methods involve cascade filtration using a set of membranes, including ultrafiltration, and flow field flow fractionation with external field application, which allows coupling with several detectors (Bouby et al., 2008, 2012; Baalousha et al., 2006, 2011; Cuss et al., 2018, 2020; Wang et al., 2020). Tangential filtration allowing the treatment of large-volume sample is also applied (Lind et al., 2006; Salbu, 2006; Salbu et al., 2018). In addition to filtration-based methods, the migration species are also analyzed using dialysis, gel-filtration and size-exclusive chromatography (Markich, 2002; Markich and Brown 2019) frequently coupled with mass-spectrometric detection of elements in each fraction. In particular, such method was applied by (Itoh et al., 1996) to quantify uranyl complexes with natural dissolved organic matter in fresh surface water.
Other methods of physical fractionation such as ion-exchange chromatography and electrophoresis were used to separate \({\text{UO}}_{2}^{{2 + }}\) and U4+, even in concentrations commonly detected in natural surface waters (Rollin and Eklund, 2000). Capillary zone electrophoretic study was applied by (Pacheсo and Havel, 2001) to identify uranyl complexation with humic acids. Electrophoretic system hyphenated to mass spectrometry made it possible to fractionate the redox species of uranium together with iron and plutonium for concentrations close to those in natural waters (Graser et al., 2015), as well as provided insight into interactions in the colloid–radionuclide system (Claveranne-Lamolère et al., 2011). However, these unique techniques did not receive the wide distribution owing to their high cost and time consumption.
None of the methods provides unambiguous and complete information on the metal migration species. Two or more methods are usually combined or a scheme of species fractionation is applied (Batley, 1989; Markich, 2002; Salbu, 2006).
Thermodynamic calculations also significantly contribute in understanding the mechanisms of formation of different migration species. The problem of applicability of thermodynamic calculations for the migration of radioactive elements in nature is also of great significance. There are several thermodynamic databases such as PRODATA (based on NEA-OECD TDB database), Thermochimie, LLNL, Minteq, PSI/NAGRA, and others (Ragoussi and Costa, 2019; Reiler and Decostes, 2020; vminteq.lwr.kth.se, and others), which are modified or supplemented constantly, for instance, for calcium and magnesium uranyl carbonates (Guillaumont et al., 2003). Modeling of the same water composition using different databases could yield different results (Mahoney, 2008). Correction of ionic strenghts of solutions during modeling taking into account specific complexes is crucial for these calculations (Reiller and Descostes, 2020; Toropov et al., 2020).
Extensive information on definite object analyzed for migration element species does not help in contribute to searching for the main factors of radionuclide transfer. At present, the multivariate statistics is applied not only to exploration geochemistry but also to many other scientific challenges (Rollinson, 2014). However, it is too soon to say that such calculations have become routine and widely used (Radomskaya et al., 2017). The combine use of such methods makes it possible to avoid incorrect interpretation of assayed consistencies/patterns, as well as help in the case of ignoring some statistical tools, if they do not confirm the author’s hypothesis. The absence of clear relations between water geochemistry parameters and radionuclide speciation during analysis of data array encouraged us to apply the methods of multivariate statistics for searching of unrevealed geochemical dependences and interpret it properly.
The aim of the work is to study in detail the influence of geochemical parameters on the uranium and thorium distribution, as well as their speciation in the natural waters of the Semipalatinsk test site (STS).
OBJECTS AND METHODS
The study object is STS in Kazakhstan, which is unique in a combined influence of anthropogenic (nuclear tests, experiments with radioactive matters) and environmental factors. As known, this region is ascribed to the uranium-bearing province and some its localities (for instance, the Karabulak Stream valley) reveal uranium anomalies (Gorlachev et al., 2020; Kayukov, 2008). The chemical composition and content of anthropogenic radionuclides of STS have been studied previously (Govenko et al., 2012; Yessilkanov et al., 2020; Performance of Complex… 2016; Toropov and Panin, 2011; Kazakova, 2005, and others). It was shown that anthropogenic radionuclides (cesium, plutonium, and others) occur in reliably detectable amounts in the surface waters of STS and represent a potential hazard for ecosystems and human, moving beyond the test site (Bakhtin et al., 2017; Subbotin and Dubasov, 2013; Toropov, 2018, 2020; Gorbunova and Subbotin, 2012, and others). Thereby, the migration of natural radionuclides (uranium and thorium) in given aquatic systems with account for their speciation remained poorly studied. In particular, there are only a few data on uranium isotopes in well waters of STS (Vintro et al., 2009).
The wide diversity of water and geochemical environments (topographic features, surface discharge of groundwater, mountains, closed water bodies of different depth) suggests a specific behavior of radionuclides in each definite case. This territory comprises natural waters of different composition: from fresh to saline waters, enriched and poor in dissolved organic matter. Thereby, the uranium and sometimes thorium contents exceed clarke values.
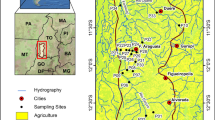
The STS territory is located in northeastern Kazakhstan, in the eastern part of the Kazakh Upland. Samples were taken at the main test fields of the STS (Fig. 1):
—The “Experimental field” site includes collapse funnels filled with water, such as crater lake V-1 (point 1).
—The “Telkem” and “Balapan” are artificial lakes formed by explosions: Telkem-1 and Telkem-2 (points 2–3); “Atomic” Lake, as well as the Chagan River (points 4–5);
The “Degelen” field is confined to the eponymous mountain massif. In this area, we sampled water streams in tunnels (horizontal excavations for nuclear tests (points 6–12)), as well as the Uzynbulak (point 13) and Karabulak (points 14–19) streams. The Degelen Mountains comprise the largest number of water objects and are located in the southern part of STS. The most part of the massif is made up of granites. Water inflow tunnels at the “Degelen” area are restricted to the filtration zone above groundwater level, at 600–700 m asl. Discharge at the entries of these tunnels varies from a few to several hundreds of liters per a minute (Kazakova, 2005; Performance of a Complex… 2016).
