Abstract—
A physicochemical study of natural hydrous magnesium orthophosphates bobierrite Mg3[PO4]2 · 8H2O and kovdorskite Mg2[PO4](OH) · 3H2O from the Kovdor carbonatite massif, Kola Peninsula, Russia, was carried out using X-ray diffraction, infrared spectroscopy, Raman spectroscopy, and thermal analysis. The enthalpies of dehydration of the minerals were measured using a NETZSCH DSC 204 F1 differential scanning calorimeter, the enthalpies of formation from the elements for bobierrite (–6167 ± 16 kJ/mol) and kovdorskite (–3251 ± 10 kJ/mol) were determined by high-temperature melt solution calorimetry on a Setaram high-temperature heat-flux Tian–Calvet microcalorimeter. The values of their standard entropies and Gibbs energies of formation are estimated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Bobierrite Mg3[PO4]2 · 8H2O is a natural hydrous Mg phosphate belonging to the vivianite group. It occurs as colorless, yellow, white, and pale blue wedge-shaped split crystals, rosettes, fan-shaped aggregates, and spherulites, which line (together with collinsite, kovdorskite, and other rare phosphates) the walls of cavities in dolomite carbonatites among apatite ores and phoscorites, and as an alteration product of the phoscorites and apatite-group minerals. The crystal structure of bobierrite consists of single [MgO2(H2O)4] and doubled (sharing edges) [Mg2O6(H2O)4] octahedrons connected with one another through \({\text{PO}}_{4}^{{3 - }}\) tetrahedrons to form layers parallel to (010). The neighboring layers are linked by the hydrogen bonds of water molecules (Takagi et al., 1986). Bobierrite crystallizes in the monoclinic system, space group C2/c.
Kovdorskite is a relatively rare hydrous Mg phosphate, whose formula is Mg2[PO4](OH) · 3H2O. This mineral sometimes contains small amounts of Fe and Mn. It was first found in 1969 in dolomite carbonatite of the Kovdor iron deposit in the Kola Peninsula, in cavities with dolomite, magnetite, hydrotalcite, manasseite, pyrite, bobierrite, and collinsite (Kapustin et al., 1980). The structure of kovdorskite is made up of clusters of four Mg octahedrons that share edges and one PO4 tetrahedron (Ovchinnikov et al., 1980). Oxygen atoms occur in the apexes shared by Mg octahedrons and PO4 tetrahedron, OH groups occur in apexes shared by three Mg octahedrons, and H2O molecules occupy the other apexes of Mg octahedrons and apexes shared by two Mg octahedrons. The clusters occur in layers parallel to (001) in structural voids and define a pseudohexagonal motif. The layers are connected through [PO4]3– tetrahedrons. Kovdorskite crystallizes in the monoclinic system, space group P2(1)/a. In contrast to bobierrite, kovdorskite contains not only crystal-hydrate H2O molecules but also structural OH groups.
Kovdorskite and bobierrite are formed, together with other Mg and Ca carbonates and phosphates, during the late hydrothermal transformations of dolomite carbonatites and apatite–magnetite phoscorites, likely at temperatures no higher than 300°C (Ponomareva and Krasnova, 1990).
The structure of kovdorskite was studied and its unit-cell parameters were determined in (Kapustin et al., 1980; Ovchinnikov et al., 1980; Ponomareva and Krasnova, 1990; Morrison et al., 2012), and bobierrite was studied in (Frazier et al., 1963; Kanazawa et al., 1979; Takagi et al., 1986; Liferovich et al., 1999). The minerals were studied by Raman and IR spectroscopy (Ponomareva and Krasnova, 1990; Liferovich et al., 1999; Frost et al., 2002; Morrison et al., 2012; Frost et al., 2013), and their thermal behavior was discussed in (Manly, 1950; Kapustin et al., 1980; Liferovich et al., 1999; Frost et al., 2013).
