Abstract
The morphology and histology of the male reproductive system of Phyllobius fulvago were described and discussed in relation to other Coleoptera species. The results show that Ph. fulvago has two-lobed flower-shaped arranged testes, each lobe with fourteen follicles. There are two types of glands in the male reproductive system in Ph. fulvago: tubular accessory glands and lobed prostate glands.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Curculionidae is known as one of the most speciose insect families with approximately 60000 species in the World and 13000 in the Palaearctic Region (Varlı, 1998). Many weevils of the genus Phyllobius Germar live on cultivated shrubs and trees, most often of the families Fagaceae, Betulaceae, Salicaceae, and Rosaceae (Pesarini, 1980). Larvae of many Phyllobius feed on the roots, while the adults feed mostly on the shoots and leaves (Dieckmann, 1980). Therefore, they can cause significant economic losses to agricultural crops and forest trees (Pesarini, 1980). Phyllobius (Ectomogaster) fulvago Gyllenhal, 1834 is widespread on oaks (Quercus spp.) across the Middle East (Avgın and Colonnelli, 2011). As this species causes economic damage feeding in numbers on many plants important for agriculture and abundant in natural landscapes, research focusing on the male reproductive system of Ph. fulvago may contribute to agricultural pest control studies.
The internal morphology of beetles is commonly used for taxonomic characterization of insect species in the same genus or different genera in the same family (Russo, 1926; Williams, 1945). The histological and morphological knowledge of the male reproductive system of Coleoptera is poor (Cerezke, 1964; Barker, 1989; Salazar et al., 2016; Özyurt Koçakoğlu et al., 2019) although several investigations of the spermatogenesis and reproductive system have been previously reported (Goldson and Emberson, 1981; Yahiro, 1996; Diefenbach et al., 1998; Sasakawa, 2007; Opitz, 2014; Senarat et al., 2019). The male reproductive system in the Coleoptera including Curculionidae consists of a pair of testes, vasa deferentia, seminal vesicles, accessory glands, prostate glands, and an ejaculatory duct (Aslam, 1961; Barker, 1989; Hoffman and Raffa, 1992; Özyurt Koçakoğlu et al., 2019). Each testicular follicle contains germ cells at different stages of development. The germarium, which is the upper part of testis, contains spermatogonia and spermatocytes. In the next zones, spermatocytes develop into spermatids and finally into mature spermatozoa (Barker, 1989; Özyurt Koçakoğlu et al., 2019). In the center of each testis, there is a seminal vesicle. Extending from the ends of the seminal vesicles, there are paired vasa deferentia. The paired accessory glands arising from the vas deferens are long blind-ended tubules. In the posterior part of the accessory glands there is a prostate gland, which opens into the vas deferens (Barker, 1989). The prostate glands have 7 or 8 lobes (Goldson and Emberson, 1981). During mating, stored sperm in the seminal vesicles is mixed with secretions of the glands before transfer to the female in the form of a spermatophore (Chapman, 1971; Barker, 1989). The vas deferens unites with the ejaculatory duct which enters the aedeagus (Barker, 1989).
Here, we describe the morphology and histology of the male reproductive system of adult Ph. fulvago for the first time. The purpose of this contribution is to offer new information about the reproductive system of Curculionidae.
MATERIALS AND METHODS
Stereomicroscopy (SM). Twenty adult males of Ph. fulvago were collected with a sweeping net from meadows in different localities of Yozgat Province of Turkey in May 2018. The samples were placed in a plastic container with good air ventilation along with food sources and were brought to the laboratory. Next, ten insects were anesthetized with ethyl acetate fumes. The dissection was done in phosphate buffer by cutting open the dorsal part of the abdomen from posterior to the anterior in order to expose the male reproductive organs which were photographed using a stereomicroscope Olympus SZX7.
Light microscopy (LM). For histological preparations, ten samples were fixed in formalin fixative for 24 hours. Next, the tissues were underwent tissue processing for eight hours. First, the 10% neutral formalin was removed by washing in tap water for a day. Next, the samples were dehydrated in subsequently graded from 50% to 100% ethanol for an hour in each alcohol series. The tissues were left in the mixture of xylene and paraffin for fifteen minutes. Then the tissues were twice infiltrated with paraffin wax. Last, tissues were embedded in histological paraffin, and 5–6 μm thick sections were taken from the tissues using microtome (Microm HM 310). Finally, the serial sections were stained with Harris’s hematoxylin and eosin (H & E) staining. The stained slides were observed and photographed under a light microscope with a digital camera Olympus BX51.
