Abstract
The distribution of dissolved major- and trace elements in the mixing zone of the Ural River and the North Caspian waters was studied based on the natural observations data from 2016–2017. Conservative behavior was established for most of major ions (Na, K, Mg, SO4) and some trace elements (Li, Rb, Cs, Sr, Co, Ni, Cu, Zn, Sb, Ga, Y, U, B, F, Cr, Ge, Mo, W) with common parameters of relationships between their concentrations and chloride content for different years. The distribution of components of the carbonate system is controlled by chemogenic precipitation of calcium carbonate at the mouth beach. This process leads to the removal from solution up to 11–17% calcium and 6–8% hydrocarbonates carried by river runoff, and a simultaneous decrease in the pH value. Nutrients are involved in the processes of biological assimilation and regeneration. The mixing zone of the Ural River and Caspian waters is characterized by: (a) phytoplankton consumption of large amounts of silicon and nitrates (up to 58–88 and 61–67%, respectively, of their content in riverine waters), (b) removal of significant part (up to 18–25%) of nitrites, and (c) additional input of phosphates into solution, presumably from pore waters of the surface layer of bottom sediments in amounts 1.5–3 times greater than those removed with river runoff. A distinctive feature of barium migration is an additional input into solution (up to 20%) at the initial stage of salinization owing to the desorption from terrigenous material. The coagulation and flocculation of organic and organo-mineral colloids lead to the removal of significant part (up to 25–100%) of dissolved manganese, iron, aluminum, and rare-earth elements runoff, as well as to the removal of lead, titanium, zirconium, and hafnium from solution in amounts 1.1–6 times greater than their contents in the river water mass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The transformation of runoff of dissolved matters in the river mouth under the influence of chemical and biological intrabasin processes, as well as mass-exchange with bottom sediments and atmosphere are an important stage in the migration of chemical elements in a global hydrological cycle. Owing to these transformations, the contents and proportions of the dissolved matters supplied in seas and oceans differ from those of riverine runoff (Lisitsyn, 1994; Gordeev, 2012).
The chemical transformation of the river runoff during mixing with seawater depend on many factors, including the composition of waters in a final sea basin. A great deal of information on the distribution of dissolved major and trace elements is mainly available for the mouths of river emptying into seas and oceans with “normal” seawater, whereas data on the river mouths of closed sea–lake basins with atypical water composition (Kaspian, Aral seas) are few in number (Demina et al., 1978; Zakharova and Savenko, 1998; Savenko, 1999; Brekhovskikh et al., 2005, 2006; Savenko et al., 2014; Brekhovskikh et al., 2017). In particular, the hydrochemical study of the Volga mouth showed (Savenko et al., 2014) that the salt and trace-element composition of the North Caspian Sea significantly differ from that of the World Ocean and is characterized by the year-to-year stability, thus determining the peculiar migration of dissolved matters in the mixing zone of the Volga and Caspian waters.
The aim of this work is to determine regularities in the transformation of dissolved matter of runoff in the mouth of the Ural River, another large river of the Caspian Sea basin, which significantly affects the chemical composition of the North Caspian waters.
The mouth area of the Ural River is of deltaic type and consists of ∼156 km long near-delta area, 500 km2 delta, and >900 km2 mouth beach (Mikhailov, 1997). In the deltaic area, the river is divided into two large branches downstream the City of Atyrau: Yaitskii and Zolotoi. The latter grades into the Ural–Caspian channel and is involved in transient navigation. The river and its tributaries are mainly fed by snow: spring flood accounts for on average ∼70% of annual water runoff. In the lower reaches, the flood lasts from the end of March to the beginning of April, and northward this period is shifted to the end of April–beginning of July (Chibilev, 2008). This results in the long-term subsequent flowing of flood waters from the lower, middle, and upper parts of the drainage system in the Ural mouth.
METHOD
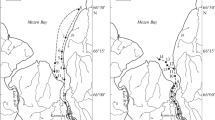
The hydrochemical studies of the Ural mouth were carried out by P.N. Makkaveev and P.V. Khlebopashev (Shirshov Institute of Oceanology of the Russian Academy of Sciences) in April 9–10, 2016 and April 14–17, 2017, during the complex expeditions on R/V Amangaliev Duisekesh. Samples were collected from 6 and 12 stations, respectively, which were arranged from the town of Atyrau along the Zolotoi branch and Ural–Caspian channel to the continental slope on the mouth beach (Fig. 1). Due to the complete absence of stratification, water samples were taken only from surface horizon.
