Abstract
All major, rock-forming lower-mantle minerals (bridgmanite, CaSi-perovskite, ferropericlase and stishovite) are “nominally anhydrous minerals” (NAMs), in which hydrogen comprises less than 1 wt % and whose chemical formula would be normally written without hydrogen. In NAMs, hydrogen occupies various defects of the crystal lattice and is bonded to structural oxygen, forming hydroxyl groups. Currently, two main techniques can be used for water determination in the mantle minerals: Fourier transform infrared spectrometry (FTIR) and secondary ion mass spectrometry (SIMS). They produce different results: determinations by SIMS are usually higher than quantifications of FTIR. As a result, the estimates of water concentrations in lower-mantle minerals vary widely. Most reliable concentrations of water are 1400–1800 ppm in bridgmanite, 10–80 ppm in ferropericlase, and 20–150 ppm in stishovite. The average concentration of water in the lower mantle is ~1500 ppm. Despite such minor concentrations in lower-mantle minerals, water forms a great reservoir within the lower mantle, probably amounting ~45.45 × 1023 grams H2O, i.e., ~3.3 times the mass of the Earth’s oceans. Some amount of water is transported into the lower mantle by subducting lithospheric slabs; this amount is balanced by the water flux from the lower mantle to the transition zone. Within areas of partial melting in the lower and upper parts of the lower mantle, as well as in some local areas, stress and thermal increase initiate release of water from lower-mantle minerals into melt. The enrichment of partial melts with H2O depends on the P–T conditions, oxygen fugacity values, and percentage of melting. It causes major geodynamic processes that are initiated within the deep Earth. The major source of the water reservoir in the lower mantle is primordial water stored early in the Earth’s evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
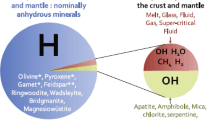
The ‘problem’ of water in the Earth’s mantle became evident in the 1980s, when H2O was first identified in upper-mantle minerals, including olivine, pyroxenes, and garnet (Smyth 1987; Bell and Rossman 1992). In these minerals, water occurs in trace amounts (<0.1 wt % H2O) and their chemical formulas would be normally written without hydrogen. These mineral phases are “nominally anhydrous minerals” (NAMs), where “water” is, in reality, hydrogen H, located in defects of the crystal lattice and bonded to structural oxygen forming hydroxyl groups.
The early studies of hydroxyl in NAMs came about during the 1950s from the electronics industry, specifically hydrogen in quartz, a phase used for electronic devices (e.g., Mitchell and Rigden, 1957; Rossman, 2006). The possibility that mantle NAMs could incorporate essential amounts of water as crystallographic defects were discussed in the early 1970s (e.g., Fyfe, 1970; Martin and Donnay, 1972), and H2O was identified in NAMs only in the late 1980s among upper-mantle minerals, with the use of IR-spectroscopy (Smyth 1987; Bell and Rossman 1992). Subsequently, based on quantitative calibration of the IR absorption bands with measurements and nuclear reaction analysis of OH in minerals, concentrations of water were found in minerals from xenoliths in kimberlites (e.g., Matsyuk and Langer 2004; Kolesnichenko et al. 2017) and from inclusions within diamond (e.g., Novella et al., 2015; Taylor et al., 2016 and references therein).
Later, a significant concentration of water (~1.4–1.5 wt % H2O) was found in natural ringwoodite, the dominant mineral in the transition zone (Pearson et al., 2014); the transition zone was thereafter considered as water-saturated, globally (Fei et al., 2017). Such as significant quantity of H2O, within this part of the mantle, would account for nearly the same amount of water as in all of the world’s oceans (Nestola and Smyth, 2016). The presence of water in the deeper mantle may affect various physical properties of the deep Earth, such as electrical conductivity, elastic properties, phase boundaries, melting, etc. At the early stages of the Earth’s history water played an important role during the solidification of the magma ocean and in core segregation. Moreover, water played a significant role during the formation of the Earth and Moon from a supraplanetary gas-dust body (Galimov, 2011). The effect of water on phase transformations in the mantle transition zone and the core-mantle boundary may lead to a significant revision of temperature distribution inside the Earth and its spatial and temporal energy balance (Litasov and Ohtani, 2007).
The presence of water below the transition zone (deeper than 660 km), in natural lower mantle minerals, is poorly constrained to date, although there is direct evidence on this subject. Diamonds from the lower mantle contain the structural impurity of hydrogen several times more than is found in lithospheric diamonds. Recently, in a diamond from Namaqualand were found crystalline inclusions of ice-VII, formed at depths of between 610–800 km (Tschauner et al., 2018). Furthermore, the development of experimental, high-pressure techniques over the last two decades has facilitated the study of synthetic lower-mantle mineral phases.
First of all, it should be said that the mineral composition of the lower mantle differs drastically to that of the upper-mantle and transition-zone compositions. In contrast to those areas, where silicates predominate, the lower mantle is composed almost entirely of oxide minerals (bridgmanite (Mg,Fe)SiO3, CaSi-perovskite CaSiO3, ferropericlase (Mg,Fe)O, stishovite SiO2 and others) and should therefore be referred to as “oxide Earth”. Our knowledge of this part of the Earth has greatly expanded in recent decades, from the studies of mineral inclusions and microxenoliths in lower-mantle diamonds (Harte et al., 1999; Kaminsky, 2012, 2017; Kaminsky et al., 2015), owing chiefly to newly developed techniques (Wirth, 2009; Silversmit et al., 2011).
It should be noted as well that the term “water”, as applied to mantle minerals, is conventional because molecules of H2O exist only in the hydrosphere, while actual hydrogen H atoms (which may also be termed “protons”) are incorporated as various species in minerals, silicate/carbonate melts and volatile-rich fluids. In most mantle mineral phases, “water” is actually a hydroxyl group, OH. In mantle melts and fluids, hydrogen may exist as gaseous H2, methane CH4, hydrogen sulfide H2S, and other, more complex molecules (Armstrong et al., 2015). However, in this paper I will use for simplicity the term “water” and recalculate all hydrogen into wt % of water.
Along with this, some minerals from the lower-mantle mafic and the carbonatitic associations are indeed hydrous minerals, containing water as a major stoichiometric component, such as hydrous aluminous Phase Egg and δ−AlOOH; dense hydrous magnesium silicates—phases “B”—Mg12Si4O19(OH)6, “D”—MgSi2O6H2 and “H”—MgSiO4H2; phlogopite KMg3(Si3AlO10)(OH)2, nahcolite NaHCO3, and cuspidine Ca4(Si2O7)(OH)2. The concentrations of water in these minerals reach 15–18 wt %, and some of minerals from the mafic association may comprise 10–30% of MORB material, subducted into the lower mantle (Ricolleau et al., 2010). However, the role of subducted mafic lithospheric material on the bulk composition in the Earth’s lower mantle is believed insignificant; only ~3% of water entering subduction zones reach the lower mantle (Bodnar et al., 2013). Herein I will concentrate on water as this relates to the compositions of minerals, comprising the lower-mantle juvenile ultramafic association (Kaminsky, 2012).
The hydrogen content of the lower mantle (as well as the upper mantle, the transition zone, and the core) has not been constant over geological time, but permanently changes in a dynamic water cycle. During the long history of the Earth, re-distribution of hydrogen among co-existing lower-mantle minerals takes place according to partition coefficients between these minerals as functions of thermodynamic conditions and mineral compositions (Peslier et al., 2017).
ANALYTICAL TECHNIQUES
Currently, two main techniques are being used for water determination in mantle minerals: Fourier transform infrared spectrometry (FTIR) and secondary ion mass spectrometry (SIMS) (Rossman, 2006). Each method has advantages and disadvantages.
FTIR (generally within the range of 3300–3700 cm–1 area of the infrared spectrum) is a non-destructive technique that allows detection of the speciation of H-defect location (e.g., Beran and Libowitzky, 2006; Libowitzky and Beran, 2006; Rossman, 2006), but requires doubly-polished samples for transmission quantitative analysis and a sample size not less than 0.5 mm. Infrared spectra, however easily obtained, are not self-calibrating, so independent methods of analysis are necessary to calibrate the spectroscopic work. Each mineral has to be calibrated for quantitative FTIR analysis; the absorption coefficients for a mineral calibrated in different laboratories, however, can vary significantly (Paterson, 1982; Pawley et al., 1993; Libowitzky and Rossman, 1996; Bolfan-Casanova, 2005; Mosenfelder et al., 2015).
Most mantle minerals are biaxial, and the IR absorbance of such minerals is anisotropic. Therefore, polarized light is typically used for quantitative FTIR analysis; and only the total absorbance of the polarized spectra (as the sum of integrated areas of OH bands in the polarized infrared spectra measured along three principal axes of the oriented mineral grain) is proportional to mineral water content (Libowitzky and Rossman, 1996; Libowitzky and Beran, 2006). It has been proposed to use the corrected average of unpolarized absorbances of multiple grains as the total absorbance to calculate mineral water concentration (Kovács et al., 2008; Sambridge et al., 2008). This method does not need the polarizer and hence the mineral grain is not required to be orientated, which sometimes can greatly simplify sample analysis procedures (Hui et al., 2016).