Scanning electron microscopy (SEM). For scanning electron microscopy (SEM), ten adult samples were fixed in 2.5% glutaraldehyde (pH 7.2, phosphate buffered), rinsed with phosphate buffer three times, and the tissues were dehydrated in ethanol series from 50% to 100% at room temperature. The tissues were dried with hexamethyldisilazane, mounted on aluminum stubs, and finally coated with gold in a Polaron SC 502 sputter coater. The samples were examined with a JEOL JSM 6060 LV SEM at 10 kV, and digital photos were taken.
RESULTS
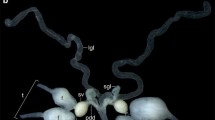
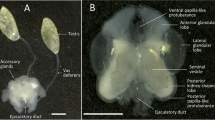
The male reproductive system of adult Phyllobius fulvago comprises a pair of testes, a pair of seminal vesicles, a pair of vasa deferentia, a pair of prostate glands, a pair of accessory glands, an ejaculatory duct, and an aedeagus (Fig. 1, a, b). The testes are located dorsolaterally in the abdominal cavity. Each testis has two separate flower-shaped lobes (Fig. 1, Fig. 2). The testis is enclosed by epithelial sheath with a flat surface, and the testis surface is covered with trachea and tracheole networks (Fig. 2, a). Histological examinations have shown that each testis lobe is composed of 14 testicular follicles (Fig. 2, b). In each of these follicles, different stages of sperm development (spermatogenesis) are seen. Sperm development is observed from the periphery to the center of each testis lobe (Fig. 2, b; Fig. 3, a). There is a seminal vesicle in the center of each testicular lobe (Fig. 3, b). At larger magnifications of LM, different stages of the germ cells which are spermatogonia, spermatocytes, spermatids, and spermatozoa are observed in each follicle (see Fig. 3, a). In the growth zone, towards the periphery of each follicle, spermatogonia and spermatocyte cysts, which are known as spherical sac, are found. At this stage (spermatogonia and spermatocyte cysts) part of the sperm’s tail is located at the back of the head and is quite short (Fig. 4, a, b).
Phyllobius fulvago Gyll., general morphology (a; SM) and histological structure (b; LM) of male reproductive system and testes. (Ae) aedeagus, (Ag) accessory gland, (Ed) ejaculatory duct, (Fl) testis follicle, (Mb) muscle bundles, (Pg) prostate gland, (Sv) seminal vesicle, (Tf) testis lobe, (Vd) vas deferens, (LM) light microscope, (SM) stereomicroscope.
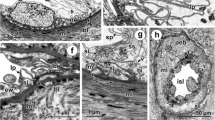
Phyllobius fulvago Gyll.: (a) trachea (Tr) and tracheoles (Tl) on the surface of the testis sheath, SEM; (b) cross section of a testis lobe and seminal vesicle (LM; ×100). (SEM) scanning electron microscopy. For other designations see Fig. 1.
Phyllobius fulvago Gyll., LM: (a) differentiation zones in a testis follicle (×200), (b) connection of the seminal vesicle to the testis (×400). (Ep) epithelial cell, (Sb) sperm bundles, (Sg) spermatogonia, (Sp) spermatocytes, (St) spermatids, (Sz) spermatozoa. For other designations see Fig. 1.