The comparison of water levels near the waterway station in the town of Atyrau and analysis of spatial distribution of dissolved nutrients during surveys showed (Makkaveev et al., 2018) that in spite of close sampling dates, obtained data characterize different phases of hydrological mode. In 2017, the mouth part of the river contained winter low waters, which were repulsed by the subsequent wave of the flood water formed in the upper reaches. In 2016, the flood wave had already reached a mixing zone of the Ural and Caspian waters, while traces of the low water were found in the mouth beach at the periphery of the studied area.
The results of natural observations were kindly given by P.N. Makkaveev and included in situ pH measurements by Ekspert-001 ion meter, on-board calorimetric determination of concentrations of dissolved nutrients, as well as sampling and preparation of water samples for analyzing in stationary conditions. The value of total alkalinity (Alk ≈ HCO3) was determined in samples filtered through a dense paper filter using volumetric acidometric method, while concentrations of other main ions were determined by capillary electrophoresis, and content of fluorides, by direct ion metric method with fluoride ion-selective electrode. Concentrations of other trace elements were analyzed by inductively coupled plasma mass spectrometry on an Agilent 7500ce spectrometer in solutions filtered immediately after sampling through a 0.45-μm membrane filter in polypropylene flasks preliminarily filled with aliquots of 5 N nitric acid of analytical grade (0.25 per 10 mL of sample). For measurements, the highly mineralized samples were diluted with 2% nitric acid of analytical grade to obtain the content of dissolved matters within 300–500 mg/L. Measurement error was no worse than ±3%.
RESULTS AND DISCUSSION
Obtained results (Tables 1–5) made it possible to distinguish the types of distribution of dissolved components in the Ural mouth and to quantify tendencies in the transformation of runoff of dissolved matters during mixing with North Caspian waters.
The conservative behavior of elements is described by the common linear relationships between concentration of i component and chloride contents for surveys of 2016 and 2017,
which span the entire range of chlorinity up to the southern boundary of the North Caspian sea (Table 6). The conservative behavior is controlled by the hydrodynamic mixing between the riverine and seawater masses and is observed for most major ions (Na, K, Mg, SO4), trace alkalis (Li, Rb, Cs), strontium, some heavy metals (Co, Ni, Cu, Zn, Sb), hydrolyzate elements (Ga, Y, U), and anionogenic elements (B, F, Cr, Ge, Mo, W). The similarity of parameters a (≈ i concentrations in the riverine waters, mg/L) and b (slope coefficient) of equation (1) for different years and the agreement between the extrapolation to the southern boundary of the North Caspian sea and corresponding concentrations obtained from multi-year observation data on the Volga mouth (Savenko et al., 2014) indicate that the distribution of conservative components was stable in time and that the chemical variability of river runoff exerted no significant influence on the trends of their migration in river–sea water mixing zone.
Nonconservative behavior indicates an additional input or removal of matter from solution owing to intrabasin chemical or biological processes. Such behavior is typical of components of carbonate system (Ca, HCO3), nutrients (Si, P–PO4, N–NO2, N–NO3), barium, heavy metals associated with organic and organo-mineral colloids (Mn, Fe, Pb), and majority of hydrolyzate elements (Al, Ti, Zr, Hf, rare-earth elements). The quantitative characteristics of this distribution in the Ural mouth, as well as in the mixing zone of the Volga and Caspian waters (Savenko et al., 2014) to greater extent depend on the chemical variability of river runoff than those of conservative components (Table 6).
For components of the carbonate system at chlorinity > 1.3–1.5 g/L, the removal from solution reached 17 and 11% of calcium input with river runoff in 2016 and 2017, and 8 and 6%, respectively, for hydrocarbonates (Fig. 2). It is reasonable to explain this by the precipitation of calcium carbonate:
which also produces the similar pH dependence on chloride content decreasing with CO2 release.
Concentrations of components of the carbonate system as functions of chloride concentration in the mixing zone of the Ural River and Caspian Sea. (1) 2016; (2) 2017. Here and in Figs. 3–7, dash means the calculated lines of conservative mixing.
The possibility of formation of chemogenic calcium carbonate in the North Caspian Sea, especially in the sites of local pH increase during photosynthesis, was confirmed by natural experimental data (Savenko, 2007) in the Volga mouth: the degree of water saturation in calcium carbonate sharply increased from 1–2 in fresh waters to 4.5 in the mouth beach at chloride content ∼400 mg/L, and then remained at the same level. The scales of this phenomenon can be estimated by analyzing the composition of suspended matter in the North Caspian Sea and on other mouth beaches of the arid zone, where significant amounts of chemogenic calcite, sometimes up to 10–20%, were frequently observed in suspended matter during intense photosynthesis (Khrustalev, 1978, 1989). The intense formation of aragonite on shallow sites of the near-channel shoal of the Volga delta was noted for the first time by Radushev (1957).