Totally 19 samples in were discussed in our study discussed. Water was sampled in compliance with generally accepted methods in pure plastic bags, avoiding entrance of impurities. Size fractionation was carried out in situ by filtration through membranes of 450 nm and 10 kDa (corresponds to ~ 3 nm) with prefiltration through nylon 10 μm mesh filter. The filters and in some cases, colloidal concentrate were stored for further studies. Thus, we distinguished suspended forms (difference between sample after prefiltration and filtration through 450-nm filter), colloid forms (difference between samples filtered through 450 nm and 10 kDa membranes), and dissolved forms (<10 kDa).
The total dissolved solids (TDS), their pH, and redox potential (Eh) of waters were determined using an Anion 4100 laboratory liquid analyzer. Water Eh was measured using silver–chloride reference electrode with subsequent recalculation for standard hydrogen electrode (SHE) at 25°С. The content of major ions in water was determined by titration (\({\text{HCO}}_{3}^{ - },\) \({\text{CO}}_{{\text{3}}}^{{2 - }}\)), Cl–, Ca, Mg, photometry \(\left( {{\text{SO}}_{4}^{{2 - }}} \right),\) optical-emission spectrometry on an iCAP 6300 Duo and Optima8300DV (Na, K, Ca, Mg), as well as by ionic chromatography on a Dionex-2000 (Cl–, \({\text{SO}}_{4}^{{2 - }}\)). The total organic carbon (TOC) was estimated from permanganate and bichromate index (titration and photometry, respectively). Some samples were analyzed using high-temperature catalytic oxidation on a Vario TOC cube, as well as using semiquantitative method: measurement of UV absorption (UV Schimadzu 1800) in the region from 225 to 280 nm, which is indicator for humic substance. The U and Th contents were determined by ICP-MS using Elan-9000, Agilent 7600, NexION 300D, and Thermo Element XR instruments with simultaneous control using standard sample with similar matrix. The proportions of size forms of natural radionuclides were established mainly from difference between their concentrations in filtrate. If element contents in the filtrate were below the detection limits, the proportions of species were determined from their contents on filter after complete acid decomposition with recalculation for water volume passing through the membrane. The work generalizes results on samples collected during 2015–2017, while filters were analyzed in 2018–2020. Analytical studies were carried out at the laboratories of the National Nuclear Center of the Republic of Kazakhstan, National Research Tomsk Polytechnic University, Karlsruhe Institute of Technology, Germany, and Lomonosov Moscow State University by authors or with their participation.
Statistical processing aimed at recognition of the unrevealed geochemical dependences involved parametric and non-parametric correlation analyses (Pearson and Spearmen analyses, respectively), discriminant analysis with direct gradual choice of variables, and cluster analysis were carried out using hierarchical clusterization with complete linkage method (farthest neighbor clustering) through the Euclidean distance, as well as factor analysis (principal component analysis) with matrix normalization to mean-square deviation of each variable in compliance with recommendations (Cho and Choo, 2019; Farnham et al., 2003, and others) and with orthogonal varimax rotation to minimize the number of factors (Lee et al., 2020, and others). Choosing the processing methods, we avoided the duplication of mathematical mechanisms for different types of statistical analysis. Variables with lognormal distribution (in particular, data on natural radionuclides) were implemented in logarithms. Data were processed using Statistica 10 and Excel software.
Modeling of uranium species profile at different concentration of bicarbonate ions in water was carried out using Visual Minteq 3.1 software complex with built-in database of equilibrium constants at temperature of 25°С, ambient pressure of 1 atm, and partial pressure of carbon dioxide. The model was limited in рН, Eh, (\({\text{CO}}_{3}^{{2 - }}\) + \({\text{HCO}}_{3}^{ - }\)), Cl–, \({\text{SO}}_{4}^{{2 - }},\) Ca2+, Mg2+, Na+, K+, Si (H4SiO4), Fe3+, \({\text{UO}}_{2}^{{2 + }}\), and Th4+. Ionic strength of solution and anion and cation balance were calculated automatically. Ion activity was corrected according to the Davis equation with b-parameter = 0.3.
RESULTS AND DISCUSSION
The studied water bodies of STS represent reservoirs (lakes) and streams, which are required to distinguish for understanding the radionuclide migration conditions. Unlike lakes, the rivers, streams, and tunnel outflowing waterstreams are dynamic media where contrast behavior of radioactive elements can occur.
The test fields of STS are disturbed by deep-seated Kalba–Chingiz (Experimetnal field), Western Arkalyk (Telkem), and Chinrau (Balapan) faults, which determined the filtration heterogeneity of rocks. The fault influence zones are characterized by the higher fracturing and crushing zones development, which is unfavorable for the removal of radionuclide-contaminated waters. Nuclear tests significantly increased rock fracturing. While strained along fractures and cavities, waters are filled underground water basin or are discharged on the surface.
The issue of possible anthropogenic uranium input in the studied waters remained beyond the scope of this work. The continuing studies of uranium isotope ratios will make it possible to estimate more reliably estimate the anthropogenic contribution in the migration of natural radionuclides at the given object. However, it is obvious that the natural sources of uranium were predominant.
Brief Geochemistry of Natural Waters of the Semipalatinsk Test Site
The pH values (Table 1) of natural waters of STS varied within a wide range from 5.9 to 8.4, and their redox potential, from 170 to 420 mV. The pH values are neutral in most waters and weakly alkaline in the Telkem-1, Telkem-2, “Atomic” Lake, Karabulak Stream, and tunnels nos. 176, 177, and 609. The Eh values (normalized to standard hydrogen electrode and 25°С) of natural waters of STS are within +150…+450 mV, which is 200–300 mV lower than that of water–atmosphere equilibrium (Aquatic Chemistry, 1995). The studied waters are characterized by the variable redox condition.
According to classification (Ovchinnikov, 1948), waters of tunnel waterstreams and streams of STS are ascribed to fresh waters (TDS up to 1000 mg/L), except for tunnel 504 (1200 mg/L). Waters of V-1 and Telkem 2 are ascribed to the brackish waters (1960 and 8950 mg/L, respectively). Waters from Lake Telkem-1 (20520 mg/L), Lake “Atomic” and Chagan River (12 380 and 13 000 mg/L, respectively) are classed with saline waters.