Data on thermodynamic properties of these minerals are limited to information on the Gibbs free energy of formation of bobierrite, and we are aware only of a single paper (Duff, 191) in which the value of \(\Delta fG_{{}}^{0}\)(298.15 K) was determined by studying the dissolution of Mg hydroorthophosphate newberyite MgHPO4 ∙ 3H2O. Data in (Vieillard and Tardy, 1984; La Iglesia, 2009) were acquired using various estimation techniques.
Our research involved a comprehensive physicochemical and thermochemical studies of kovdorskite and bobierrite by Calvet microcalorimetry and differential scanning calorimetry.
METHODS
This study was carried out with bobierrite and kovdorskite samples from the Kovdor carbonatite massif in the Kola Peninsula, Russia. These samples were made available for us by the Fersman Mineralogical Museum, Russian Academy of Sciences (bobierrite and kovdorskite sample nos. FN288 and FN287, respectively). The bobierrite sample consisted of single colorless transparent flattened crystals and radiating aggregates up to 2 cm across. The kovdorskite sample was an aggregate of colorless transparent flattened crystals 0.5–1.5 cm long, which were taken from radiating rosettes of this mineral hosted in magnetite–dolomite carbonatite.
The chemical composition of the minerals was analyzed on a Camebax-microbeam (France) microprobe equipped with an Si(Li) EDS detector and INCA Energy Oxford analytical system. The operation conditions were 20 kV accelerating voltage and 30 nA beam current.
The X-ray diffraction identification of the minerals was made in a STOE-STADI MP (Germany) X-ray powder diffractometer with a curved Ge(III) monochromator that ensured highly monochromatic CaKα1 radiation (λ = 0.178897 Å). The data were acquired by successively covering scanning regions, using a position-sensitive linear detector (2Θ = 5°, channel width 0.02°).
The Raman spectroscopic study was conducted on an EnSpector R 532 (Russia) Raman microscope. The wavelength of the laser radiation was 532 nm, the laser output was approximately 16 mW, the holographic dispersion grating had 1800 lines/mm, the spectral resolution was 6 cm–1, and the diameter of the focal spot varied from 1 to 2.5 µm at 40× magnification. The spectra were acquired within the region of 100 to 4000 cm–1, using randomly oriented samples, at a counting time of 1 s and averaging over 50–100 positions.
The IRspectroscopic study was carried out on a FSM-1201 (LOMO, Russia) FTIR spectrometer within the range of 400 to 4000 cm–1, with the frequencies determined accurate to ±1 cm–1 in transmission mode in air at room temperature, using samples prepared in the form of suspensions in paraffinic oil.
The thermal behavior of the minerals up to 1200 K was studied on NETZSCH TG 209 F1 thermogravimetric analyzer in air flow (40 mL min–1), at a heating rate of 10 K min–1. The instrument was calibrated on the melting points of standard reference compounds (Ag, Al, Bi, and Sn 99.999% purity). The systematic inaccuracy of the weight measurements was no higher than 0.1%. The samples were weighed before the measurements on A&D GH-202 analytical scale balance accurate to 2 × 10–5 g. The masses of the samples were 5.38 mg for kovdorskite and 12.36 mg for bobierrite.
Thermochemical studies. The enthalpies of formation of the natural Mg phosphates were determined in a Setaram (France) Tian–Calvet microcalorimeter by dissolving in 2PbO ∙ B2O3 melt. The samples 1.3–8.3 (±2 × 10–3) mg were dropped from room temperature into solvent melt in a calorimeter at T = 973 K. The measured total heat involved the enthalpy of the mineral and its dissolution enthalpy [H0(973 K) − H0(298.15 K) + Δdis-solH0(973 K)] (Kiseleva and Ogorodova, 1983). In six to eight experiments with melt portions of 30–35 g, the solute/solvent ratio approached that of an infinitely diluted solution, and the mixing enthalpy thus is infinitely close to zero. The calorimeter was calibrated by dropping a standard compound (platinum), with needed data on its [H0(973 K) − H0(298.15 K)] borrowed from (Robie and Hemingway, 1995).