Dissolved nutrients migrate under the influence of production–destruction processes, in particular, the consumption of large amounts of silicon by diatom phytoplankton, the vital activity of which is supported by significant input of nitrates with river runoff and maintenance of sufficient phosphate concentrations (Fig. 3). For silicon and nitrates, the efficiency of biological assimilation is maximum and reaches, respectively, 58–88 and 61–67% of their contents in riverine waters, which is confirmed by significant interannual variations. The less intense removal of dissolved nitrites (up to 18–25% of their content in the Ural waters) is supposedly related to the oxidation by nitrobacteria. Phosphates are characterized by nontypical distribution: in the region with moderate chlorinity, they are additionally supplied in solution in amounts up to 1.5–3 times more than their removal with river runoff, which is possible only if a source of dissolved phosphates is present in the mixing zone of the riverine and sea waters. It is known that phosphorus possesses the highest remineralization rate. Therefore, such source could be pore waters of the surface layer of bottom sediments, which in the absence of stratification, enter in contact with vertically stirring water column.
Barium is intensely desorbed from terrigenous material at the initial salinization stage in amounts reaching 10.6 μg/L, or 20% of its input with riverine waters (53.0 μg/L). At chloride content >1.8 g/L at the mouth beach, its behavior becomes close to conservative (Fig. 4). Similar barium distribution with desorption zone slightly more extended by chlorinity range and a close value of its maximum excess in the solution (13.6 μg/L) are typical of the Volga mouth (Savenko et al., 2014). However, the barium content in the Volga waters (28.8 μg/L) is almost two times lower than that of the Ural waters. Barium migration in most part of the studied mouths around the world is also controlled by sorption–desorption processes (Gordeev, 2012, and others).
Heavy metals and hydrolyzate elements form strong complexes with dissolved organic matter and occur as true dissolved species and in colloid particles. Their mobility in the river mouth worldwide sharply decreases at the early stages of mixing with seawater owing to the coagulation and flocculation of organic and organo-mineral colloids (Gordeev, 2012). In the mouth of the Ural River, this group of trace elements includes manganese, iron, and aluminum, significant part of which (respectively, up to 26–38, 25–34, and 46–62% of their contents in the riverine water mass) is removed from solution within the chlorinity range of 0.7–2.5 g/L, above which their concentrations approach those of the North Caspian Sea. This group also includes lead, titanium, zirconium, and hafnium, the content of which above a local minimum in the zone of active flocculation of colloids gently increases toward the seaward boundary of the mixing zone. As a result, their highest removal from solution is, respectively, 1.1–1.2, 1.4–5.5, 3.6–6.0, and 2.7 times higher than their input with river runoff (Figs. 5, 6). Rare-earth elements occupy an intermediate position in this group: their content in the North Caspian waters is higher than those in the zone of maximum removal, but immobilization does not exceed their removal with riverine waters (Fig. 7, Table 6). Similar distribution of trace elements in colloid fraction was established in the Volga mouth (Savenko et al., 2014).
Thus, tendencies in the transformation of the runoff of dissolved matter in the Ural mouth are similar to those observed in the mixing zone of the Volga and Caspian waters and are determined by the chemical peculiarities of riverine waters and North Caspian sea.
CONCLUSIONS
(1) Most major ions (Na, K, Mg, SO4) and some trace elements (Li, Rb, Cs, Sr, Co, Ni, Cu, Zn, Sb, Ga, Y, U, B, F, Cr, Ge, Mo, W) in the Ural mouth show conservative behavior, which is described by common relationships of their concentrations with chloride content for 2016 and 2017.
(2) The distribution of components of the carbonate system is controlled by the chemogenic formation of calcium carbonate at the mouth beach, which leads to the 11–17 and 6–8% removal from solution of calcium and hydrocarbonates supplying with river runoff and to the simultaneous decrease of pH value.
(3) Migration of dissolved nutrients is controlled by the biological assimilation and regeneration: consumption of large amounts of silicon and sodium by phytoplankton (up to 58–88 and 61–67% of their content in riverine waters), removal of significant part (up to 18–25%) of nitrites, and additional influx of phosphates supposedly from pore waters of the surface layer of bottom sediments in amounts up to 1.5–3 times higher than their removal with river runoff.