The surface waters of STS have sufficiently diverse ionic composition. The waters of tunnel streams are either bicarbonate calcic or sulfate calcic in composition, except for waters of tunnel 177 ascribed to sulfate sodic–calcic type. The average \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) ratio in the considered tunnel streams is 22 and varies from 3 in tunnel 511 to 62 in tunnel 504. Thereby, the majority of sulfate–chloride ratio fall in the range from 10 to 25. In addition, this parameter shows a direct correlation with рН. This suggests that the composition of tunnel groundwaters is mainly defined by leaching of minerals from host rocks and oxidation of sulfide minerals.
Stream waters are similar by chemistry to tunnel waters, because tunnel waters recharge them. Waters of the Uzynbulak and Karabulak streams are sulfate–calcic in composition. The streams have close average compositions and an in-between attitude relative to the chemistry of tunnel waters. Thus, mixing of waters of small streams and springs feeding the streams of the Degelen Mountains results in the “smoothing” of their chemistry. This can be taken into account in estimating the downstream migration of radioactive elements.
Surface water bodies of the studied area are confined to the influence zone of continental salinization with variations of \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) from 1.4 in V-1 waters, to 1.2 in Lake Telkem-1, and up to 0.7 in Lake “Atomic” and Chagan River, and 0.5 in Lake Telkem-2. These waters are chloride and chloride–sulfate in composition. Predominant anions, as well as sodium proportionally increase with the growth of TDS in the water bodies, while Ca/Mg ratio, in contrast, decreases.
The content of TOC varied from 4 to 12 mg/L in tunnel waters, from 10 to 16 mg/L in streams, and reached 35 mg/L in the lakes(crater lake V-1). The measurements in some samples were not quantitative due to the low sensitivity of methods of TOC determination in samples with complex matrix and high salt contents. However, humic and fulvic acids were detect qualitatively and estimated semiquantitatively from the UV absorption spectrum.
The saturation index indicates that the streams and tunnel waters are oversaturated with respect to beidellite, clinoptilolite, diaspor, ordered dolomite, ferrite, goethite, hematite, illite, kaolinite, muscovite, and quartz, and are in equilibrium with calcite, aragonite, and chalcedony. Lake waters were oversaturated relative to the above-mentioned minerals and additionally to albite, analcime, chrysolite, K-feldspar, laumontite, and magnesite. Waters of all studied objects were oversaturated relative to uranophane and thorianite.
Migration of Natural Radionuclides
The thorium content in natural waters of STS varies within two orders of magnitude: from 0.012 μg/L in the Chagan River to 2.1 and 3.1 μg/L in the streams of tunnels 504 and 511, respectively. The uranium content in the studied natural waters also varies within two orders of magnitude. Thereby, the concentration of this radioactive element in water bodies is much lower: from 5.5 (V-1) to 66 μg/L (Telkem-2). The uranium content in the Karabulak and Uzynbulak streams of the Degelen Mountains is 60 and 81 mg/L, respectively. The tunnel streams have much higher uranium content: from 39 (tunnel 511) to 825 μg/L (tunnel 504), averaging 543 mg/L. The uranium contents in the Chagan River and Lake “Atomic” were similar: 28 and 36 mg/L, respectively, as well as their chemical composition.
Thorium and uranium are mainly transported in suspended form. The thorium content transported with suspended matter of streams and lakes is practically identical, whereas the uranium content shows significant variations. In particular, the share of uranium migrating in stream waters with colloid particles is approximately three times higher than that of the lakes.
The fraction of uranium migrating in the colloid form in water streams of the Degelen Mountains is not very high, from 5 to 15%. However, with allowance for the high bulk concentrations of this radioactive element and specifics of element migration in colloid form, this may indicate a significant removal of uranium with colloids. The fraction of uranium migrating in colloid form was higher in the V-1 water body and Karabulak stream. This is explained by the highest contents of organic matter and colloids among all studied objects.
The Th/U ratio in water samples was calculated for understanding its indicator role in studying geochemical processes and intensity of migration of these radioactive elements in aquatic systems. The Th/U ratio is widely applied in mineral geochemistry and rock dating. The theoretical and experimental Th/U ratios in the Earth’s crust at present fall within 3–4 (Degueldre and Joyce, 2020) with sufficiently wide range in the rocks of different composition. However, the Th/U ratio in hydrosphere in most experimental studies (Rikhvanov, 2017) is much lower than unity (Th/U = 10–3–10–5).
The Th/U ratio in the studied aqueous objects accounted for 1.0–1.1 × 10–3 for tunnel waters and streams, and is almost three times higher, 2.8 × 10–3, in the lakes. This can be explained by the lowered water exchange in the lakes, which provides favorable conditions for thorium transition into colloidal state. In contrast, this can be related to the more intense coprecipitation of uranium with secondary minerals, which reached saturation in brackish water of lakes and precipitated. A change of Th/U ratio in natural waters is considered as a distinguished marker of the influence of terrigenous component in the formation of migration species of radionuclides.
The sulfate ion to chloride ion ratio was indicative of an intensity of sulfide oxidation in streams and tunnel waterstreams and evaporative concentration in lakes, whereas the Ca/Mg ratio marks secondary minerals formation. The values of saturation indexes of minerals indicate that calcium is more intensely consumed for mineral precipitation, while magnesium is more efficiently introduced with terrigenous flux.
Identification of Unrevealed Geochemical Dependences of Radionuclide Migration by Multivariate Statistics
State of the art research of elements migration is faced often with the large geochemical data arrays, which is difficult to interpret and to decipher (disclose) trends using acknowledged instruments. The presence of geochemical anomalies, rather small data sets for expensive and exclusive studies demonstrate the need for non-parametric statistical methods.
Given the scope and heterogeneous origin of obtained data, correlation analysis was carried out in two modes: the Pearson parametric correlation (with Student t-test, Fig. 2a) and Spearman non-parametric rank correlation (Fig. 2b), using pleades method for the highest pair correlation coefficients. Similar interpretation of correlation analysis is frequently used for analysis of a great deal of variables (Malikova et al., 2020, and others). In the considered data set, the distribution of such components as organic carbon, iron, and speciations of radioactive elements were not controlled by the normal distribution law. Therefore, for these components, the rank correlation is more suitable.