The enthalpies of dehydration of the hydrous phosphates were measured on a NETZSCH DSC 204 F1 differential scanning calorimeter in a flow if high-purity nitrogen (40 mL min–1) at a heating rate of 10 K min–1. The instrument was calibrated for temperature and heat flux in compliance with recommendations of the ASTM E967 and ASTM E2253 standards. The standards were high-purity (99.999%) compounds whose parameters of phase transitions were determined highly accurately: cyclohexane C6H12, Hg, Ga, benzoic acid С6H5COOH, KNO3, In, Sn, Bi, Pb, CsCl, and Zn. The systematic inaccuracies of the calibration curves were 0.1°C and 5% heat. The measurements were carried out in standard aluminum containers with punctured lids. The DSC curves thus acquired were processed with the NETZSCH Proteus Thermal Analysis software. The masses of the compounds were 4–6 mg.
The following equipment was used in the course of this study: Camebax-microbeam microprobe at the Fersman Mineralogical Museum, Russian Academy of Sciences; STOE STADI MP X-Ray powder diffractometer and FSM-1201 IR Furrier spectrometer, EnSpector R532 Raman microscope, and Setaram Tian–Calvet microcalorimeter at the Geological Faculty of the Moscow State University; and NETZSCH TG 209 F1 thermogravimetric analyzer and NETZSCH DSC 204 F1 differential scanning calorimeter at the Chemical Faculty of the Moscow State University.
RESULTS AND DISCUSSION
Samples
Data of chemical analysis of the samples are presented in Table 1. The chemical formulas were normalized to three cations for kovdorskite and five cations for bobierrite and are Mg1.97Mn0.01[PO4]1.02(OH)0.90 ∙ 2.96H2O and Mg2.97Fe0.02Mn0.01[PO4]2.00 ∙ 8.20H2O, respectively. These formulas are close to the theoretical ones for kovdorskite Mg2[PO4](OH) · 3H2O and bobierrite Mg3[PO4]2 · 8H2O. All thermodynamic constants were calculated in this work for the minerals corresponding to their theoretical formulas.
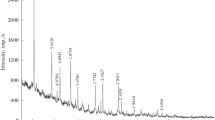
The X-ray diffraction spectra of the natural hydrous phosphates (Figs. 1, 2) are consistent with data on natural samples from the Kovdor Massif in the RRUFF (RRUFF project) international database and correspond to bobierrite and kovdorskite.
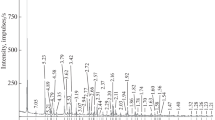
The Raman spectra of the minerals (Figs. 3b, 4b) are consistent with the spectra of bobierrite in the database RRUFF # R 060681 (RRUFF project) and in (Frost et al., 2002) and the spectra of kovdorskite in RRUFF # R 060579 (RRUFF project) and (Morrison et al., 2012; Frost et al., 2013).
The Raman spectrum of the bobierrite (Fig. 3b) is close to that of vivianite, a hydrous Fe phosphate, Fe3[PO4]2 ∙ 8H2O, which was studied in (Ogorodova et al., 2017). The region of 900–1100 cm–1 shows an intense line of totally symmetric valence vibration at 953 cm–1 and low-intensity lines of triply degenerate antisymmetric stretching vibrations of the [PO4]3– ion at 1002, 1075, and 1100 cm–1. The region 400–600 cm–1 includes lines pertaining to triply degenerate deformation vibrations (at 558 cm–1 and a shoulder at 584 cm–1) and doubly degenerate vibrations of the PO4 tetrahedron. The frequency region below 400 cm–1 includes cation translational vibrations and lattice modes. The high-frequency region shows low-intensity lines corresponding to valence vibrations of the water molecule at 3121, 3208, 3429, and 3439 cm–1. The occurrence of molecular water in the mineral is confirmed by the presence of a scattering line corresponding to the deformation vibrations of the water molecule (1627 cm–1).