Due to the limited brightness of a thermal source in FTIR, the spatial resolution and signal-to-noise ratio (SNR) are quite low. One way to resolve this limitation is to use synchrotron radiation (SR), which is some order of magnitude more intense than a conventional source. Therefore, the SNR of the spectra using SR-FTIR is much higher than that of conventional FTIR. The beam size could be reduced to a few micrometers in the mid-IR region, to increase the spatial resolution without degradation of the SNR (Della Ventura et al., 2014). This suggests that even small features of the spatial distribution of water in NAMs may be mapped using SR-FTIR techniques. In addition, the SR has several key advantages, such as continuous distribution of intensity over the entire infrared range, high degree of polarization and high stability (Della Ventura et al., 2014). Most laboratories, however, have difficulty in accessing synchrotron sources, and hence the SR-FTIR is far less widely used than conventional FTIR.
In contrast, SIMS can be performed on samples polished on a single side, but is partly destructive. Its advantage is the simultaneous measurement of water concentration and H isotopes, as well as other volatiles (Cl, F, C, S). It requires suitable standards for a given mineral. SIMS has become a well-used technique for quantitative analysis of water in NAMs since the 2000s (e.g., Hauri 2002; Koga et al., 2003; Aubaud et al., 2007; Mosenfelder et al., 2011).
Concentrations of water established with the use of SIMS systematically overestimate FTIR data in lower-mantle minerals by a factor of several times (Tables 1 and 2). The same effect of overestimating water content by SIMS was found in other minerals, such as feldspar (Mosenfelder et al., 2015). Bolfan-Casanova (2005) surmises that this effect exists because SIMS cannot discriminate the hydrogen counts from microscopic inclusions distributed within the sample, while the IR technique allows possible discrimination between contamination and water bound in fluid inclusions; their conclusions were based upon this recognition in natural perovskitic phases. In any case, FTIR data seem more reliable than those for SIMS, and below I will base any conclusions mainly upon FTIR data for water in mantle minerals.
Attempts have been made to use other techniques in performing analyses for water determination in lower-mantle minerals. For example, micro-Raman spectroscopy (RS) was used by Kleppe and Jephcoat (2006) because this method allows for the study of smaller grains than is required for FTIR (~1 μm); it is also easier to use RS with high-pressure diamond-anvil cell (DAC) studies. Recently, Nisr et al. (2017) studied water-rich stishovite by RS methods, but they were unable to quantify the results. There have also been attempts to use nuclear magnetic resonance (NMR) and thermogravimetric analysis (TGA) for water determination in stishovite (Spektor et al., 2011, 2016). The initial data showed a general agreement with SIMS.
WATER IN LOWER-MANTLE MINERALS
General
Data on water in synthetic lower-mantle minerals are scarce and sometimes contentious. Most representative results are obtained for bridgmanite (Mg,Fe)SiO3, the major lower-mantle mineral. It should be noted that experimental measurements and theoretical calculations of water solubility in minerals cannot directly represent the actual water content of the lower mantle. These only constrain the maximum water concentrations in minerals, and thus the storage capacity of the mantle.
Bridgmanite
The first attempt to evaluate the presence and the concentration of water in bridgmanite was performed by Meade et al. (1994) with the use of synchrotron-induced micro-IR techniques. They examined single crystals of almost pure (0.33 wt % Al2O3) bridgmanite that were grown from an H2O-rich (50 wt %) melt of MgSiO3, at a pressure of 27 GPa and a temperature of 1830°C. Two absorption bands were observed at 3483 and 3423 cm–1, associated with structurally bound hydroxyl; these bands were used in future by other researches as well. The concentration of hydrogen in the samples was established, using Paterson (1982) absorption coefficients for hydrogen in silicates as 700 ± 170 H atoms per 106 Si atoms. This work was the first indication of a possible hydrogen reservoir in the lower mantle.
In subsequent experiments with the use of FTIR, the concentration of water in Al-free bridgmanite was found to be very low, at 1–2 ppm (Bolfan-Casanova et al., 2003; Bolfan-Casanova, 2005). The established concentration, most likely, is understated for natural bridgmanite because along with bridgmanite, ringwoodite occurred in the system, which does not occur in the lower mantle and likely concentrates the major part of any water present. More representative data on bridgmanite were obtained by Litasov et al. (2003) who also utilized FTIR techniques. In their experiments, pure bridgmanite, associated with melt only, contained 104 ppm H2O; a result that seems more reliable compared to the data of Bolfan-Casanova et al. (2003) and Bolfan-Casanova (2005). In experiments with bridgmanite containing 2, 4.4 and 7.2 wt % Al2O3, the determined concentration was 101, 1101 and 1440 ppm H2O, respectively, as a function of increasing content of Al which replaces Si.
In addition, Litasov et al. (2003) measured hydrogen concentrations in bridgmanite from systems, modelling the MORB (24.50–16.77 wt % Al2O3 and 23.40–26.23 wt % FeO) and pyrolite (5.50–5.80 wt % Al2O3 and 7.39–6.09 wt % FeO) compositions. For the MORB system, water concentrations in bridgmanite are the same as in the Al-free system (47–110 ppm), while pyrolitic bridgmanite, which may be considered as an analogue to natural lower-mantle bridgmanite, contains 1460–1780 ppm H2O.
These results indicate that in the lower mantle natural bridgmanite, which usually has high concentrations of Al (up to 12.6 wt %; Kaminsky, 2012), may contain significant quantities of water. This is caused by the increase of oxygen vacancies, in the bridgmanite crystal structure, with the increase of the Al3+ content (2VISi4+ → 2VIAl3+ + VO). This creates favorable conditions for hydroxyl OH– incorporation into the bridgmanite structure.
There were also analyses of water in bridgmanite with the use of SIMS (Murakami et al., 2002; Sanehira et al., 2002; Inoue et al., 2016). The results were 0.2–0.8 wt % H2O, i.e., more than one order of magnification higher than for the FTIR determinations.
The existing data on the solubility of water in bridgmanite are summarized in Table 1 and Fig. 1. One can see that they vary very widely, from 1–2 ppm to almost 1 wt %. The contrasting results may be due to differences in sample bulk compositions, synthesis conditions and, first of all, the analytical techniques. FTIR determinations are located in the lower end of the concentration range, while all SIMS data are at the higher end. One may agree with Bolfan-Casanova (2005) who considers strongly overstated SIMS data as the result of the presence of microscopic post-genetic inclusions, distributed within bridgmanite. However, the absence of micrometer-size fluid inclusions was demonstrated by monitoring the profile of hydrogen-intensity with sputter time during the course of SIMS measurements by Litasov et al. (2003). Possibly, the inclusions are nano-sized.
Concentrations of water in synthetic bridgmanite determined by various techniques. Data from Meade et al. (1994), Murakami et al. (2002), Sanehira et al. (2002), Bolfan-Casanova et al. (2003), Bolfan-Casanova (2005), Litasov et al. (2003), Inoue et al. (2010), Hernández et al. (2013), Panero et al. (2015).
In any case, based on most reliable data, two major groups of bridgmanite may be distinguished by concentrations of water within them. The first group, Al-free bridgmanite has concentrations of H2O near 100 ppm. The second group of Al-containing bridgmanite (>2 wt % Al2O3) has higher concentrations of H2O. The samples associated with ringwoodite and majorite should be excluded from any consideration because they were synthesized at conditions transitional between the lower mantle and the transition zone. Most reliable analogs of natural lower-mantle bridgmanite samples contain from 1400 ppm to 1800 ppm H2O. Exactly such concentrations may be accepted for water in natural bridgmanite within the lower mantle because Al-free bridgmanite does not occur in natural lower-mantle samples (Kaminsky, 2012).
The model of water incorporation in bridgmanite is the oxygen vacancy mechanism (OVM), according to which Al replaces Si in the octahedral site, only where oxygen vacancies are created to balance the charge:
The oxygen vacancies may be occupied by water:
(Navrotsky 1999; Keppler and Bolfan-Casanova 2006) or form Al3+ – H+ pairs (Ross et al., 2003; Panero et al., 2015) (Fig. 2).
Post-Perovskite
The water partitioning between bridgmanite and post-perovskite in the D'' layer was studied using first-principles calculations of total energy and abinitio lattice dynamics simulations to compute the quasi-harmonic free energies (Townsend et al., 2016). They found that, at ambient mantle geotherm conditions, water favors Al-bearing post-perovskite over Al-bearing bridgmanite by a factor of 3.2 : 1. These results suggest that post-perovskite may contain as much as 1–2 wt % H2O (Townsend et al., 2016) and that the D'' layer may host primordial water.