(4) Barium is additionally supplied in solution (up to 20%) at the initial salinization stage owing to the desorption from terrigenous material, but shows nearly conservative behavior at the mouth beach.
(5) Coagulation and flocculation of colloids, which contain heavy metals and hydrolyzate elements forming strong complexes with dissolved organic matter, lead to the removal of significant part (up to 25–100%) of runoff of dissolved manganese, iron, aluminum, and rare-earth elements, as well as to the extraction from solution of lead, titanium, zirconium, and hafnium in amounts 1.1–6 times higher than their contents in the riverine water.
REFERENCES
V. F. Brekhovskikh, V. D. Kazmiruk, and A. V. Savenko, “Transformation of the dissolved matter discharge in the near–mouth region of the Volga River,” Geochem. Int. 43 (6), 619–626 (2005).
V. F. Brekhovskikh, E. V. Ostrovskaya, D. N. Katunin, and Z. V. Volkova, “Influence of the Volga runoff on the heavy metal distribution in its mouth seashore,” Meteorol. Gidrol., No. 2, 88–97 (2006).
V. F. Brekhovskikh, E. V. Ostrovskaya, Z. V. Volkova, et al., Pollutants in Waters of the Volga–Caspian Basin (Astrakhan, 2017) [in Russian].
V. S. Brezgunov and V. I. Ferronskii, “Concentrations of microelements in the Caspian Sea and different occurrence types of dissolved elements in marine environment (based on expedition data of 1995),” Water Res. 31 (1), 68–72 (2004).
A. A. Chibilev, Ural Basin: History, Geography, and Ecology (UrO RAN, Yekaterinburg, 2008) [in Russian].
L. L. Demina, V. V. Gordeev, and L. S. Fomina, “Fe, Mn, Zn, and Cu species in the river water and suspended matter and their variations in mixing zone of river and sea waters: evidence from the rivers of the Black, Azov, and Caspian seas,” Geokhimiya, No. 8, 1211–1229 (1978).
V. V. Gordeev, River–Sea System Geochemistry (Moscow, 2012) [in Russian].
Yu. P. Khrustalev, Trends of the Sedimentation in Intracontinental Seas of the Arid Zone(Nauka, Leningrad, 1989) [in Russian].
Yu. P. Khrustalev, Trends of the Modern Sedimentation in the Northern Caspian Area, (RGU, Rostov-on-Don, 1978) [in Russian].
A. P. Lisitsyn, “Marginal oceanic filters,” Okeanologiya 34 (5), 735–748 (1994).
P. N. Makkaveev, V. V. Gordeev, P. O. Zav’yalov, and A. K. Kurbaniyazov, “Hydrochemical and hydrological conditions in the lower reaches of the Ural River and in the mouth area of the Caspian Sea at the beginning of flood time, Meteorol. Gidrol., No. 10, 108–116 (2018).
V. N. Mikhailov, Mouths of the Russia’s River and Adjacent Countries: Past, Present, and Future (GEOS, Moscow, 1997) [in Russian].
V. I. Radushev, “Chemogenic carbonate formation in rivers of the arid zone,” Dokl. Akad. Nauk SSSR 114 (1), 180–181 (1957).
A. V. Savenko, “Strontium behavior in the mixing zone of the Volga and Caspian Sea waters,” Water Res. 26 (2), 220–223 (1999).
A. V. Savenko, “Assessment of the degree of water saturation in the Volga mouth area in calcium carbonate, Geology of Seas and Oceans. Proceedings of 17thInternational Conference (School) on Marine Geology (GEOS, Moscow, 2007), Vol. 3, pp. 179–181 [in Russian].
A. V. Savenko, V. F. Brekhovskikh, and O. S. Pokrovskii, “Migration of dissolved trace elements in the mixing zone between Volga River water and Caspian seawater: results of observations over many years,” Geochem. Int. 52 (7), 533–547 (2014).
E. A. Zakharova and V. S. Savenko, “Fluoride and boron of the Volga River and the Caspian Sea waters mixing zone,” Geochem. Int. 36 (2), 180–182 (1998).
ACKNOWLEDGMENTS
This work was supported by the Russian Foundation for Basic Research (project no. 16–05–00369).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Bogina
Rights and permissions
About this article
Cite this article
Savenko, A.V., Pokrovsky, O.S. Transformation of Dissolved Matter Runoff in the Ural River Mouth. Geochem. Int. 58, 947–958 (2020). https://doi.org/10.1134/S0016702920070101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702920070101