The Raman spectrum of the kovdorskite (Fig. 4b) is similar in general configuration to the bobierrite spectrum. The frequency region of 950–1100 cm–1 shows an intense line of totally symmetric valence vibrations at 966 cm–1 and low-intensity lines of triply degenerate antisymmetric stretching vibrations of the [PO4]3– ion at 1057 and 1093 cm–1. The region of 400–600 cm–1 includes lines corresponding to triply degenerate deformation vibrations (456 and a shoulder at 479 cm–1) and to doubly degenerate deformation vibrations (539 and a shoulder at 576 cm–1) of the PO4 tetrahedrons. The region below 400 cm–1 shows lines of cation translational vibrations and lattice modes. The region of the stretching vibrations of the OH group exhibits low-intensity lines pertaining to the vibrations of the water molecule (at 2986, 3219, and 3383 cm–1). The frequency of 1642 cm–1 corresponds to the deformation vibrations of the water molecule. The occurrence of the OH group in the structure of the mineral is reflected in the kovdorskite spectrum as an intense narrow line at 3686 cm–1.
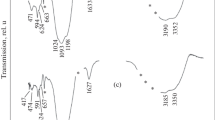
The IR absorption spectra of the bobierrite (Fig. 5c) and kovdorskite (Fig. 6f) are consistent with data on samples of these minerals from the Kovdor Massif (Chukanov, 2014): nos. P39 and P107 for the bobierrite and kovdorskite, respectively. Analogous data are presented in (Frost et al., 2002; Morrison et al., 2012; Frost et al., 2013) and in the database RRUFF no. 050505.1 (RRUFF project)
The IR absorption spectrum of the bobierrite (Fig. 5c) is also similar to the IR spectrum of the group-forming mineral vivianite (Ogorodova et al., 2017). The region of the valence vibrations of the OH group shows a broad intense absorption band with one narrow (3474 cm–1) and three smoothed-out components at 3285, 3187, and 3110 cm–1. An intense band corresponding to the deformation vibrations of water molecules shows a maximum at 1594 cm–1 and a shoulder at 1660 cm–1. The intense band corresponding to the valence vibrations of the [РО4]3– ion consists of two components with maxima at approximately 1010 and 1037 cm–1 and two shoulders at approximately 976 and 1079 cm–1. The intense lines with maxima at 709 and 855 cm–1 correspond to the librational vibrations of the water molecules. The absorption bands at 547 and 574 cm–1 and a shoulder at 549 cm–1 pertain to triply degenerate deformation vibrations of the PO4 tetrahedron. The band with a frequency of 474 cm–1 corresponds to the doubly degenerate deformation vibration of [РО4]3–.
The high-frequency region of the IR absorption spectrum of the kovdorskite (Fig. 6f) shows an intense broad absorption band of the valence vibrations of molecular water, with two maxima at 3207 and 3402 cm–1 and an intense narrow band at 3691 cm–1, which corresponds to the valence vibrations of the OH groups that coordinate the cations. A band of deformation vibrations of the water molecules is registered at about 1658 cm–1. An intense absorption band corresponding to the valence vibrations of the [РО4]3– ion has a maximum at a frequency of 1037 cm–1 and two shoulders at 1018 and 1067 cm–1. The absorption lines at approximately 652 and 782 cm–1 and a shoulder at 812 cm–1 pertain to the librational vibrations of the hydroxyl groups and water molecules. The low-frequency region shows lines of triply and double degenerate deformation vibrations of the [РО4]3– ion at 564, 453, and 421 cm–1.