In regions of the lowermost mantle where upwelling primordial hydrous post-perovskite transforms to bridgmanite, water would be liberated, and might generate melts at the base of the mantle by dehydration reactions, analogous to dehydration melting reactions across the 410 and 660 km discontinuities of the mantle transition zone.
CaSi-Perovskite
There is little data on water in CaSi-perovskite. This mineral accommodates comparable with bridgmanite AlSi and OHO defects (Akber-Knutson and Bukowinski, 2004) and theoretically may concentrate the same amounts of water under lower-mantle conditions. Only a single water analysis was carried out with the use of SIMS for an amorphized CaSi-perovskite, grown in a natural peridotitic composition at 25.5 GPa and 1600–1650°C, and finding 0.4 wt % H2O (Murakami et al., 2002). At the same time, there are doubts concerning water solubility in CaSi-perovskite, with suggestions that this mineral phase should essentially be anhydrous (Németh et al., 2017).
Ferropericlase
Data on water solubility in ferropericlase are scarce and contradictory. Initially, Bolfan-Casanova et al. (2000) reported that hydrogen dissolves more readily into ferropericlase than into bridgmanite. This corresponded to the first determinations of the concentration of water in ferropericlase (fe = 0.076), with the use of SIMS at ~2000 ppm (Murakami et al. 2002). However, the analyses utilizing FTIR spectroscopy, performed for ferropericlase of the same composition, gave results two orders of magnitude lower, ~20 ppm (Bolfan-Casanova et al., 2002). All subsequent analyses of ferropericlase were made with FTIR (Bolfan-Casanova et al., 2003, 2006; Keppler and Bolfan-Casanova, 2006; Litasov 2010). Joachim et al. (2013) analyzed low-pressure pure periclase under water-saturated conditions utilizing both FTIR and Raman spectral methods and obtained 3.4–3.5 ppm and 8.4–9.3 ppm respectively, which is in good agreement (Table 2).
The most reliable results demonstrate the solubility of water in ferropericlase similar to natural composition within the range of 10–80 ppm H2O, almost two orders of magnitude lower than in bridgmanite. At the same time abinitio calculations with the stoichiometric composition of periclase demonstrate that it should be practically dry (Merli et al., 2016; Muir and Brodholt, 2018). This contradicts data by Bolfan-Casanova et al. (2003) who found, for 24 GPa and 1400°C, \(D_{{{\text{water}}}}^{{{{{\text{Per}}} \mathord{\left/ {\vphantom {{{\text{Per}}} {{\text{Bridg}}}}} \right. \kern-0em} {{\text{Bridg}}}}}}\) = 60. However, theoretical calculations lead to the opposite conclusion that water at lower-mantle conditions (24–80 GPa) prefers bridgmanite to periclase with \(D_{{{\text{water}}}}^{{{{{\text{Per}}} \mathord{\left/ {\vphantom {{{\text{Per}}} {{\text{Bridg}}}}} \right. \kern-0em} {{\text{Bridg}}}}}}\) in the 0.31–0.56 interval (Merli et al., 2016).
The addition of Al2O3 virtually does not influence the solubility of water in ferropericlase. Pure periclase with 0.9–1.2 wt % Al2O3, synthesized at 25 GPa, contains 11–25 ppm H2O, while ferropericlase (fe = 0.12) with 0.9–2.9 wt % Al2O3 contains 14–79 ppm H2O (Litasov, 2010). It should be taken into account that in natural ferropericlase the Al2O3 content does not exceed 0.13 wt % (Kaminsky, 2017).
The variations of the iron content within a range of fe = 0.01–0.13 also have little effect on the water solubility (Bolfan-Casanova et al., 2006). At the same time, the ferric iron content, as was indicated by Mössbauer spectra, decreases with pressure, while the OH concentration increases (Keppler and Bolfan-Casanova, 2006). This indicates that H+ in ferropericlase replaces Fe3+ defects. It was suggested that the incorporation of water in ferropericlase has a form of isolated OH groups (Keppler and Bolfan-Casanova, 2006).
The OH solubility in ferropericlase can be well explained by the incorporation of hydrogen as isolated hydroxyl groups via the reduction of ferric iron (Bolfan-Casanova et al., 2002). In this case:
This substitution mechanism was found to be energetically favorable in olivine and wadsleyite, based on atomistic simulations (Wright and Catlow, 1996); it is likely that it remains the same in ferropericlase (Fig. 3).
Another, alternative mechanism of incorporation water in ferropericlase is related to intrinsic point defects in its structure (Demouchi et al., 2007). Under hydrous conditions, charge neutrality conditions may look like:
where \(({\text{OH}})_{{\text{O}}}^{ + }\) defects are essentially interstitial protons.
In the natural environment, both ferric iron and hydrogen are present in the system. Therefore, another possible charge neutrality condition is:
where \({{{\text{\{ (OH)}}_{{\text{O}}}^{ + } - {\text{V}}_{{{\text{Me}}}}^{{2 - }}{\text{\} }}}^{ - }}\) are defect associates formed between a proton and a metal vacancy. For this charge neutrality condition, the concentration of \({{{\text{\{ (OH)}}_{{\text{O}}}^{ + } - {\text{V}}_{{{\text{Me}}}}^{{2 - }}{\text{\} }}}^{ - }}\) defects must be twice the number of unassociated vacancies (Demouchi et al., 2007).
Stishovite
Data on water in stishovite are mostly obtained for not the lower-mantle but the transition-zone conditions, where stishovite associates with ringwoodite, majorite and clinopyroxene (Pawley et al., 1993; Bolfan-Casanova et al., 2000; Chung and Kagi, 2002; Bromiley et al., 2006; Litasov et al., 2007). The SIMS analyses (as well as thermogravimetric analyses, TGA), like for other minerals, show extreme results (Spektor et al., 2011, 2016). Thought more reliable are those data for stishovite in association with bridgmanite, obtained under pressure conditions over the range 24-25 GPa (Panero et al., 2003; Litasov et al., 2007). All current data obtained for stishovite are summarized in Table 3.
The FTIR determinations of water in stishovite, formed within the pressure range of 30–63 GPa, are 80–520 ppm. These do not differ much to the values for water in Al-containing stishovite formed within the transition zone (90–1840 ppm). No correlation between the concentrations and pressure is observed. At the same time, in samples, where stishovite associates with melt, the water concentrations remain the same, indicating an almost equal distribution of water between stishovite and melt.
Concentrations of water in stishovite clearly correlate with Al2O3 independently of pressure. Litasov et al. (2007) demonstrated that Al-free stishovite contains 8–21 ppm H2O, and with the increase of Al2O3 up to 4.36–7.62 wt %, the water concentration increases up to 1600–1840 ppm. Other experiments (Chung and Kagi, 2002; Bromiley et al., 2006) confirm this trend (Fig. 4).
Concentrations of Al in natural lower-mantle stishovite are low, less than 0.1 wt % Al2O3 (Kaminsky 2012). This allows one to estimate the water content in natural lower-mantle stishovite at 20–150 ppm.
The most likely way of incorporating hydrogen within stishovite is, as for bridgmanite, the oxygen vacancy mechanism (OVM):
with subsequent occupation of site vacancies by hydroxyl groups (Pawley et al., 1993; Chung and Kagi, 2002; Panero et al., 2003):
However, in this model less than half of the Al atoms in stishovite are charge balanced with hydrogen to satisfy the electro-neutrality conditions in the mineral (Pawley et al., 1993; Smyth et al., 1995; Chang and Kagi, 2002; Litasov et al., 2007). For this reason a second mechanism, charge coupled substitution (CCM) of Al3+ onto the large, distorted octahedral interstitial site in the stishovite structure is thought possible:
Such a mechanism is more likely under high Al concentrations (Bromiley et al., 2006) or high pressures (Litasov et al., 2007), while the first mechanism (OVM) with the formation of vacancies likely occurs at low Al concentrations, i.e., under natural conditions.
The structure of stishovite (space group P42 /mnm) consists of corner- and edge-sharing silicate octahedra SiO6 (partially replaced by octahedra AlO6) such that each Si4+ (Al3+) cation is coordinated by six oxide O2– anions and each oxide anion is coordinated by three Si4+ (Al3+) cations. The incorporation of Al3+ leads to the formation of AlO6 octahedra with an interstitial H+, replacing a part of the silicate SiO6 octahedra (Fig. 5). In a super-cell, the Al-octahedron is rotated with respect to the Si-octahedra. The charge density analysis (Ross et al., 2003) and first-principle calculations (Panero and Stixrude, 2004) demonstrated that hydrogen is most stable when bonded to the apical oxygen of the Al-octahedron (Fig. 5).
Post-Stishovite Phases
There have been no reports on the water solubility in post-stishovite phases, such as CaCl2-type SiO2 and seifertite. The structure of the high-pressure, orthorhombic post-stishovite phase with a CaCl2-type symmetry (space group P21nm) is close to that of δ-AlOOH, which contains both AlO6 and HO6 octahedra (e.g., Vanpeteghem et al., 2007). The stishovite—to—post-stishovite transition occurs at pressures of 75–80 GPa and the adiabate temperature ~2200°С; this allows for the suggestion at depths of over 1700–1800 km, the solubility of water in SiO2 is outside of the field for stishovite stability.