In the maturation zone, spermatocytes undergo two meiotic divisions and turn into spermatids (Fig. 5, a, b). At this stage, the sperm head is round and its tail is quite long and filiform (Fig. 6, a, b). Spermatids are seen in bundles together. In the differentiation zone, the spermatids turn into spermatozoa, and the sperm heads turn from oval to spindle-shaped structures (Fig. 7, a, b). In the spermatozoon head, the middle part of the head is wide, and the anterior and posterior parts are narrow (Fig. 9, b). The tail parts are long, strand-shaped, and form regular bundles (Fig. 8, a, b; Fig. 9, a). In the middle of each testicular lobe, there is a seminal vesicle (Fig. 10, a, b). The wall of the seminal vesicle is surrounded with a monolayer epithelium, and there are sperm bundles in the lumen (Fig. 10, a). The sperm stored in the seminal vesicles is mixed with accessory and prostate glands secretions before transfer to the female insect. The sperm bundle travels from the seminal vesicle to the vas deferens. The vas deferens wall is surrounded with monolayer cubic epithelium and muscle layer from the inside (Fig. 11, a, b). Toward the middle of the vas deferens, there are pairs of long, curved, blind-ended accessory glands. Also, trachea and tracheole networks are found on the surface (Fig. 12, b). The accessory glands are surrounded with monolayer cylindrical epithelium with large spherical nuclei, and secretory material is found in the lumen (Fig. 12, a; Fig. 13, a, b). Below the accessory glands, there are multi-lobed prostate glands (Fig. 14, a, b). The epithelium of the prostate glands consists of cuboidal cells with round nuclei (Fig. 14, a). A lower concentration of secretory material is found in the lumen as compared to the accessory glands (see Fig. 12, a; Fig. 14, a). The prostate glands are connected to the ejaculator duct by a thin vas deferens duct (see Fig. 14, a, b). The thickness of the muscle layer in the ejaculator duct wall is considerably larger than that of other ducts (Fig. 15, a, b). The nuclei and streaks in the muscle layer are clearly distinguished (see Fig. 15, a). The ejaculator duct is connected to the aedeagus via dense muscle bundles just below the duct (Fig. 16, a, b). The muscles are involved in the retraction and protraction of the aedeagus which is a sclerotized tube.
DISCUSSION
The results of this study show that the general morphology of the male reproductive system of Phyllobius fulvago is typical of that in the beetles of the suborder Polyphaga (Aslam, 1961; Barker, 1989; Hoffman and Raffa, 1992; Werner et al., 2002; Wu et al., 2017; Özyurt Koçakoğlu et al., 2019) The general appearance of Phyllobius fulvago testes is similar to that of other curculionids (Aslam, 1961; Barker, 1989; Hoffman and Raffa, 1992; Özyurt Koçakoğlu et al., 2019). However, the morphology of the testis varies between species. The testes in Ph. fulvago are flower-shaped, while Dendroctonus monticolae (Curculionidae: Scolytinae) has the testes bean-shaped (Cerezke, 1964). Rhysodes comes (Lewis, 1888) of the Adephaga (Rhysodidae) has ovoid testes (Yahiro, 1996). Spasalus silvarum (Polyphaga: Passalidae) has fusiform testes (Salazar et al., 2016). The testes of Tentyria cypria (Polyphaga: Tenebrionidae) look like four-leaf clover (İzzetoğlu and Gülmez, 2018).
Each testis consists of a number of follicles which contain the male germ cells; the number of follicles in each testis and the number of male accessory glands in the examined species of Coleoptera are given in Table 1.
The follicles of the sexually mature males are full of sperm cysts, within which spermatogenesis occurs (Phillips, 1970; Wu et al., 2017). Sperm development in Ph. fulvago testis proceeds as described by Cerezke (1964), Salazar et al. (2016), Senarat et al. (2019) and Özyurt et al. (2019) for other beetles.
Each testicular lobe of Ph. fulvago is connected to the vas deferens via the seminal vesicle. The same structures were found in other Curculionidae: Listronotus bonariensis, Hypothenemus hampei and Tanymecus dilaticollis (Goldson and Emberson, 1981; Barker, 1989; Rubio et al., 2008; Özyurt Koçakoğlu et al., 2019)
The morphology of the male accessory glands is known to vary between species (Barker, 1989). Phyllobius fulvago has a pair of long, tubular accessory glands and a pair of multilobed prostate glands. In Dendroctonus monticolae, some of the accessory glands are tubular, and others have four to six lobes (Cerezke, 1964). Listronotus bonariensis has large accessory glands and prostate glands with seven or eight lobes (Barker, 1989). The accessory glands in Phyllobaenus pallipennis are trilobed (Opitz, 2014). Isohydnocera aegra and Wolcottia sobrina have accessory glands swollen at base and particularly long (Opitz, 2014). Axina bifasciata has biramous accessory glands of which the medial pair is much shorter than the lateral one (Opitz, 2014). In Cardiostychus gabonicus, the medial gland is biramous and lateral gland is uniramous (Opitz, 2014). Clerus mutillarius has medial glands paired and not branched, but its lateral glands are paired biramously (Opitz, 2014). In Epiclines basalis, the medial gland is not branched and highly diverticulated at base, and the lateral gland is biramous (Opitz, 2014). Neoscrobiger patricius has spheroid lobe at the base of the medial gland, and its lateral pair is not branched (Opitz, 2014). In Omadius semicarinatus, the medial pair is not branched, and the lateral pair has a basal vesicle (Opitz, 2014). In Thanasimus dubius and Th. formicarius, medial pair is biramous and the lateral pair is not branched, but in Th. ceylonicus both glands are biramous (Opitz, 2014). Trichodes alvearius has a short medial pair but the lateral pair is very long (Opitz, 2014). In Trogodendron fasciculatum, the medial glands are much longer than the lateral ones (Opitz, 2014). In Cymatoderella collaris, the medial pair is not branched but the lateral one is biramous (Opitz, 2014). In C. inornata the medial pair is not branched but lateral gland is bilobed and very long (Opitz, 2014). In Tanymecus dilaticollis, the accessory glands have large and blind-ended tubules, and the prostate glands have multilobed lobes (Özyurt Koçakoğlu et al., 2019). These glands have two functions in general: formation of sperm fluid and formation of the spermatophore (Sehnal, 1985; Gillott, 2005; Klowden, 2008; İzzetoğlu and Gülmez, 2018).