Figures 7 and 8 exhibit the results of thermal analysis of the minerals. The TG and DTG curves of the bobierrite (Fig. 7) show that it is dehydrated in two stages. The mineral starts to rapidly lose mass at a temperature close to 340 K, and water removal from it is completed at 980 K. During the first stage at 340–510 K, the mass loss is ~31.2% (about seven water molecules are removed), and the loss during the second stages is ~4.7% (the last remaining water molecule is lost). The IR spectrum of bobierrite heated to 510 K (Fig. 5b) is remarkably modified: the high-temperature region contains no intense band at 3474 cm–1, the overall intensity of the absorption band of the valence vibrations of the water molecule is much lower, as also is the intensity of the absorption band of deformation vibrations at 1642 cm–1, and the spectrum has no absorption bands related to librational vibrations at 709 and 855 cm–1. The absorption bands at 450–590 and 950–1100 cm–1, which correspond to the deformation and valence vibrations of the [PO4]3– ion in the bobierrite structure, acquire a configuration typical of phosphate glass. The IR absorption spectrum of sample dehydrated at 980 K (Fig. 5a) indicates that a new highly crystalline phase is present: this is farringtonite Mg3[PO4]2 (Chukanov and Chervonnyi, 2016; no. P 481). The presence of newly formed farringtonite also follows from the IR spectrum of the sample calcinated at T = 980 K (Fig. 3a). This spectrum coincides with data in RRUFF no. R 130127 (RRUFF project) and (O’Neill et al., 2006).
The TG and DTG curves of the kovdorskite (Fig. 8) show four mass–loss stages: (1) at 465–550 K, which corresponds to ~15.8% loss of the sample mass (removal of ~2% water molecules), (2) 550–730 K (mass loss of ~4.6%), (3) 730–810 K (~further ~4.1% mass loss), and (4) 810–1140 K (loss of the remaining water, ~3.8%). To elucidate in greater detail the sequence of water removal from kovdorskite, we have studied the IR spectra of products obtained at various heating stages of this mineral. The IR spectra of kovdorskite heated to 550 K (point A on the TG curve, Fig. 8) shows a decrease in absorbance intensity only in the regions of deformation and valence vibrations of water. The spectrum (Fig. 6d) of the sample heated to 730 K (Fig. 8, point B) provides evidence of the total dehydroxylation of the mineral (the absence of an intense band at 3693 cm–1 correspond to absorption by hydroxyl groups), further dehydration of the sample, and the development of a glass-like phase. The IR spectrum (Fig. 6c) of the product of heating to 810 K (Fig. 8, point C) shows that the glass-like phase with a low water concentration is preserved. The spectrum (Fig. 6b) of the sample heated to 980 K (Fig. 8, point D) provides evidence of farringtonite Mg3[PO4]2 crystallization and the preservation of the remaining water. The further heating of the sample to T = 1140 K (Fig. 8, point E) leads to the complete dehydration of the farringtonite (Fig. 6a), which exactly agrees with the results of IR spectroscopy (Fig. 4a). The heating of the kovdorskite to T = 550 K thus leads to the removal of its crystal-hydrate water and part of its OH groups, and heating to 730 K results in the complete dehydroxylation of the mineral and its further dehydration with the formation of phosphate glass. The further heating to 1140 K is associated with the crystallization of anhydrous Mg phosphate.
Enthalpy of Formation
The average values of seven Tian–Calvet calorimetric measurements of [H0(973 K) − H0(298.15 K) + Δdissol.H0(973 K)] were 1956.7 ± 37.9 J/g = 420.0 ± 8.1 kJ/mol (М = 214.64 g/mol) for kovdorskite and 2078.3 ± 22.5 J/g = 845.8 ± 9.1 kJ/mol (М = 406.98 g/mol) for bobierrite (the inaccuracies are calculated at 95% probability).