WATER IN LOWER-MANTLE MINERAL ASSOCIATIONS AND MELTS
The partition coefficients of water between lower-mantle minerals at the upper border of the lower mantle, based upon first-principle calculations for 24.3 GPa (Hernández et al., 2013), are: between ringwoodite and bridgmanite \(D_{{{\text{water}}}}^{{{\text{Ringw/Bridg}}}}\) = 10–13 depending on temperature (10 at 2000 K and 13 at 1500 K), which is similar to experimental values (9–24; average ~15; Inoue et al., 2010); and between ferropericlase and bridgmanite \(D_{{{\text{water}}}}^{{}}\) = 90, which also corresponds to average estimates of the water solubility in these minerals. These coefficients are calculated for the area intermediate between the lower mantle and the transition zone. However, the amount of water in each phase and, accordingly, partition coefficients vary strongly with depth and with total content of water, which is modelled as Al3+–H+ pairs. Their formation is closely related to configurational entropy, whose role is dominant at lower water concentrations; with increasing water concentration its effect becomes less favoured in a balance between volumetric and entropic effects on the free energy (Muir and Brodholt, 2018). As a result, if the total water content in the lower mantle is low (~100 ppm), then the water is preferentially incorporated into bridgmanite as Al3+–H+ pairs. For ~1000 ppm of total water, bridgmanite remains the major water concentrator at depths of greater than 1000 km, while above this depth CaSi-perovskite becomes the dominant host up to the top of the lower mantle. For very high concentrations of water (∼5000 ppm), the depth range where CaSi-perovskite is the main host for water, is extended to about 1200 km. It should be noted that in the proposed model the incorporation of H+ is suggested in a form of Al3+–H+ pairs, although the hydroxyl OH model of the hydrogen incorporation is also possible (Fig. 2).
Low seismic velocity regions within the lower mantle, particularly in its deepest portion (e.g., Garnero and McNamara, 2008; Schmandt et al., 2014) indicate the existence of melts in these regions. The most likely interpretation of such regions is partial melting of the lower-mantle material. Therefore, if these low velocity regions are caused by partial melting, some water is likely present there. During the course of partial melting, which may be initiated by local stress or/and a local increase in the thermal conditions within that part of the lower mantle, water releases from lower-mantle minerals and passes into melt owing to very low partition coefficient \(D_{{\text{H}}}^{{{{{\text{Peridotite}}} \mathord{\left/ {\vphantom {{{\text{Peridotite}}} {{\text{Melt}}}}} \right. \kern-0em} {{\text{Melt}}}}}}\), experimentally established from 0.01 (Kohn and Grant, 2006) to 0.0065 (Rosentahl et al., 2015). In such a case, one may expect the dehydration of lower-mantle phases during the course of any melting, as well as concentration of water in the melt. The enrichment of partial melts with H2O depends on the P–T conditions, oxygen fugacity values, and percentage of melting and may result in complete miscibility of water and silicate melt. At the same time, as first-principles molecular dynamics simulations of hydrous MgSiO3 melt demonstrate, pressure has a profound influence on speciation of the water component. It changes from simple hydroxyls and water molecules at low pressure to a much greater variety of species at high pressure, such as Si–O–H–O–Si polyhedral linkages, –O–H–O–H– chains and O–H–O edge decoration of SiO6 octahedra (Mookherjee et al., 2008).
The lower-mantle melts are likely to be positively buoyant and may upwell (Sakamaki et al., 2006; Hirschmann et al., 2012). At some levels melts may be neutrally buoyant and remain at some levels and spread laterally (Lee et al., 2007), like it was determined seismically at the 410-km discontinuity (Schmandt et al., 2014). In other cases, they may form mantle plumes, indicated by ocean-island basalts (OIB) enriched with high 3He/4He ratio and with anomalous isotopic compositions of other noble gases (e.g., Graham, 2002; Mukhopadhyay, 2012). On the other hand, during the Earth’s first billion years, deep and hot melting of the lower mantle may have resulted in the generation of dense liquids that crystallized and sank into the lower mantle, with the formation of “primordial”, undegassed lower-mantle reservoirs forming large low shear velocity provinces (LLSVP) (Lee et al., 2010).
WATER BALANCE IN THE LOWER MANTLE
The concentration and distribution estimates of water in the Earth still have many uncertainties, particularly for the lower mantle and the core. In the early years of studying the mantle Ringwood (1966) suggested that the high amount of water in the Earth’s interior possibly exceeds its total amount in the lithosphere, hydrosphere and atmosphere. The approximate estimates of the average water concentration in the entire mantle were, at that time, at 0.1–0.2 wt % (Harris and Middlemost, 1969; Wyllie, 1970). The first determinations of water in lower-mantle minerals gave a basis for the conclusion that the Earth’s lower mantle may be one of the major reservoirs of water in the Earth (Meade et al., 1994; Murakami et al., 2002). Litasov et al. (2003) were the first to make a quantitative estimate of water storage capacity in the lower mantle at 3.42 × 1021 kg, i.e., ~2.5 times the present ocean mass. Further estimates vary from 0.2 to 4.3 times the mass of the ocean (Bodnar et al., 2013; Nestola and Smyth 2016), although the estimations of water content in the lower mantle were more modest than previously, from 10 to 100 ppm (Keppler and Bolfan-Casanova, 2006; Litasov and Ohtani, 2007; Mottl et al., 2007; Inoue et al., 2010; Bodnar et al., 2013). The presence of 20% of stishovite in the lower-mantle assemblage is likely to increase this estimate up to 600 ppm H2O (Litasov and Ohtani, 2007).
After the discovery of high water concentrations in the transition-zone, as evidenced in ringwoodite at 1.4–1.5 wt % H2O (Pearson et al., 2014), the expectations of elevated water content in the lower mantle became higher. Nestola and Smyth (2016) estimated the average concentration of water in the lower mantle at 2000 ppm which leads to the total amount of water in this area at 6.10 × 1024 grams, i.e., ~7 times the ocean mass. Peslier et al. (2017) calculated that if the lower mantle consists of 80% bridgmanite with ~800 ppm H2O, 11% ferropericlase with ~2000 ppm H2O, 8% CaSiO3 with 3500 ppm H2O, and 1% of hydrous minerals with ~20 000 ppm H2O (according to Ohtani 2015), then it contains on average 1340 ppm H2O and has a mass of 40.6 × 1023, i.e., 2.9 times the mass of the oceans.
For the Earth’s core early estimates of water, based on high values of a partition coefficient between silicate melt and liquid iron under high pressure, as well as on the possible presence of iron hydride in the core, resulted in the volume equal to 100 times the ocean mass; the core was thereafter considered as the main water reservoir in the Earth (Okuchi, 1997; Williams and Hemley, 2001; Saxena et al., 2004). In more recent calculations, that consider the high solubility of hydrogen in liquid iron metals, the concentration of water in the core is suggested at 80–500 ppm to ~1000 ppm, and the mass from 5.75 × 1023 grams H2O to 17.4 × 1023 grams H2O, i.e., up to ~12 times that of the Earth’s oceans (Nestola and Smyth, 2016; Peslier et al., 2017).
A summary on estimates of concentrations and mass of water in the deep Earth, reflecting variations therein, is presented in Table 4. The proportion of lower-mantle water in the Earth’s balance varies from 1% (Mottl et al., 2007) to 50% (Nestola and Smyth, 2016). My estimate for the lower mantle, considering most reliable data on water concentrations in major lower-mantle minerals (1400–1800 ppm in bridgmanite, 10–80 ppm in ferropericlase, and 20–150 ppm in stishovite) and the dominance of bridgmanite in the composition of the lower mantle, is ~1500 ppm. In such a case the total mass of water in the lower mantle should be 45.45 × 1023 grams H2O, i.e., ~3.3 times the ocean masses.
Two things should be considered in addition. First, in all calculations of the water content in the entire lower mantle, only NAMs were considered as concentrators of water. However, there may be other mineral phases that play host to hydrogen in the lower mantle (particularly in its lowermost part, the D'' layer), first of all iron hydrides. Hydrogen, along with other elements lighter than Fe and Ni, such as N, C, O, Si, and S comprise together approximately 5–10% of the core’s mass, where it forms iron hydride (Stixrude et al., 1997; McDonough, 2014). The D'' layer, occupying the lowermost 200 km of the lower mantle, is the area, in which iron-rich liquid metal from the outer core, containing light elements, infiltrates into the lowermost mantle and forms a series of assemblages comprising native Fe0, iron carbides, nitrides, and hydrides (Kaminsky, 2017). There may not only be not FeH hydride, but some other compounds; for example, FeH2, FeH3, FeH5 (Pepin et al., 2014) and Fe3H5, Fe3H13, FeH6 (Bazhanova et al., 2012; Kvashnin et al. 2018), which were found to be stable at lower-mantle pressure conditions.