Pleades of water chemistry parameters of natural waters and migration forms of natural radionuclides in STS natural waters based on correlation analysis. (a) Pearson parametric correlation; (b) Spearmen rank correlation. Hereinafter: (MCC) main chemical composition, (TDS) total dissolved solids, (ThS, US) suspended Th and U species, respectively. (ThC, UC) thorium and uranium colloid forms, (ThD, UD) thorium and uranium dissolved forms, (ТОС) total organic carbon.
Both versions of correlation analysis demonstrated strong correlation between major chemical parameters of water: TDS, chloride and sulfate ions, as well as major cations, including calcium, magnesium, sodium, and potassium. These components were united in a separate paragenetic block marked by red. Within this group, the average correlation coefficient is 0.97, varying from 0.93 to 0.99. These values are slightly lower (average r is 0.90) in non-parametric correlation. This is especially noticeable for chloride-ions, where the correlation coefficients with other components were from 0.78 to 0.88. This can be explained by the presence of waters of different genesis, where evaporative concentration was not exclusive.
A transition from parametric to rank correlation also shows an increase of a negative correlation between Ca/Mg ratio and major chemical parameters (from ‒0.70 to –0.91). A similar increase of correlations was noted for pairs pН–Eh (from –0.71 to –0.74), Fe–TOC (from 0.56 to 0.71), and \({\text{HCO}}_{3}^{ - }\)–\({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) (from –0.65 to –0.84). Non-parametric correlation of Eh with uranium, thorium, dissolved thorium and \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) ratio shows a weakening up to the reliable level (0.50). We believe that the volume of data array and range of obtained pH-Eh values do not allow us to accept these trends as reliable. Thus the revealed correlation pairs for such volume of data (n = 19) could be nonsense. It is known that thorium is not redox-sensitive element, although its migration is determined by solubility of its compounds, which is directly correlated with pH. As to uranium, it should be noted that during migration in form of uranyl carbonate complexes, uranyl ions are strongly shielded by ligands, which prevents the change of U6+ oxidation state even in the presence of sulfide sulfur. Other natural reducing barriers seem to be insufficiently contrasting and uranium demonstrates a transit migration through oxidizing and reducing environment (Mahskovtsev et al., 2010).
One more significant difference between two calculated versions of correlation analysis is the appearance of moderate Th–U positive correlation for non-parametric correlation. Thereby, this is expressed both for bulk content (r = 0.72), and their dissolved (r = 0.76) and suspended species (r = 0.73). The dissolved species of both radionuclides positively correlate with \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}},\) which reflects their increased content owing to leaching from rocks and the prevalence of sulfide oxidation process over the evaporative concentration.
In general, data obtained using non-parametric correlation are better described by basic water geochemistry parameters of the water-rock interaction and specifics of trace element migration during water metamorphism process. It is known that a monotonous change of definite parameter causes the geochemical processes to behave in a non-linear fashion. For instance, with increase of organic content in waters, the fraction of complexed elements tends to increase until the aggregation of organic matter occurs and it is transformed into suspended state. This is also the case for the precipitation of elements with accomplishment of saturation with respect to their minerals, change of the oxidation state of polyvalent elements as well as other processes. Thus, the rank correlation provides a more correct assessment of heterogeneous data arrays (Makinen, 1991; Reimann et al., 2017, and others).
The strong correlations of natural radionuclide species with \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) and Ca/Mg ratios in the absence of significant correlations with major components show the high sensitivity of these geochemical markers.
The problems of migration of colloidal forms of the studied elements, the influence of iron content and organic matter, as well as the role of pH value and redox condition of natural waters unfortunately were not completely clarified during consideration of correlations. Undoubtedly, colloid transport, as the element migration in aquatic systems in general, depends on the above mentioned factors, which follows from the numerous studies of natural and model objects (Cuss et al., 2018; Equeenuddin et al., 2020, and others). It remains unclear how this can be determined within a definite range of geochemical environments, which have existed in the studied water reservoirs and streams. Cluster, factor, and discriminant analyses were regarded as the most suitable tools for solution of this issue. Similar approach was successfully applied by (Yusupov et al., 2021) in searching for biogeochemical trends of bromine migration in the ecosystems of urbanized territories.
When distinguishing clusters by variables (Fig. 3a), two large groups of components are defined. The first group includes рН, Eh, TDS, TOC, iron, calcium, sulfate ion, and bulk uranium content. This suggests that the intensity of uranium introduction in water is determined by рН–Eh pair together with organic matter. Uranium as more mobile element then thorium is remains in dissolved and colloid forms, while thorium is tend to transition into suspended state or absorbed on suspended matter, and coprecipitated with other mineral phases. The redistribution of species is rather determined by other components involved in other cluster. The second group includes uranium and thorium species, such geochemical descriptors as Ca/Mg and \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}},\) hydrocarbonate ions and some other major components. The clear association of \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) with dissolved uranium form is confirmed by the distribution of forms of this radionuclide in the tunnel streams of the Degelen Mountains. This area is characterized by the maximum content of sulfate ion together with the high uranium content dominated by dissolved species. The colloid forms of both radionuclides united in a single cluster are most close to this association in terms of the Euclidean distance. The formation of the colloid fraction of radionuclides is more expressed in tunnel waters, which contain mainly inorganic colloids formed during rock weathering. Since this process is associated with leaching and further sulfide minerals oxidation, these components show a tight correlation.
Cluster analysis of chemical composition and migration forms of radionuclides in natural waters of STS. Hereinafter: (V-1) crater lake V-1, Experimental field (point 1, Fig. 1), (UZ) Uzynbulak Stream (point 13), (KB) Karabulak Stream within the “Degelen” site (points 14–18), (KBcf) confluence site of the Karabulak Stream tributaries (point19), (Сh) Chagan River (point 5), (AL) “Atomic” Lake (point 4), (Tel-1, Tel-2) Telkem-1 and Telkem-2 lakes (points 2–3), (T104…Т609) tunnel waterstreams of the “Degelen” site (points 7–12). (I, II, III, IV) clusters of water bodies based on water geochemistry parameters. (a) Clusterization by studied parameters; (b) clusterization by water bodies
The reservoirs of the studied territory are characterized by the negligible content of uranium and thorium colloid forms. Given the fact that the lakes have the negligible content (close to the detection limit) of natural radionuclides and are highly salinized, the role of organic matter can be underestimated in experimental studies. It is also necessary to take into account the complexation capacity of thorium and uranium, which migrate as complexes with humic matters, according to calculated data on similar aquatic systems (Toropov et al., 2020; Kolpakova et al., 2018).