The data thus acquired were used to calculate the standard enthalpy of formation of the hydrous magnesium phosphates, using reactions (1) and (2) and Eqs. (3) and (4)
where ΔH = [H0(973 K)−H0(298.15 K) + Δdissol.H0(973 K)] is calorimetric data on the minerals, MgO, P2O5, Al2O3, and Al(OH)3 (Table 2); the required values of Δf\(H_{{el}}^{0}\)(298.15 K) of the latter are reported in the same table. The calculated enthalpies of formation of kovdorskite and bobierrite are given in Table 3.
Enthalpy of Dehydration
Data obtained by studying water removal from kovdorskite and bobierrite using differential scanning calorimetry are presented in Figs. 9 and 10. The heat corresponding to the first dehydration stage and reflected in the DSC curve of bobierrite (Fig. 9) is Q = 1097 J/g = 447 kJ/mol. The DSC curve for kovdorskite (Fig. 10) shows three endothermic effects related to water removal from the mineral. The measured heat values correspond to the stepwise mass loss were Q1 = 443 J/g = 95 kJ/mol (removal of the crystal-hydrate water and partly OH groups); Q2 = 194 J/g = 42 kJ/mol (removal of the crystal-hydrate water and remaining OH groups); Q3 = 49 J/g = 11 kJ/mol (loss of crystal-hydrate water).
Gibbs Energy of Formation
To calculate the Gibbs energy of formation of the hydrous phosphates from elements, we have evaluated the standard entropy values not available from the literature (Table 3). The calculations were carried out according to Latimer’s method, with regard for the average entropy values for cations and anions in the solid compounds and the entropy contribution of the crystal-hydrate water (Naumov et al., 1971). The estimates of So(298.15 K) and the enthalpies of formation determined in the course of this research were used to calculate Δf\(G_{{el}}^{0}\)(298.15 K) for bobierrite and kovdorskite (Table 3). The Gibbs energy value thus obtained (–5472 ± 16 kJ/mol) are close to the value determined in studying exchange reactions in (Duff, 1971) and reported without error (–5450.5 kJ/mol).
CONCLUSIONS
Chemical analyses of Martian meteorites found on the Earth and data of Martian surface scanning by the Spirit and Opportunity Mars exploration rovers of NASA have shown that phosphorus content in the Martian lithosphere is roughly one order of magnitude higher than the average concentration of this element in terrestrial rocks (Dreibus et al., 1996; Wänke and Dreibus, 1998). Ample evidence indicates that hydrous phosphates are currently present on Mars and that the surface of this planet contained water in the historical past. The thermodynamic parameters we obtained for kovdorskite and bobierrite can be recommended to use in simulating mineral-forming processes in the Martial lithosphere.
In the Earth’s lithosphere, bobierrite occurs much more widely than kovdorskite and is formed in a great diversity of geological environments: in dolomite veins hosted in apatite ores and phoscorites (as in the iron ore quarry at the Kovdor deposit, Russia; Liferovich et al., 1999), as an alteration product of primary phosphate minerals in granite pegmatites in Bendata, Portugal (Garate-Olabe et al., 2012) and in Wodgina, Australia (Mason and Dunn, 1974); and in phosphorus-rich sedimentary iron ores in Kerch, Crimea, Russia. Fine-grained crusts of this mineral are produced by chemical alterations in guano at Mejillones, Chile (Atencio et al., 2012) and in elephant teeth at Edgerton, Minnesota, United States (Gruner and Stauffer, 1943). Kovdorskite is endemic to the Kovdoir Massif, although this mineral was found at the deposit as fairly large spectacular crystals and crystal aggregates up to 10 cm (Liferovich et al., 1999). The thermodynamic characteristics of kovdorskite and bobierrite reported in this publication can be used to constrain the stability fields of minerals and to elucidate the reasons for their highly uneven distribution in the Earth’s lithosphere.
REFERENCES
D. Atencio, N. V. Chukanov, F. Nestola, T. Witzke, J. M. Coutinho, A. E. Zadov, and G. Färber, “Mejillonesite, a new acid sodium, magnesium phosphate mineral, from Mejillones, Antofagasta, Chile,” Am. Mineral. 97, 19–25 (2012).