Second, it should be noted that during the Earth’s geological history the water content in the mantle was not always constant. As the cosmochemical analysis (Albarede, 2009) demonstrates, the ancient Earth’s mantle was essentially dry, and only later obtained water during the course of the bombardment by ice meteorites. During the solidification of the magma ocean, the Earth’s mantle may have contained water in the amount from ~1 (Elkins-Tanton, 2008) to ~10 (Hamano et al., 2013) ocean masses.
SOURCES OF THE LOWER-MANTLE WATER
There are three possible sources of water in the Earth’s lower mantle:
(1) Primordial water stored early in the Earth’s evolution;
(2) Water transported from the surface and oceans by descending slabs;
(3) Water expelled from a hydrogen-saturated outer core (Litasov et al., 2003).
In view of the paucity of data on water exchange between the lower mantle and the core, the most popular mechanism for the presence of water in the lower mantle is that introduced by subduction of slabs, during which various water-containing minerals and sediments of the oceanic core may supply water right down to the core-mantle boundary and perhaps even into the core (Ohtani et al., 2001, 2015; Ohtani et al., 2005; Komabayashi et al., 2004; Ohira et al., 2014; Peslier et al., 2017). It is suggested that billions of years of plate tectonics have recycled lithosphere into the deep mantle with the potential to deliver hydrous components. Such a mechanism cannot be confirmed by numerical calculations, not less any talk about the enigmatic character of water exchange between subducted hydrous minerals and NAMs of the lower mantle.
The existing estimates of water mass transported by subducting slabs into the mantle vary from 9 to 18 × 1011 kg/year (Litasov and Ohtani, 2007), but only a small portion of this mass reaches the lower mantle. Bodnar et al. (2013) calculated the amount of the total flux of water into the lower mantle from the subduction of oceanic crust and lithosphere at 5 × 1010 kg/year, that corresponds to 2.25 × 1023 grams for 4.5 Ga, if we suggest the subduction process started very early in the Earth’s history. This amount is only a minor part of the total mass of water in the lower mantle (Table 4). Moreover, it is exactly the same amount of water that the lower mantle supplies to the transitional zone (Bodnar et al., 2013), i.e., the flux of water into the lower mantle in the supposed subduction process is fully balanced by the outgoing flux.
A significant (and possibly major) part of water within the lower mantle should be primordial, representing the actual nature of the ancient lower mantle. Primordial water, along with other volatile elements, originated from a cosmochemical reservoir; and the solid Earth may have stored up to ~3000 ppm water (Marty, 2012; Halliday, 2013). The enrichment of the lower mantle in water was caused by iron reduction from the FeO form, during the formation of a massive metallic iron core (Galimov, 2011). Subsequently, owing to the general Earth’s degassing, the amount of water in the Earth decreased, but the solubility of water in minerals allowed the lower mantle to retain a significant hydrogen content. The existence of primordial remnants within the lower mantle is supported by the isotopic composition of noble gases from OIBs, which are considered as samples derived from the lower-mantle (Graham, 2002; Mukhopadhyay, 2012). Helium-isotope (3He/4He) ratios, found in OIBs, are more than 30 times the present-day ratio of our Earth’s atmosphere (e.g., Füri et al., 2010). High 3He/4He ratios in OIBs indicate the presence of relatively undegassed regions in the deep mantle, as compared to the upper mantle, which retain a greater proportion of their primordial He (e.g., Stuart et al., 2003; Jackson et al., 2010). Such primordial areas are likely to contain primordial water, and recent studies of hydrogen isotopic composition support this idea. Isotopically low D/H ratios in Hawaiian lavas, as well as in lavas from Baffin Island, Canada and Iceland (δD more negative than –218‰ SMOW), which represent volcanic rocks from the early Iceland mantle plume and contain the highest recorded terrestrial 3He/4He ratios, confirm the existence, in the deep Earth, of primitive undegassed areas, which inherited their isotopic characteristics directly from the protosolar nebula (Hauri, 2002; Hallis et al., 2015). These areas are most likely the sources of the lower-mantle water reservoir.
CONCLUSIONS
(1) All major lower-mantle minerals (bridgmanite, CaSi-perovskite, ferropericlase, stishovite) are nominally anhydrous minerals (NAMs), in which hydrogen occupies various defects of the crystal lattice and is bonded to structural oxygen, forming hydroxyl groups. Most reliable concentrations of water are 1400–1800 ppm in bridgmanite, 10–80 ppm in ferropericlase, and 20–150 ppm in stishovite.
(2) Within areas of low seismic velocity and thermal anomalies that locally result in an increased temperature, partial melting of the lower mantle is envisaged to occur, whereby water releases from lower-mantle minerals to pass into melt, which may then either buoyantly rise, remain neutral or sink, depending on the P–T conditions, oxygen fugacity values, and percentage of melting. These effects cause major geodynamic processes that are initiated within the deep Earth.
(3) The average concentration of water in the lower mantle is ~1500 ppm. In its lowermost part (the Dʺ layer) the water content is likely to be higher owing to the possible presence, in this layer, of iron hydrides. The total amount of water in the lower-mantle reservoir is ~45.45 × 1023 grams H2O, i.e., ~3.3 times the mass of the Earth’s oceans.
(4) The major source of the water reservoir in the lower mantle is primordial water stored in primitive undegassed areas within the deep Earth, distinguished by isotopic characteristics (high 3He/4He and low D/H ratios) inherited directly from the protosolar nebula.
REFERENCES
S. Akber-Knutson and M. S. T. Bukowinski, “The energetics of aluminum solubility into MgSiO3 perovskite at lower mantle conditions,” Earth Planet. Sci. Lett. 220, 317–330 (2004). doi 10.1016/S0012–821X(04)00065–2
F. Albarede, “Volatile accretion history of the terrestrial planets and dynamic implications,” Nature 461, 1227–1233 (2009). doi 10.1038/nature08477
L. S. Armstrong, M. M. Hirschmann, B. D. Stanley, E. G. Falksen, S. D. Jacobsen, “peciation and solubility of reduced C–O–H–N volatiles in mafic melt: implications for volcanism, atmospheric evolution, and deep volatile cycles in terrestrial planets,” Geochim. Cosmochim. Acta 171, 283–302 (2015). doi 10.1016/j.gca.2015.07.007
C. Aubaud, A. C.Withers, M. M. Hirschmann, Y. Guan, L. A. Leshin, S. J. Mackwell, and D. R. Bell, “Intercalibration of FTIR and SIMS for hydrogen measurements in glasses and nominally anhydrous minerals,” Am. Mineral. 92 (5–6), 811–828 (2007).
Z. G. Bazhanova, A. R. Oganov, and O. Gianola, “Fe–C and Fe–H systems at pressures of the Earth’s Inner Core,” Physics-Uspekhi 55 (5), 489–497 (2012). doi 10.3367/UFNe.0182.201205c.0521
D. R. Bell and G. R. Rossman, “Water in the Earth’s mantle: The role of nominally anhydrous minerals,” Science 255, 139l–1397 (1992).
A. Beran and E. Libowitzky, “Water in natural mantle minerals II: Olivine, garnet and accessory minerals,” Water in Nominally Anhydrous Minerals, Ed. by H. Keppler and J. R. Smyth, Rev. Mineral. Geochem. 62(1), 169–191 (2006). doi 10.2138/rmg.2006.62.8. 10.2138/rmg.2006.62.8
R. J. Bodnar, T. Azbej, S. P. Becker, C. Cannatelli, A. Fall, and M. J. Severs, “Whole Earth geohydrologic cycle, from the clouds to the core: the distribution of water in the dynamic Earth system,” The Web of Geological Sciences: Advances, Impacts, and Interactions, Ed. by M. E. Bickford, Geol. Soc. Am. Spec. Pap. 500, 431–461 (2013). doi 10.1130/2013.2500(13).10.1130/ 2013.2500(13)
N. Bolfan-Casanova, “Water in the Earth’s mantle,” Mineral. Mag. 69, 229–257 (2005). doi 10.1180/0026461056930248
N. Bolfan-Casanova, H. Keppler, and D. C. Rubie, “Water partitioning between nominally anhydrous minerals in the MgO–SiO2–H2O system up to 24 GPa,” Earth Planet. Sci. Lett. 182, 209–221 (2000).
N. Bolfan-Casanova, S. Mackwell, H. Keppler, C. McCammon, and D. C. Rubie, “Pressure dependence of H solubility in magnesiowüstite up to 25 GPa: Implications for the storage of water in the Earth’s lower mantle,” Geophys. Res. Lett. 29 (10), 1449 (2002).