The clusterization of water objects (Fig. 3b) seems to be reasonable from the view point of water geochemistry and migration of radioactive elements. Since clusters were distinguished with allowance for the distribution of speciations and chemical composition, the objects were arranged mainly according to their genesis. This is related to the more expressed influence of major-component composition. In particular, water body V-1, which has the most contrasting composition, formed cluster with none of the studied objects. The water bodies of the Telkem site formed a single cluster, as did the Chagan River and “Atomic” Lake. The Chagan River flows from bicarbonate and is very close to it in terms of chemical composition and distribution of radionuclide forms. Based on the analysis, the water bodies are subdivided into four clusters with specific geochemical features (Fig. 3b).
I. Waters with higher TDS, and concentrations of TOC, iron, and contents of suspended uranium and thorium species: Uzynbulak Stream, confluence of the Karabulak Stream tributaries, and tunnel 511.
II. Weakly acid waters with lowered content of bicarbonate ions and higher level of sulfate ions, as well as higher Th/U ratio (three times higher than in the first clusters): tunnels 504, 503, and 104, areas of the Karabulak stream within the Degelen Mountains.
III. Weakly alkaline waters with the lowest contents of iron and TOC among tunnel streams: tunnels 176, 609, 165, and 177.
IV. Lakes with brackish and saline waters, as well as the Chagan River with the strong predominance of suspended Th and U.
Thus, factors of water composition formation and migration of radionuclides in them are highlighted during clusterization of objects by selected method. It is possible that analysis of other data, for instance, intensity of water exchange, biological productivity of landscapes, content of colloids, zeta potential (electrophoretic mobility), as for instance was made in (Dinu, 2020), would reveal predominant factors more clearly.
It is very important how the landscape-geochemical conditions affect the intensity of trace element migration. A combined (sometimes opposite) influence of factors justifies the application of factor analysis. The distribution of the studied geochemical parameters by values of factor loads (principal component analysis) is shown in Fig. 4.
Distribution of factor loads by components of natural waters. (а) factors 1 and 2, (b) factors 3 and 4. Dashed line shows line with reliable factor load r = 0.7. Points lying beyond the dashed line have reliable factor load (additionally shown by red). Numerals in parentheses along axes show the fraction of explained dispersion, in %. Raw data are normalized and transformed using varimax rotation.
In total, we distinguished four factors, which explain 92% of dispersion of experimental data.
Analysis of obtained trends allowed us to distinguish main factors, which determine the chemical composition and migration of thorium and uranium in natural waters of STS:
• factor 1 (44% of total dispersion): relationship of major chemical components of waters, metamorphism of water types due to the evaporative concentration from hydrocarbonate calcic to chloride sodic water types;
• factor 2 (24%): redox processes, change of chemical composition of water under water–rock interaction, rate of leaching aquifer rocks and oxidation of sulfide minerals, as well as the water exchange intensity;
• factor 3 (15%): accumulation of organic matter in tunnel waters and streams; water enrichment in iron and thorium;
• factor 4 (9%): influence of terrigenous component, thorium absorption on suspended matter, changing of the Th/U ratio.
There is a clear differentiation between lakes and streams, which is explained by significant difference in mechanisms of their waters formation and in trends of chemical variations. The most contrasting points were waterstreams from tunnels 504 and 511, as well as water body V-1, which are characterized by the peculiar features of migration of natural radioactive elements and their ratios.
The first and second factors in total reflect main trends, which determine the water chemistry and are typical of any water types. The comparison of the factors showed that two former factors moderately correlate (r = 0.63), which suggest their related genesis. The third and fourth factors reflect the peculiar features and specifics of definite aqueous objects. In spite of their subordinate role, these factors explain 24% of total dispersion and provide insight into factors that determine the radionuclide migration and their speciation.
It was established that \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) and Ca/Mg ratios are independent parameters and can be used to trace the accumulation of radioactive elements depending on geochemical environment. The рН and Eh values are determined by a common factor, with an opposite sign. Thereby, the influence of redox is expressed more clearly. In spite of the insufficiently reliable description of uranium distribution by selected parameters (r < 0.7 for all values), some geochemical parameters of water or their combinations are expressed in other applications of multivariate statistical analysis. Thus, it was concluded that the uranium migration and distribution of its species are caused by multifactor influence.
The Th content and Th/U ratio in the STS natural waters operate as a single factor. The Th precipitation through absorption, precipitation of its insoluble compounds, and retention by colloid particles constrain its migration. Into a pair of U–Th elements, exclusively thorium properties determine the ratio of these elements in water. Colloid forms of both studied radionuclides in the factor load maps plot closely for all factors. It is possible that the migration of the colloid fraction of uranium and thorium is determined by a single mechanism, which, however, was weakly expressed in the studied objects against the background of more clearly expressed and strong factors.
Bicarbonate-ion and uranium fall in diametrically opposite planes of factor loads. According to thermodynamic modeling, the uranyl carbonate complexes are the predominant species of this element (Toropov et al., 2020, and others), but this component has no direct influence on the intensity of its migration. We suggest that natural waters are excessive in inorganic carbon relative to the uranyl carbonate complexes formation (Fig. 5).
Change of uranium migration forms depending on the content of bicarbonate-ion (based on modeling results). The model is limited by elements and physicochemical parameters with values shown in Table 1 (average content for streams). Uranium concentration in calculation was taken to be 55 mg/L (sum of fractions passing through 450-nm membrane). Experimental value on \({\text{HCO}}_{3}^{ - }\) for streams are shown by blue line.
Modeling of a change of uranium migration forms depending on the content of bicarbonate-ions was carried out within a range from 10 to 1000 mg/L. The figure shows a fragment up to 500 mg/L. Within the entire concentration range, the uranyl carbonate complexes are predominant uranium species with strongly subordinate content of uranyl hydroxyl complexes, which increases with a decrease of carbonate content in the model system.