N. V. Chukanov, Infrared Spectra of Mineral Species: Extended Library (Springer-Verlag GmbH, Dordrecht–Heidelberg–New York–London, 2014).
N. V. Chukanov and A. D. Chervonnyi, Infrared Spectroscopy of Minerals and Related Compounds (Springer Cham, Heidelberg–New York–Dordrecht–London, 2016).
G. Dreibus, T. Jagoutz, B. Spettel, and H. Wänke, “Phosphate-mobilization on Mars? Implications from leach experiments on SNCs,” Lunar Planet. Sci. Conf. 27, 323 (1996)
E. J. Duff, “Orthophosphates. Part VIII. The transformation of newberryite into bobierrite in aqueous alkaline solutions,” J. Chem. Soc. A, 2736–2740 (1971).
M. D. Dyar, E. R. Jawin, E. Breves, G. Marchand, M. Nelms, M. D. Lane, S. A. Mertzman, D. L. Bish, and J. L. Bishop, “Mössbauer parameters of iron in phosphate minerals: Implications for interpretation of martian data,” Am. Mineral. 99, 914–942 (2014).
A. W. Frazier, J. R. Lehr, and J. P. Smith, ‘The magnesium phosphates hannayite, schertelite and bobierrite,” Am. Mineral. 48, 635–641 (1963).
R. L. Frost, W. Martens, P. A. Williams, and J. T. Kloprogge, “Raman and infrared spectroscopic study of the vivianite-group phosphates vivianite, baricite and bobierrite,” Mineral. Mag. 66 (6), 1063–1073 (2002).
R. L. Frost, A. López, Y. Xi, A. Granja, R. Scholz, and R. M. F. Lima, “Vibrational spectroscopy of the phosphate mineral kovdorskite—Mg2(PO4)(OH) ⋅ 3H2O,” Spectrochim. Acta Part. A114, 309–315 (2013).
I. Garate-Olabe, E. Roda-Robles, P.-P. Gil-Crespo, A. Pesquera-Pérez, R. Vieira, and A. Lima, “Estudio textural y mineralogico del Dique de Cuarzo con Fosfatos de Folgosinho (Guarda, Portugal),” Macle junio revista de la sociedad espanola de mineralogia 16, 220–221 (2012).
J. W. Gruner and C. R. Stauffer, “A unique occurrence of bobierrite, Mg3(PO4)2 ⋅ 8H2O,” Am. Mineral. 28, 339–340 (1943).
T. Kanazawa, T. Umegaki, and M. Shimizu, “The synthesis of Mg3(PO4)2 ⋅ 8H2O and its new polymorphism,” Bull. Chem. Soc. Jpn. 52, 3713–3717 (1979).
Yu. L. Kapustin, A. V. Bykova, and Z. V. Pudovkina, “Kovdorskite–a new mineral,” Zap, Ross. Mineral. O-va, No, 3, 341–347 (1980).
I. A. Kiseleva and L. P. Ogorodova, “An application of high-temperature dissolution calorimetry for determination of enthalpies of formation of hydroxyl-bearing minerals by the example of talc and tremolite,” Geokhimiya, No. 12, 1745–1755 (1983).
A. La Iglesia, “Estimating the thermodynamic properties of phosphate minerals at high and low temperature from the sum of constituent units,” Estud. Geológ. 65 (2), 109–119 (2009).
R. P. Liferovich, Ya. A. Pakhomovsky, V. N. Ykovenchuk, A. N. Bogdanova, and A. Yu. Bakhchisaraitsev, “Vivianite and bobierrite group minerls from the Kovdor massif,” Zap. Ross. Mineral. O-va, No. 6, 109–117 (1999).
R. L. Manly, “The differential thermal analysis of certain phosphates,” Am. Mineral. 35, 108–115 (1950).
B. Mason and P. J. Dunn, “An unusual occurrence of bobierrite at Wodgina, Western Australia,” Miner. Record. No. 5, 265–267 (1974).