N. Bolfan-Casanova, H. Keppler, and D. C. Rubie, “Water partitioning at the 660 km discontinuity and evidence for very low water solubility in magnesium silicate perovskite,” Geophys. Res. Lett. 30 (17), 1905 (2003). doi 10.1029/2003GL017182
N. Bolfan-Casanova, C. A. McCammon, and S. J. Mackwell “Water in transition zone and lower mantle minerals. In: Eds.),” Earth’s Deep Water Cycle, Ed. by S. D. Jacobsen, and S. van der Lee, Geophys. Monogr. Ser. 168, 57–68 (2006).
G. D. Bromiley, F. A. Bromiley, and D. W. Bromiley “On the mechanism for H and Al incorporation in stishovite,” Phys. Chem. Miner. 33, 613–621 (2006). doi 10.1007/s00269–006–0107–9
J. I. Chung H. Kagi, “High concentration of water in stishovite in the MORB system,” Geophys. Res. Lett. 29(21), 2020 (2002). doi 10.1029/2002GL015579
G. Della Ventura, A. Marcelli, and F. Bellatreccia “SR–FTIR microscopy and FTIR imaging in the Earth sciences,” Rev. Mineral. Geochem. 78, 447–479 (2014). doi 10.2138/rmg.2014.78.11
S. Demouchy, S. J. Mackwell, and D. L. Kohlstedt “Influence of hydrogen on Fe–Mg interdiffusion in (Mg,Fe)O and implications for Earth’s lower mantle,” Contrib. Mineral. Petrol. 154 (3), 279–289 (2007). doi 10.1007/s00410–007–0193–9
L. T. Elkins-Tanton, “Linked magma ocean solidification and atmospheric growth for Earth and Mars,” Earth Planet. Sci. Lett.271, 181–191 (2008). doi 10.1016/ j.epsl.2008.03.062
H. Fei, D. Yamazaki, M. Sakurai, N. Miyajima, H. Ohfuji, T. Katsura, and T. Yamamoto “A nearly water–saturated mantle transition zone inferred from mineral viscosity,” Sci. Adv. 3, e1603024 (2017).
E. Füri, D. R. Hilton, S. A. Halldórsson, P. H. Barry, D. Hahm, T. P. Fischer, and K. Grönvold, “Apparent decoupling of the He and Ne isotope systematics of the Icelandic mantle: the role of He depletion, melt mixing, degassing fractionation and air interaction,” Geochim. Cosmochim. Acta (74), 3307–3332 (2010).
W. S. Fyfe, “Lattice energies, phase transformations and volatiles in the mantle,” Phys. Earth Planet. Inter. 3, 196–200 (1970).
E. M. Galimov, “Formation of the Moon and the Earth from a Common Supraplanetary Gas–Dust Cloud (Lecture Presented at the XIX All–Russia Symposium on Isotope Geochemistry on November 16, 2010),” Geochem. Int. 49 (6), 537–554 (2011).
E. J. Garnero and A. K. McNamara, “Structure and dynamics of the Earth’s lower mantle,” Science, 320, 626–628 (2008). . doi 10.1126/science.1148028
D. W. Graham, “Noble gas isotope geochemistry of mid–ocean ridge and ocean island basalts: characterization of mantle source reservoirs,” Rev. Mineral. Geochem. 47(1), 247–317 (2002). doi 10.2138/rmg.2002.47.8
A. N. Halliday, “The origins of volatiles in the terrestrial planets,” Geochim. Cosmochim. Acta 105, 146–171 (2013). doi 10.1016/j.gca.2012.11.015
L. J. Hallis, G. R. Huss, K. Nagashima, G. J. Taylor, S. A. Halldórsson, D. R. Hilton, and M. J. Mottl “Evidence for primordial water in Earth’s deep mantle,” Science 350(6262), 795–797 (2015). doi 10.1126/science.aac4834
K. Hamano, Y. Abe, and H. Genda “Emergence of two types of terrestrial planet on solidification of magma ocean,” Nature 497, 608–610 (2013). doi 10.1038/nature12163
P. G. Harris and E. A. K. Middlemost, “The evolution of kimberlites,” Lithos 3, 77–88 (1970).
B. Harte, J. W. Harris, M. T. Hutchison, G. R. Watt, and M. C. Wilding “Lower mantle mineral associations in diamonds from Sao Luiz, Brazil,” Mantle Petrology: Field Observations and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd, Ed. by Y. Fei et al., Geochem. Soc. Spec. Publ. 6, 125–153 (1999).
E. Hauri, “SIMS analysis of volatiles in silicate glasses, 2: isotopes and abundances in Hawaiian melt inclusions,” Chem. Geol. 183 (1–4), 115–141 (2002). doi 10.1016/S0009–2541(01)00374–6
E. R. Hernández, D. Alfe, and J. Brodholt “The incorporation of water into LM perovskites: A first–principles study,” Earth Planet. Sci. Lett. 364, 37–43 (2013). doi 10.1016/j.epsl.2013.01.005
M. M. Hirschmann, A. C. Withers, P. Ardia, and N. T. Foley “Solubility of molecular hydrogen in silicate melts and consequences for volatile evolution of terrestrial planets,” Earth Planet. Sci. Lett. 345, 38–48 (2012). doi 10.1016/j.epsl.2012.06.031
H. J. Hui, Y. J. Xu, and M. Pan “On water in nominally anhydrous minerals from mantle peridotites and magmatic rocks,” Sci. China Earth Sci. 59 (6), 1157–1172 (2016). doi 10.1007/s11430–016–5308–6
T. Inoue, T. Wada, R. Sasaki, and H. Yurimoto, “Water partitioning in the Earth’s mantle,” Phys. Earth Planet. Inter. 183, 245–251 (2010). doi 10.1016/j.pepi.2010.08.003
T. Inoue, S. Kakizawa, K. Fujino, T. Kuribayashi, T. Nagase, S. Gréaux, Y. Higo, N. Sakamoto, H. Yurimoto, T. Hattori, and A. Sano, “Hydrous bridgmanite: water storage capacity in the lower mantle,” Advances in High–Pressure Research- III: Towards Geodynamic Implications-20 16. Novosibirsk, Russia (Novosibirsk, 2016), p. 13.
M. G. Jackson, R. W. Carlson, M. D. Kurz, P. D. Kempton, D. Francis, and J. Blusztajn “Evidence for the survival of the oldest terrestrial mantle reservoir,” Nature 466, 853–856 (2010). doi 10.1038/nature09287
B. Joachim, A. Wohlers, N. Norberg, E. Garde’s, E. Petrishcheva, and R. Abart, “Diffusion and solubility of hydrogen and water in periclase,” Phys. Chem. Miner. 40 (1), 19–27 (2013). doi 10.1007/s00269–012–0542–8
F. Kaminsky, “Mineralogy of the lower mantle: A review of ‘super-deep’ mineral inclusions in diamond,” Earth-Sci. Rev. 110 (1–4), 127–147 (2012). doi 10.1016/j.earscirev.2011.10.005
F. V. Kaminsky, The Earth’s Lower Mantle: Composition and Structure (Springer, 2017).
F. V. Kaminsky, R. Wirth, and A. Schreiber “A microinclusion of LM rock and some other LM inclusions in diamond,” Can. Mineral. 53 (1), 83–104 (2015). doi 10.3749/canmin.1400070
H. Keppler and N. Bolfan-Casanova “Thermodynamics of water solubility and partitioning,” Water in Nominally Anhydrous Minerals, Ed. by H. Keppler and J. R. Smyth, Rev. Mineral. Geochem. 62 (1), 193–230 (2006). doi 10.2138/rmg.2006.62.9.10.2138/rmg.2006.62.9
A. K. Kleppe and A. P. Jephcoat, “Raman spectroscopic studies of hydrous and nominally anhydrous deep mantle phases,” Earth’s Deep Water Cycle, Ed. by S. D. Jacobsen and S. van der Lee, Geophys. Monogr. Ser. 168, 69–93 (2006). doi 10.1029/168GM07.10.1029/168GM07
K. Koga, E. Hauri, M. Hirschmann, and D. Bell, “Hydrogen concentration analyses using SIMS and FTIR: Comparison and calibration for nominally anhydrous minerals,” Geochem., Geophys., Geosyst. 4 (2), 1019 (2003). doi 10.1029/2002GC000378
S. C. Kohn and K. J. Grant, “The partitioning of water between nominally anhydrous minerals and silicate melts,” Water in Nominally Anhydrous Minerals, Ed. by H. Keppler and J. R. Smyth, Rev. Mineral. Geochem. 62 (1), 231–241 (2006). doi 10.2138/rmg.2006.62.10. 10.2138/rmg.2006.62.10
M. V. Kolesnichenko, D. A. Zedgenizov, K. D. Litasov, I. Yu. Safonova, and A. L. Ragozin, “Heterogeneous distribution of water in the mantle beneath the central Siberian Craton: Implications from the Udachnaya kimberlite pipe,” Gondwana Res. 47, 249–266 (2017). doi 10.1016/j.gr.2016.09.011
T. Komabayashi, S. Omori, and S. Maruyama, “Petrogenetic grid in the system MgO–SiO2–H2O up to 30 GPa, 1600 degrees C: applications to hydrous peridotite subducting into the Earth’s deep interior,” J. Geophys. Res B 109, B03206 (2004). doi 10.1029/2003JB002651
I. Kovács, J. Hermann, H. St. C. O’Neill, J. F. Gerald, M. Sambridge, G. Horvath, “Quantitative absorbance spectroscopy with unpolarized light: Part II. Experimental evaluation and development of a protocol for quantitative analysis of mineral IR spectra,” Am. Mineral. 93 (5–6), 765–778 (2008).