The subdivision of the studied water objects by hydrodynamic intensity is a priori most contrasting indicator of radionuclide migration specifics. The clear distribution of thorium and uranium species over these groups can be observed even from the raw data. Differentiation of uranium and thorium migration in tunnel waters, riverstreams, and lakes is also clearly observed from results of factor and cluster analysis. Therefore, we additionally carried out discriminant analysis (Fig. 6) with less obvious discriminators: the content of colloid forms of radionuclide transport and concentration of organic matter. Raw data are grouped based on the median value of colloid forms into new data set: “more active colloid transport of radionuclides in colloid form” and “minimum share of U and Th colloid forms” groups. In a similar way, waters were divided based on their organic matter content into “organic-rich waters”, “organic-poor waters” groups.
The highest reliable differences are determined as follows (in order of decreasing):
—root 1 (significance of function of 25.8, χ2 = 145.5): the content of suspended Th, Eh, Fe, and Ca species, fraction of colloid Th species;
—root 2 (significance of function 7.7, χ2 = 68.3): content of suspended Th species, \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}},\) ratio and Cl–.
These parameters ultimately contributed to the intracluster correlation. Among major components, calcium determines the maximum difference between discriminant groups, which differ in the share of radionuclides colloid forms.
Groups of object separated by concentration of organic matter in water in the developed discrimination model are located at the greater distance than groups separated by content of colloid matters. Such distribution deserves attention, since the range of measured concentrations of organic matters was insignificant. Group of “organic-poor waters” involved mainly tunnel waters, where the source of natural radionuclides differs from that of water bodies and streams.
Thorium species (suspended and colloid) in this model serve as indicators of colloid transport. The dependence of suspended Th content on pH and Eh was also revealed from cluster analysis. Undoubtedly, an addition of data on other aqueous objects with contrasting geochemistry to the available database will significantly improve the understanding of factors responsible for the migration of natural radionuclides in natural waters.
CONCLUSIONS
Selected instruments confirm that the species of natural radionuclides in water are able to transform within a change of geochemical environment. These methods are also informative for understanding the spatiotemporal distribution pattern of radionuclides as well as radiation hazardous territories and radioactive waste disposal sites status prediction. The use of non-parametric correlation for limited data sets makes it possible to exclude nonsense correlations, which are occur during conjoin change of two variables having no cause–and–effect relationships. Migration in form of colloid particles is more typical for thorium than for uranium. Migration forms of the given radionuclide also serve as indicators of colloid-facilitated transport according to discriminant analysis. Factor analysis showed that the \({{{\text{SO}}_{4}^{{2 - }}} \mathord{\left/ {\vphantom {{{\text{SO}}_{4}^{{2 - }}} {{\text{C}}{{{\text{l}}}^{ - }}}}} \right. \kern-0em} {{\text{C}}{{{\text{l}}}^{ - }}}}\) and Ca/Mg ratios are more informative in estimating the effect on migration of natural radionuclides than distinct components. The peculiarities of migration of transuranic radionuclides in waters with contrasting flow-fluid dynamics (streams and lakes) are clearly defined by the cluster and factor analyses.
The great advantage of described methods of analysis of geochemical data is their visual expression. Such approach suggests an opportunity to study natural processes not through the lens of data arrays, but provides efficient and intuitive base for the identification of revealed mechanisms. Extensive and complex examination of raw basic data and involvement of analytical tools and multivariate statistics make it possible to discuss the revealed dependences not as particulate assumptions, but as justified and reliable trends.
REFERENCES
Y. V. Alekhin, E. A. Ivleva, S. M. Ilina, and L. Z. Lakshtanov, “Experimental fundamentals of the colloid hydrogeochemistry of continental runoff,” Geochem. Int. 58 (9), 1050–1060 (2020).
Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd Edition, Ed. by W. Stumm and J. J. Morgan (Wiley, New York, 1995).
M. F. Baalousha, V. D. Kammer, M. Motelica-Heino, M. Baborowski, C. Hofmeister, and P. Le Coustumer, “Size-based speciation of natural colloidal particles by flow field flow fractionation, inductively coupled plasma-mass spectroscopy, and transmission electron microscopy, X-ray energy dispersive spectroscopy: Colloids-trace element interaction,” Environ. Sci. Technol. 40 (7), 2156–2162 (2006).
M. Baalousha, B. Stolpe, and J. R. Lead, “Flow field-flow fractionation for the analysis and characterization of natural colloids and manufactured nanoparticles in environmental systems: A critical review,” J. Chromatogr. A. 1218 (27), 4078–4103 (2011).
L. V. Bakhtin, I. V. Ivanova, E. Yu. Pestov, A. M. Romanov, “Differences in radionuclide migration in geological media,” Vestn. NYATs RK, 2, 146–149 (2017).
G. E. Batley, Trace Element Speciation Analytical Methods and Problems (CRC Press, 1989).
E. Bolea, M. P. Gorriz, M. Bouby, F. Laborda, J. R. Castilloa, and H. Geckeis, “Multielement characterization of metal-humic substances complexation by size exclusion chromatography, asymmetrical flow field-flow fractionation, ultrafiltration and inductively coupled plasma-mass spectrometry detection: A comparative approach,” J. Chromatogr. A. 1129 (2), 236–246 (2006).
M. Bouby, H. Geckeis, and F. W. Geyer, “Application of asymmetric flow field-flow fractionation (AsFlFFF) coupled to inductively coupled plasma mass spectrometry (ICPMS) to the quantitative characterization of natural colloids and synthetic nanoparticles,” Anal. Bioanal. Chem. 392 (7–8), 1447–1457 (2008).
M. Bouby, N. Finck, and H. Geckeis, “Flow field-flow fractionation (FlFFF) coupled to sensitive detection techniques: a way to examine radionuclide interactions with nanoparticles,” Mineral. Mag. 76 (7), 2709–2721 (2012).
B. W. Cho, and O. C. Choo, “Geochemical behavior of uranium and radon in groundwater of Jurassic granite area, Icheon, Middle Korea,” Water 11, 1278 (2019).
C. Claveranne-Lamolre, J. Aupiais, G. Lespes, J. Frayret, E. Pili, F. Pointurier, and M. Potin-Gautier, “Investigation of uranium-colloid interactions in soil by dual field-flow fractionation/capillary electrophoresis hyphenated with inductively coupled plasma-mass spectrometry,” Talanta 85 (5), 2504–2510 (2011).