S. M. Morrison, R. T. Downs, and H. Yang, “Redetermination of kovdorskite, Mg2(PO4)(OH) ⋅ 3H2O,” Acta Crystallogr. Sect. E 68, i12–i13 (2012).
G. B. Naumov, B. N. Ryzhenko, and I. L. Khodakovsky, A Handbook of Thermodynamic Data (for Geologists) (Atomizdat, Moscow, 1971) [in Russian].
A. Navrotsky and W. J. Coons, “Thermochemistry of some pyroxenes and related compounds,” Geochim. Cosmochim. Acta 40, 1281–1295 (1976).
A. E. O’Neill, D. Uy, and M. Jagner, “Characterization of phosphates found in vehicle-aged exhaust gas catalysts: a Raman study,” SAE Paper # 2006-01-0410 (2006).
L. P. Ogorodova, L. V. Melchakova, I. A. Kiseleva, and I. A. Belitsky, “Thermochemical study of natural pollucite,” Thermochim. Acta 403, 251–256 (2003).
L. P. Ogorodova, I. A. Kiseleva, L. V. Mel’chakova, M. F. Vigasina, and E. M. Spiridonov, “Calorimetric determination of the enthalpy of formation for pyrophyllite,” Russ. J. Phys. Chem. A85 (9), 1492–1494 (2011).
L. Ogorodova, M. Vigasina, L. Mel’chakova, V. Rusakov, D. Kosova, D. Ksenofontov, and I. Bryzgalov, “Enthalpy of formation of natural hydrous iron phosohate: vivianite,” J. Chem. Thermodyn. 110, 193–200 (2017).
V. E. Ovchinnikov, L. P. Solov’eva, Z. V. Pudovkina, Yu. L. Kapustin, and N. V. Belov, “Crystalline structure of kovdorskite Mg2(PO4)(OH) ⋅ 3H2O,” Dokl. Akad Nauk SSSR 255 (2), 351–354 (1980).
E. V. Ponomareva and N. I. Krasnova, “New data on kovdorskite—Mg2(PO4)(OH) ⋅ 3H2O,” Zap. Vsesoyuz. Mineral. O-va, No. 6, 92–100 (1990).
R. A. Robie and B. S. Hemingway, “Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures,” U.S. Geol. Surv. Bull. No. 2131, (1995). RRUFF Project. Database of Raman Spectroscopy, X-ray Diffraction and Chemistry of Minerals, http://www.rruff.info/
S. Takagi, M. Mathew, and W. E. Brown, “Crystal structures of bobierrite and synthetic Mg3(PO4)2 ⋅ 8H2O,” Am. Mineral. 71, 1229–1233 (1986).
S. V. Ushakov, K. V. Helean, A. Navronsky, and L. A. Boatner, “Thermochemistry of rare-earth orthophosphates,” J. Mater. Res. 16 (9), 2623–2633 (2001).
P. Viellard and Y. Tardy, “Thermochemical properties of phosphates, Phosphate Minerals, Ed. by J. O. Nriagu, and P. B. Moore (Springer-Verlag, Berlin, 1984), pp. 171–198.
H. Wänke and G. Dreibus, “Chemical composition and accretion history of terrestrial planets,” Philos. Trans. R. Sos. London Ser. A 325, 545–557 (1998).
Funding
This study was supported by the Russian Foundation for Basic Research, project no. mk-18-29-12128.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Kurdyukov
Rights and permissions
About this article
Cite this article
Ogorodova, L.P., Gritsenko, Y.D., Vigasina, M.F. et al. Natural Magnesium Hydrous Orthophosphates Bobierrite and Kovdorskite: FTIR, Raman, Thermal, and Thermochemical Study. Geochem. Int. 58, 189–199 (2020). https://doi.org/10.1134/S0016702920020093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702920020093