A. G. Kvashnin, I. A. Kruglov, D. V. Semenok, and A. R. Oganov, “Iron superhydrides FeH5 and FeH6: stability, electronic properties and superconductivity,” J. Phys. Chem. C 122, 4731–4736 (2018). doi 10.1021/acs.jpcc.8b01270
C.-T. A. Lee and W.-P. Chen, “Possible density segregation of subducted oceanic lithosphere along a weak serpentinite layer and implications for compositional stratification of the Earth’s mantle,” Earth Planet. Sci. Lett. 255 (3–4), 357–366 (2007). doi 10.1016/j.epsl.2006.12.022
C.-T. A. Lee, P. Luffi, T. Höink, J. Li, R. Dasgupta, and J. Hernlund, “Upside–down differentiation and generation of a ‘primordial’ lower mantle,” Nature 463, 930–933 (2010). doi 10.1038/nature08824
E. Libowitzky and A. Beran, “The structure of hydrous species in nominally anhydrous minerals: Information from polarized IR spectroscopy,” Water in Nominally Anhydrous Minerals, Ed. by H. Keppler and J. R. Smyth, Rev. Mineral. Geochem. 62(1), 29–52 (2006). doi 10.2138/ rmg.2006.62.2.10.2138/rmg.2006.62.2
E. Libowitzky and G. R. Rossman, “Principles of quantitative absorbance measurements in anisotropic crystals,” Phys. Chem. Miner. 23, 319–327 (1996).
K. D. Litasov, “The influence of Al2O3 on the H2O content bearing in periclase and ferropericlase at 25 GPa,” Russ. Geol. Geophys. 51 (6), 827–834 (2010).
K. D. Litasov and E. Ohtani, “Effect of water on the phase relations in Earth’s mantle and deep water cycle,” Advances in High–Pressure Mineralogy, Ed. by E. Ohtani, Geol. Soc. Am. Spec. Pap. 421, 115–156 (2007). doi 10.1130/2007.2421(08).10.1130/2007.2421(08)
K. Litasov, E. Ohtani, F. Langenhorst, H. Yurimoto, T. Kubo, and T. Kondo, “Water solubility in Mg–perovskites and water storage capacity in the lower mantle,” Earth Planet. Sci. Lett. 211, 189–203 (2003). doi 10.1016/S0012–821X(03)00200–0
K. D. Litasov, H. Kagi, A. Shatskiy, E. Ohtani, D. L. Lakshtanov, J. D. Bass, and E. Ito, “High hydrogen solubility in Al–rich stishovite and water transport in the lower mantle,” Earth Planet. Sci. Lett. 262, 620–634 (2007). doi 10.1016/j.epsl.2007.08.015
R. F. Martin and G. Donnay, “Hydroxyl in the mantle,” Am. Mineral. 57(1), 554–570 (1972).
B. Marty, “The origins and concentrations of water, carbon, nitrogen and noble gases on Earth,” Earth Planet. Sci. Lett. 313–314, 56–66 (2012). doi 10.1016/j.epsl.2011.10.040
S. S. Matsyuk and K. Langer, “Hydroxyl in olivines from mantle xenoliths in kimberlites of the Siberian platform,” Contrib. Mineral. Petrol. 147 (4), 413–437 (2004). doi 10.1007/s00410–003–0541–3
W. F. McDonough, “Compositional Model for the Earth’s Core,” Treatise on Geochemistry, 2nd edition, Ed. by R. W. Carlson, (Elsevier, 2014), pp. 559–576. doi 10.1016/B978–0–08–095975–7.00215–1
C. Meade, J. A. Reffner, and E. Ito, “Synchrotron infrared absorbance measurements of hydrogen in MgSiO3 perovskite,” Science 264, 1558–1560 (1994).
M. Merli, C. Bonadiman, V. Diella, and A. Pavese “Lower mantle hydrogen partitioning between periclase and perovskite: A quantum chemical modeling,” Geochim. Cosmochim. Acta 173, 304–318 (2016). doi 10.1016/j.gca.2015.10.025
E. W. J. Mitchell and J. D. Rigden, “The effects of radiation on the near infra-red absorption spectrum of α–quartz,” Philosoph. Mag. 2, 941–956 (1957).
M. Mookherjee, L. Stixrude, and B. Karki “Hydrous silicate melt at high pressure,” Nature 452 (7190), 983–986 (2008). doi 10.1038/nature06918
J. L. Mosenfelder and G. R. Rossman, “Analysis of hydrogen and fluorine in pyroxenes: I. Orthopyroxene,” Am. Mineral. 98(5–6), 1026–1041 (2013). doi 10.2138/am.2013.4291
J. L. Mosenfelder, G. R. Rossman, and E. A. Johnson “Hydrous species in feldspars: a reassessment based on FTIR and SIMS,” Am. Mineral. 100, 1209–1221 (2015). doi 10.2138/am–2015–5034
J. L. Mosenfelder, M. Le Voyer, G. R. Rossman, Y. Guan, D. R. Bell, P. D. Asimow, and J. M. Eiler, “Analysis of hydrogen in olivine by SIMS: evaluation of standards and protocol,” Am. Mineral. 96, 1725–1741 (2011). doi 10.2138/am.2011.3810
M. J. Mottl, B. T. Glazer, R. I. Kaiser, and K. J. Meech, “Water and astrobiology,” Chem. Erde, Geochem. 67 (4), 253–282 (2007). doi 10.1016/j.chemer.2007.09.002
J. M. R. Muir and J. P. Brodholt, “Water distribution in the lower mantle: implications for hydrolytic weakening,” Earth Planet. Sci. Lett. 484, 363–369 (2018). doi 10.1016/j.epsl.2017.11.051
S. Mukhopadhyay, “Early differentiation and volatile accretion recorded in deep mantle neon and xenon,” Nature 486 (7401) (101–U124) (2012). doi 10.1038/nature11141
M. Murakami, K. Hirose, H. Yurimoto, S. Nakashima, and N. Takafuji, “Water in Earth’s lower mantle,” Science 295 (5561), 1885–1887 (2002). doi 10.1126/science.1065998
A. Navrotsky, “A lesson from ceramics,” Science 284 (5421), 1788–1789 (1999). doi 10.1126/science.284.5421.1788
P. Németh, K. Leinenweber, H. Ohfuji, T. Groy, K. J. Domanik, I. J. Kovács, J. S. Kovács, and P. R. Buseck “Water-bearing, high-pressure Ca-silicates,” Earth Planet. Sci. Lett. 469, 148–155 (2017). doi 10.1016/j.epsl.2017.04.011
F. Nestola and J. R. Smyth, “Diamonds and water in the deep Earth: a new scenario,” Int. Geol. Rev. 58 (3), 263–276 (2016). doi 10.1080/00206814.2015.1056758
C. Nisr, S.-H. Shim, K. Leinenweber, A. Chizmeshya, “Raman spectroscopy of water-rich stishovite and dense high-pressure silica up to 55 GPa,” Am. Mineral. 102, 2180–2189 (2017). . doi 10.2138/am–2017–5944
D. Novella, N. Bolfan-Casanova, F. Nestola, and J. W. Harris, “H2O in olivine and garnet inclusions still trapped in diamonds from the Siberian craton: implications for the water content of cratonic lithosphere peridotites,” Lithos 230, 180–183 (2015). doi 10.1016/ j.lithos.2015.05.013
I. Ohira, E. Ohtani, T. Sakai, M. Miyahara, N. Hirao, Y. Ohishi, and M. Nishijima, “Stability of a hydrous δ‑phase, AlOOH–MgSiO2(OH)2, and a mechanism for water transport into the base of lower mantle,” Earth Planet. Sci. Lett. 401, 12–17 (2014). doi 10.1016/ j.epsl.2014.05.059
E. Ohtani, “Water in the mantle,” Elements 1(1), 25–30 (2005).
E. Ohtani, “Hydrous minerals and the storage of water in the deep mantle,” Chem. Geol. 418, 6–15 (2015) doi 10.1016/j.chemgeo.2015.05.005
E. Ohtani, M. Toma, K. Litasov, T. Kubo, and A. Suzuki, “Stability of dense hydrous magnesium silicate phases and water storage capacity in the transition zone and lower mantle,” Phys. Earth Planet. Inter. 124, 105–117 (2001).
T. Okuchi, “Hydrogen partitioning into molten iron at high pressure: Implications for Earth’s core,” Science 278, 1781–1784 (1997).