Complex of Scientific-Technical and Engineering Works to Bring the Former Semipalatinsk Test Site in a Safe State, Ed. by N. A. Nazarbaev, V. S. Shkol’nikov, E. G. Batyrbekov et al., (Nats. Yad. Ts. RK, Kurchatov, 2016) [in Russian].
C. W. Cuss, M. W. Donner, I. Grant-Weaver, T. Noernberg, R. Pelletier, R. N. Sinnatamby, and W. Shotyk, “Measuring the distribution of trace elements amongst dissolved colloidal species as a fingerprint for the contribution of tributaries to large boreal rivers,” Sci. Total Environ. 642 (6), 1242–1251 (2018).
C. W. Cuss, C. N. Glover, M. B. Javed, A. Nagel, and W. Shotyk, “Geochemical and biological controls on the ecological relevance of total, dissolved, and colloidal forms of trace elements in large boreal rivers: review and case studies,” Environ. Rev. 28, 138–163 (2020).
C. Degueldre and M. J. Joyce, “Evidence and uncertainty for uranium and thorium abundance: A review,” Prog. Nucl. Energy. 124 (4), 103299 (2020).
M. I. Dinu, “Geochemical features of element speciation in natural waters of the Valdai Rise (March–November, 2019),” Geochem. Int. 58 (12), 1379–1385 (2020).
Environmental Colloids and Particles: Behavior, Separation and Characterization, Ed. by K. J. Wilkinson and J. R. Lead (John, Wiley & Sons, 2007).
S. M. Equeenuddin, S. Akhtar, F. Bastia, S. S. Rout, and P. J. Saikia, “Role of colloid in metal transport in river water around Jaduguda uranium mines, Singhbhum shear zone,” J. Earth Syst. Sci. 129 (1), (2020). https://doi.org/10.1007/s12040-019-1262-y
I. M. Farnham, K. H. Johannesson, A. K. Singh, V. F. Hodge,and K. J. Stetzenbach “Factor analytical approaches for evaluating groundwater trace element chemistry data,” Anal. Chim. Acta. 490(1–2), 123–138 (2003).
E. Gorbunova and S. Subbotin, “Study of radionuclide transport by underground water at the Semipalatinsk Test Site,” The New Uranium Mining Boom, Ed. by B. Merkel and M. Schipek (Springer, Berlin–Heidelberg, 2012), pp, 335–342.
I. Gorlachev, P. Kharkin, M. Dyussembayeva, S. Lukashenko, G. Gluchshenko, L. Matiyenko, D. Zheltov, A. Kitamura, and N. Khlebnikov, “Comparative analysis of water contamination of the Shagan river at the Semipalatinsk test site with heavy metals and artificial radionuclides,” J. Env. Radioact. 213, 106110 (2020).
P. V. Govenko, A. A. Amirov, and S. N. Lukashenko, “Study of mechanisms determining the trace-element composition of surface water streams of the Degelen mountains,” Semipalatinsk Test Site, Radiation Inheritance and Prospects for Development. Proc. 5th International Conference (2012), pp. 71–73.
C. Graser, N. Banik, K. A. Bender, M. Lagos, C. M. Marquardt, V. Montoya, and H. Geckeis, “Sensitive redox speciation of iron, neptunium, and plutonium by capillary electrophoresis hyphenated to inductively coupled plasma sector field mass spectrometry,” Anal. Chem. 87, 9786–9794 (2015).
R. Guillaumont, T. Fanghänel, J. Fuger, I. Grenthe, V. Neck, D. A. Palmer, and M. H. Rand, Update on the Chemical Thermodynamics of Uranium, Neptunium, Plutonium, Americium and Technetium, Ed. by F. J. Mompean, (Elsevier Science, Michigan, 2003).
S. M. Ilina, S. A. Lapitskiy, Y. V. Alekhin, J. Viers, M. Benedetti, and O. S. Pokrovsky, “Speciation, size fractionation and transport of trace elements in the continuum soil water–mire–humic lake–river–large oligotrophic lake of a Subarctic watershed,” Aquat. Geochem. 22, 65–95 (2016).
A. Itoh, C. Kimata, H. Miwa, H. Sawatari, and H. Haraguchi, “Speciation of trace metals in pond water as studied by liquid chromatography/inductively coupled plasma mass spectorometry,” Bull. Chem. Soc. Jpn. 69 (12), 3469–3473 (1996).
P. G. Kayukov, Study of Radiation Environment at the Territory of Republic of Kazakhstan. Report of 2004–2008 (Volkovgeologiya, Almaty, 2008) [in Russian].
Yu. I. Kazakova, “Anthropogenic fracturing and chemical composition of waters of the filtration zone of the Degelen mountainous massif,” Vestn. NYATs RK, No. 4, 84–89 (2005).
M. N. Kolpakova, O. L. Gaskova, and O. S. Naimushina, “Ebeity Lake, Russia: chemical-organic and mineral composition of water and bottom deposits,” Izv. Tomsk. Politekhn. Univ. Inzh. Geores. 329 (1), 111–123 (2018).
B. D. Lee, C. H. Jeong, Y. C. Lee, Y. J. Lee, and J. H. Yang, “Statistical Analysis and Thermodynamic Equilibrium Modelling for Chemical Composition of Groundwater and Spring Water at Jeju Island, South Korea,” Water 777 (12), 1–18 (2020).
O. C. Lind, D. H. Oughton, B. Salbu, L. Skipperud, M. A. Sickel, and J. E. Brown, “Transport of low 240Pu/239Pu atom ratio plutonium–species in the Ob and Yenisey Rivers to the Kara Sea,” Earth Planet. Sci. Lett. 251 (1–2), 33–43 (2006).
J. J. Mahoney and R. T. Jakubowski, “Assessment of uranyl sorption constant on ferrihydrite—comparison of model derived constants and updates to the diffuse layer model database,” Uranium, Mining and Hydrogeology, Ed. by B. Merkel and A. Hasche-Berger (Springer, Berlin–Heidelberg, 2008), pp. 919–928.
J. Makinen, “Similarity analysis using rank in till geochemistry. Bull. Geol. Soc. of Finland. 63 (1), 49–57 (1991).
I. N. Malikova, V. D. Strakhovenko, and M. T. Ustinov, “Uranium and thorium contents in soils and bottom sediments of lake Bolshoye Yarovoye, western Siberia,” J. Env. Radioact. 211, 106048 (2020).