In Phyllobius fulvago, the ejaculatory duct is a thick-walled muscular tube with inner epithelium. This duct opens to the aedeagus. No distinct intima was observed in our study. Similar structures were found in Dendroctonus monticolae, Listronotus bonariensis and Tanymecus dilaticollis (Cerezke, 1964; Barker, 1989; Özyurt Koçakoğlu et al., 2019).
REFERENCES
Aslam, N.A., An assessment of some internal characters in the higher classification of the Curculionidae, Trans. Royal Entomol. Soc. London, 1961, vol. 113, p. 417.
Avgın, S.S. and Colonnelli, E., Curculionoidea (Coleoptera) from southern Turkey, Afr. J. Biotechnol., 2011, vol. 10, no. 62, p. 13555.
Barker, G.M., Functional anatomy of the reproductive system of Listronotus bonariensis (Kuschel), NZ Entomol., 1989, vol. 12, no. 1, p. 34.
Cerezke, H.F., The morphology and functions of the reproductive systems of Dendroctonus monticolae Hopk. (Coleoptera: Scolytidae), Canad. Entomol., 1964, vol. 96, no. 3, p. 477.
Chapman, R.F., The Insects: Structure and Function, London: English Universities Press, 1971.
Dieckmann, L., Beiträge zur Insektenfauna der DDR: Coleoptera–Curculionidae (Brachycerinae, Otiorhynchinae, Brachyderinae), Beitr. Entomol., 1980, vol. 30, p. 145.
Diefenbach, L.M.G., Redaelli, L.R., and Gassen, D.N., Characterization of the internal reproductive organs and their state as diapause indicator in Phytalus sanctipauli Blanchard, 1850 (Coleoptera, Scarabaeidae), Rev. Bras. Biol., 1998, vol. 58, no. 3, p. 541.
Gillott, C., Entomology. Third Edition, Springer, 2005.
Goldson, S.L. and Emberson, R.M., Reproductive morphology of the Argentine stem weevil, Hyperodes bonariensis (Coleoptera: Curculionidae), N.Z. J. Zool., 1981, vol. 8, p. 67.
Hoffman, G.D. and Raffa, K.F., Maturation of the male pales weevil (Coleoptera: Curculionidae) reproductive system and its effect on male response to females, Ann. Entomol. Soc. Am., 1992, vol. 85, no. 5, p. 571.
İzzetoğlu, G.T. and Gülmez, M., Macroscopic and histological structures of testes in three different Tentyria species, Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi, 2018, vol. 21, no. 3, p. 433
Kheirallah, D.A. and El-Samad, L.M., Spermatogenic alterations in the ground beetle Trachyderma hispida (Coleoptera: Tenebrionidae) induced by ceramic industrial pollution, Afr. Entomol., 2019, vol. 27, no. 2, p. 418
Klowden, M.J., Physiological Systems in Insects. Second Edition, Moscow, Idaho: University of Idaho, 2008.
Lodos, N., Önder, F., Pehlivan, E., Atalay, R., Erkin, E., Karsavuran, Y., Tezcan S., and Aksoy, S., Faunistic Studies on Curculionidae (Coleoptera) of Western Black Sea, Central Anatolia and Mediterranean Regions of Turkey, İzmir: Meta Basım Matbaacılık Hizmetleri, 2003.