W. R. Panero and L. P. Stixrude, “:Hydrogen incorporation in stishovite at high pressure and symmetric bonding in δ–AlOOH,” Earth Planet. Sci. Lett. 221, 421–431 (2004).
W. R. Panero, L. R. Benedetti, and R. Jeanloz “Transport of water into the lower mantle: role of stishovite,” J. Geophys. Res 108, 2039 (2003). doi 10.1029/2002JB002053
W. R. Panero, J. S. Pigott, D. M. Reaman, J. E. Kabbes, and Z. Liu, “Dry (Mg,Fe)SiO3 perovskite in the Earth’s lower mantle,” J. Geophys. Res. Solid Earth 120 (2), 894–908 (2015).
M. S. Paterson, “The determination of hydroxyl by infrared absorption in quartz, silicate glasses and similar materials,” Bull. Minéral. 105, 20–29 (1982).
A. R. Pawley, P. F. McMillan, and J. R. Holloway, “Hydrogen in stishovite, with implications for mantle water contents,” Science 261, 1024–1026 (1993). doi 10.1126/science.261.5124.1024
D. G. Pearson, F. E. Brenker, F. Nestola, J. McNeill, L. Nasdala, M. T. Hutchison, S. Matveev, K. Mather, G. Silversmit, S. Schmitz, B. Vekemans, and L. Vincze, “Hydrous mantle transition zone indicated by ringwoodite included within diamond,” Nature 507, 221–224 (2014). doi 10.1038/nature13080
C. M. Pépin, A. Dewaele, G. Geneste, P. Loubeyre, and M. Mezouar, “New iron hydrides under high pressure,” Phys. Rev. Lett. 113 (26), 265504 (2014). doi 10.1103/PhysRevLett.113.265504
A. H. Peslier, M. Schönbächler, H. Busemann, and S.‑I. Karato, “Water in the Earth’s interior: distribution and origin,” Space Sci. Rev. 212(1–2), 743–810 (2017). doi 10.1007/s11214–017–0387–z
A. Ricolleau, J.-P. Perrillat, G. Fiquet, I. Daniel, J. Matas, A. Addad, N. Menguy, H. Cardon, M. Mezouar, and N. Guignot, “Phase relations and equation of state of a natural MORB: Implications for the density profile of subducted oceanic crust in the Earth’s lower mantle,” J. Geophys. Res 115, B08202 (2010). doi 10.1029/2009JB006709
A. E. Ringwood, “The chemical composition and origin of the earth,” Advances in Earth Science, Ed. by P. M. Hurle (M IT Press, Cambridge, 1966), pp. 287–356.
A. Rosenthal, E. H. Hauri, and M. M. Hirschmann “Experimental determination of C, F, and H partitioning between mantle minerals and carbonated basalt, CO2/Ba and CO2/Nb systematics of partial melting, and the CO2 contents of basaltic source regions,” Earth Planet. Sci. Lett. 412, 77–87 (2015). doi 10.1016/j.epsl.2014.11.044
N. L. Ross, G. V. Gibbs, and K. Rosso “Potential docking sites and positions of hydrogen in high-pressure silicates,” Am. Mineral. 88 (10), 1452–1459 (2003).
G. R. Rossman, “Analytical methods for measuring water in nominally anhydrous minerals,” Water in Nominally Anhydrous Minerals, Ed. by H. Keppler and J. R. Smyth, Rev. Mineral. Geochem. 62(1), 1–28 (2006). doi 10.2138/rmg.2006.62.1.10.2138/rmg.2006.62.1
T. Sakamaki, A. Suzuki, and E. Ohtani “Stability of hydrous melt at the base of the Earth’s upper mantle,” Nature 439, 192–194 (2006). doi 10.1038/nature04352
M. J. Sambridge, F. Gerald, I. Kovács, H.St.C. O’Neill, and J. Hermann, “Quantitative absorbance spectroscopy with unpolarized light: Part I. Physical and mathematical development,” Am. Mineral. 93, 751–764 (2008).
T. Sanehira, T. Irifune, T. Inoue, and N. Nishiyama, “Synthesis of hydrous aluminous perovskite (in Japanese),” Abstracts of the 43th High Pressure Conference in Matsuyama, Japan, Rev. High Pressure Sci. Technol. 12, 208 (2002).
S. K. Saxena, H.-P. Liermann, and G. Shen, “Formation of iron hydride and high–magnetite at high pressure and temperature,” Phys. Earth Planet. Inter. 146, 313–317 (2004). doi 10.1016/j.pepi.2003.07.030
B. Schmandt, S. D. Jacobsen, T. W. Becker, Z. Liu, and K. G. Dueker, “Dehydration melting at the top of the lower mantle,” Science 344, 1265–1268 (2014).
G. Silversmit, B. Vekemans, K. Appel, S. Schmitz, T. Schoonjans, F. E. Brenker, F. Kaminsky, and L. Vincze, “Three-dimensional Fe speciation of an inclusion cloud within an ultra-deep diamond by confocal µ–XANES: evidence for late stage overprint,” Analyt. Chem. 83, 6294–6299 (2011). doi 10.1021/ac201073s
J. R. Smyth, “β–Mg2SiO4: A potential host for water in the mantle?,” Am. Mineral. 72, 1051–1055 (1987).
J. R. Smyth, R. J. Swope, and A. R. Pawley “H in rutile–type compounds: II. Crystal chemistry of Al substitution in H–bearing stishovite,” Am. Mineral. 80, 454–456 (1995).
K. Spektor, J. Nylen, E. Stoyanov, A. Navrotsky, R. L. Hervig, K. Leinenweber, G. P. Holland, and U. Häussermann, “Ultrahydrous stishovite from high–pressure hydrothermal treatment of SiO2” PNAS, Proc. Natl. Acad. Sci. USA 108 (52), 20918–20922 (2011). doi 10.1073/pnas.1117152108
K. Spektor, J. Nylen, R. Mathew, M. Eden, E. Stoyanov, A. Navrotsky, K. Leinenweber, and U. Häussermann, “Formation of hydrous stishovite from coesite in high pressure hydrothermal environments,” Am. Mineral. 101, 2514–2524 (2016). doi 10.2138/am-2016-5609
L. Stixrude, E. Wasserman, and R. E. Cohen “Composition and temperature of Earth’s inner core,” J. Geophys. Res.102 (B11), 24, 729–24, 739 (1997).
F. M. Stuart, S. Lass-Evans, J. G. Fitton, and R. M. Ellam, “High 3He/4He ratios in picritic basalts from Baffin Island and the role of a mixed reservoir in mantle plumes,” Nature 424, 57–59 (2003).
L. A. Taylor, A. M. Logvinova, G. H. Howarth, Y. Liu, A. H. Peslier, G. R. Rossman, Y. Guang, Y. Chen, and N. V. Sobolev, “Low water contents in diamond mineral inclusions: proto-genetic origin in a dry cratonic lithosphere,” Earth Planet. Sci. Lett. 433, 125–132 (2016). doi 10.1016/j.epsl.2015.10.042
J. P. Townsend, J. Tsuchiya, C. R. Bina, S. D. Jacobsen, “Water partitioning between bridgmanite and postperovskite in the lowermost mantle,” Earth Planet. Sci. Lett. 454, 20–27 (2016). doi 10.1016/j.epsl.2016.08.009
O. Tschauner, S. Huang, E. Greenberg, V. B. Prakapenka, C. Ma, G. R. Rossman, A. H. Shen, D. Zhang, M. Newville, A. Lanzirotti, and K. Tait “Ice-VII inclusions in diamonds: evidence for aqueous fluid in Earth’s deep mantle,” Science 359 (6380), 1136–1139 (2018). doi 10.1126/science.aao3030
C. B. Vanpeteghem, A. Sano, K. Komatsu, E. Ohtani, and A. Suzuki, “Neutron diffraction study of aluminous hydroxide δ-AlOOD,” Phys. Chem. Miner. 34 (9), 657–661 (2007). doi 10.1007/s00269-007-0180-8
Q. Williams and R. J. Hemley, “Hydrogen in the deep earth,” Annu. Rev. Earth Planet. Sci. 29, 365–418 (2001). doi 10.1146/annurev.earth.29.1.365
R. Wirth, “Focused Ion Beam (FIB) combined with SEM and TEM: advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale,” Chem. Geol. 261, 217–229 (2009). doi 10.1016/j.chemgeo.2008.05.019
K. Wright and C. R. A. Catlow, “Calculations on the energetics of water dissolution in wadsleyite,” Phys. Chem. Miner. 23 (1), 38–41 (1996).
P. J. Wyllie, “Ultramafic rocks and the upper mantle,” Mineral. Soc. Am. Spec. Pap. 3, 3–32 (1970).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article was translated by the author.
Rights and permissions
About this article
Cite this article
Felix V. Kaminsky Water in the Earth’s Lower Mantle. Geochem. Int. 56, 1117–1134 (2018). https://doi.org/10.1134/S0016702918120042
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702918120042