S. J. Markich, “Uranium speciation and bioavailability in aquatic systems: an overview,” The Sc. World J. 2, 707–729 (2002).
S. J. Markich and P. L. Brown, “Actinide speciation and bioavailability in fresh and marine waters,” Enc. Inorg. Bioinorg. Chem., (2019). doi.org/https://doi.org/10.1002/9781119951438.eibc2559
G. A. Mashkovtsev, A. K. Konstantinov, A. K. Miguta, M. V. Shumilin, and V. N. Shchetochkin, Uranium of the Russian Interior (VIMS, Moscow, 2010) [in Russian].
M. L. Pacheco and J. Havel, “Capillary zone electrophoretic (CZE) study of uranium(VI) complexation with humic acids,” J. Radioanal. Nucl. Chem. 248, 565–570 (2001).
C. J. Placzek, J. M. Heikoop, B. House, B. S. Linhoff, and M. Pelizza, “Uranium isotope composition of waters from South Texas uranium ore deposits,” Chem. Geol. 437, 44–55 (2016).
V. I. Radomskaya, D. V. Yusupov, L. M. Pavlova, A. G. Sergeeva, and E. N. Voropaeva, “Using multivariate statistical analysis to study the ecological and geochemical properties of soils of Blagoveshchensk city,” Uch. Zap. Kazan. Univ. Ser. Est. Nauki. 159 (4), 602–617 (2017).
M. E. Ragoussi and D. Costa, “Fundamentals of the NEA Thermochemical Database and its influence over national nuclear programs on the performance assessment of deep geological repositories”, J. Environ. Radioact. 196, 225–231 (2019).
P. E. Reiller and M. Descostes, “Development and application of the thermodynamic database PRODATA dedicated to the monitoring of mining activities from exploration to remediation,” Chemosphere 251, 126301 (2020).
C. Reimann, P. Filzmoser, K. Hron, P. Kynčlová, and R. G. Garrett, “A new method for correlation analysis of compositional (environmental) data—a worked example,” Sci. Total Env. 607–608, 965–971 (2017).
L. P. Rikhvanov, “Radioactivity and radioactive elements as factors of geologicam medium and its application in the Earth’s science,” Razved. Okhr. Nedr, 12, 55–61 (2017).
S. Rollin and U. Eklund, “Determination of U(VI) and U(IV) by ion chromatography-inductively coupled plasma mass spectrometry and its application to kinetic studies,” J. Chromatogr. 884A, 131–141 (2000).
H. R. Rollinson, Using Geochemical Data: Evaluation, Presentation, Interpretation (Routledge, New York, 2014).
B. Salbu, “Speciation of radionuclides in the environment,” In Encyclopedia of Analytical Chemistry (2006), pp. 1–24. https://doi.org/10.1002/9780470027318.a6308
B. Salbu, V. Kashparov, O. C. Lind, R. Garcia-Tenorioc, M. P. Johansen, D. P. Child, and R. C. Sanchof, “Challenges associated with the behavior of radioactive particles in the environment,” J. Env. Radioact. 186, 101–115 (2018).
S. B. Subbotin, and Yu. V. Dubasov, “Radioactive contamination of water of the Degelen Mountain Massif,” Radiochemistry 55 (6), 647–654.
A. S. Toropov, “Cesium-137 speciation in natural waters of the Semipalatinsk test site in the model and natural experiments,” Vestn. Zabaikal’sk. Gos. Univ. 23 (12), 59–68 (2017).
A. S. Toropov, “Fractionation of technogenic radionuclides species in water bodies of Semipalatinsk Test Site,” Bull. Tomsk Polytech. Univ. Geo Assets Eng. 329 (6), 74–84 (2018).
A. S. Toropov, “Migration forms of anthropogenic radionuclides in tunnel waters at the Degelen Mountains, Semipalatinsk Test Site,” Geochem. Int. 58 (3), 342–351 (2020).
A. S. Toropov and M. S. Panin, “State of groundwaters of the Semipalatinsk test nuclear site (ecological assessment of chemical composition),” Proc. 7 th International Conference “Science and Education–2011”, 2, 243–249 (2011).
A. S. Toropov, E. A. Soldatova, and L. P. Rikhvanov, “Forms of radionuclides (U and Th) migration in natural waters under different geochemical conditions based on computational and experimental data,” Bull. Tomsk Polytech. Univ. Geo Assets Eng. 331 (12), 7–21 (2020).
A. M. Ure and C. M. Davidson, Chemical Speciation in the Environment (Blackwell Science, Glasgow, 2002).
L. Vintró, P. I. Mitchell, A. Omarova, M. Burkitbayev, H. Jiménez Nápoles, and N. D. Priest, “Americium, plutonium and uranium contamination and speciation in well waters, streams and atomic lakes in the Sarzhal region of the Semipalatinsk Nuclear Test Site, Kazakhstan,” J. Env. Radioact. 100 (4), 308–314 (2009).
Y. Wang, C. W. Cuss, and W. Shotyk, “Application of asymmetric flow field-flow fractionation to the study of aquatic systems: Coupled methods, challenges, and future needs,” J. Chromatogr. A. 1632, 461600 (2020).
G. M. Yessilkanov, M. T. Dyusembaeva, L. P. Rikhvanov, N. Zh. Mukhamediyarov, and A. Zh. Tashekova, “Ecological–geochemical features of groundwaters of the Semipalatinsk test site,” Ekol. Prom. Rossii 24 (11), 30–35 (2020).
D. V. Yusupov, L. P. Rikhvanov, N. V. Baranovskaya, L. A. Dorokhova, and A. F. Sudyko, “Bromine in the Poplar leaves of urban areas: natural and anthropogenic sources of scattering,” Bull. of the Tomsk Polytech. Univ. Geo Assets Eng. 332 (1), 76–87 (2021).
Funding
The reported study was funded by Russian Foundation for Basic Research (project no. 19-33-60030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by M. Bogina
Rights and permissions
About this article
Cite this article
Toropov, A.S., Yessilkanov, G.M. Advanced Instruments for Identifying Geochemical Dependences of Radionuclide Migration in Natural Waters. Geochem. Int. 60, 266–278 (2022). https://doi.org/10.1134/S0016702922010116
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702922010116