Opitz, W., Morphologic studies of the alimentary canal and internal reproductive organs of the Chaetosomatidae and the Cleridae (Coleoptera: Cleroidea) with comparative morphology and taxonomic analyses, Insecta Mundi, 2014, vol. 0342, p. 1.
Özyurt Koçakoğlu, N., Candan, S., and Güllü, M., The histomorphological structure of the male reproductive system of maize leaf weevil Tanymecus dilaticollis Gyllenhal, 1834 (Coleoptera: Curculionidae), Microsc. Res. Tech., 2019, vol. 82, p. 1345.
Pesarini, C., Le specie paleartiche occidentali della tribu Phyllobiini (Coleoptera: Curculionidae), Bull. Zool. Agr. Bachicolt., 1980, vol. 15, p. 49.
Phillips, D.M., Insect sperm: Their structure and morphogenesis, J. Cell Biol., 1970, vol. 44, p. 243.
Rubio, J.D.G., Bustillo, P.A.E., Vallejo, E.L.F., Acuña, Z.J.R., and Benavides, M.P., Alimentary canal and reproductive tract of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae, Scolytinae), Neotrop. Entomol., 2008, vol. 37, no. 2, p. 143.
Russo, G., Contributo alla conoscenze degli scolytidi. Studio morfobiologico del Cheatoptelius vestitus (Muls. e Rey) Funchs e dei suoi simbionti, Boll. Lab. Zool. Gen. Agric. Portici, 1926, vol. 19, p. 103.
Salazar, K., Dias, G., Boucher, S., Lino-Neto, J., and Serrão, J.E., Morpho-anatomy of the male reproductive tract and spermatogenesis of the South American Spasalus silvarum Kuwert (Coleoptera: Passalidae), Zoomorphology, 2016, vol. 135, no. 4, p. 487.
Sasakawa, K., Sperm bundle and reproductive organs of carabid beetles tribe Pterostichini (Coleoptera: Carabidae), Naturwissenschaften, 2007, vol. 94, no. 5, p. 384.
Sehnal, F., Morphology of insect development. Annu. Rev. Entomol., 1985, vol. 30, p. 89.
Senarat, S., Kettratad, J., Jiraungkoorskul, W., Thaochan, N., Pengsakul, T., Poolpraseit, P., and Dokchan, P., Light microscopic evidence of spermatogenesis of firefly, Pyrocoelia tonkinensis Olivier, 1886 (Coleoptera: Lampyridae), J. Appl. Sci., 2019, vol. 18, no. 1, p. 12.
Varlı, S.V., Balıkesir Geniş Yapraklı Ormanlarında Zarar Yapan Polydrusus Germ. (Coleoptera: Curculionidae) Türleri Üzerinde Araştırmalar, Doktora Tezi, Uludağ Üniversitesi, Fen Bilimleri Enstitüsü, Bursa, 1998.
Werner, M., Tscheulin, T., Speck, T., Zissler, D., and Peschke, K., Ultrastructure and motility pattern of the spermatozoa of Aleochara curtula (Coleoptera, Staphylinidae), Arthropod Struct. Dev., 2002, vol. 31, p. 243.
Williams, J.L., The anatomy of the internal genitalia of some Coleoptera, Proc. Entomol. Soc. Wash., 1945, vol. 47, p. 73.
Wu, Y.F., Wei, L.S., Anthony Torres, M., Zhang, X., Wu, S.P., and Chen, H., Morphology of the male reproductive system and spermiogenesis of Dendroctonus armandi Tsai and Li (Coleoptera: Curculionidae: Scolytinae), J. Insect Sci., 2017, vol. 17, no. 1, p. 1.
Yahiro, K., Comparative morphology of the alimentary canal and reproductive organs of the terrestrial Caraboidea (Coleoptera: Adephaga) part 2, Entomol. Sci., 1998, vol. 1, no. 1, p. 47.
ACKNOWLEDGMENTS
We thank Gazi Üniversity Academic Writing Center for linguistic revision of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Erbey, M., Koçakoğlu, N.Ö. & Candan, S. Histomorphology of the Male Reproductive System and Spermatogenesis of Phyllobius (Ectomogaster) fulvago Gyllenhal, 1834 (Coleoptera, Curculionidae): A Light and Scanning Electron Microscope Study. Entmol. Rev. 101, 23–44 (2021). https://doi.org/10.1134/S0013873821010036
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0013873821010036