Abstract
A method for synthesis of nitrogen-doped graphene (N-graphene) with high specific surface area has been developed. In this method, magnesium oxide is used as a template where a carbon layer modified with nitrogen atoms is deposited. Nitrogen–carbon–mineral composites (N–C/MgO) containing from 5 to 27 wt % of nitrogen-doped carbon have been synthesized. After dissolving magnesium oxide, N-graphene with a specific surface area of 1300–1400 m2 g–1 has been synthesized for the first time. The synthesis of N-graphene has been characterized by electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Graphene is a two-dimensional crystal composed of hexagonally bonded carbon atoms [1]. Graphene possesses unique electrical conductivity and mechanical properties. The development of such materials and methods for their preparation is very important because of the great potential of their use in electrochemical processes and catalysis [2, 3]. The application of graphene can be extended by modifying it with nitrogen atoms. Compared to carbon atoms, nitrogen atoms embedded in the carbon network of graphene are able to form stronger bonds with the atoms of the supported metals (Pd, Pt, Ni). This feature of N-graphene opens up possibilities for the synthesis of highly dispersed, up to atomic, metal particles [4, 5].
In principle, oxides SiO2, γ-Al2O3, and MgO can be used as the starting oxide on which N-graphene is formed. However, silica gel (SiO2) has narrow pores, and it is very difficult to cover these pores uniformly with a thin layer of modified carbon. Using γ-Al2O3 as a template, we managed to uniformly carbonize the inner surface of the oxide pores with a thin layer of carbon. However, it was not possible to etch alumina from such a synthesized carbon–mineral composite [6].
It has been shown [7] that the use of magnesium oxide as a substrate makes it possible to obtain a uniform distribution of graphene layers.
Graphene modified with nitrogen can be fabricated by two methods. The first method is the direct synthesis of N-graphene from a mixture of methane and ammonia [8, 9]. The synthesis of N-graphene was carried out on a fine crystalline metal (nickel or copper) foil by the chemical vapor deposition (CVD) method.
The second method consists of postsynthesis treatment of graphene samples using plasma [10, 11] or chemical reactions [12, 13].
In [14], graphene, both undoped and nitrogen-doped, was grown on a polished MgO (100) single crystalline substrate. The crystal was 10 × 5 × 0.5 mm in size. Benzene and pyridine were used as precursors. The synthesized doped graphene had a small number of monolayers (from two to five) and a high nitrogen content. Apparently, this method can find application in microelectronics.
A method has been proposed for producing graphene from acetonitrile as a nitrogen-containing precursor on MgO micrograins [16, 17]. The nitrogen content in the synthesized graphene has not been specified. The synthesized carbon material has a low specific surface area, 175 m2 g–1; therefore, it should be multilayer (10–15 layers). Such a carbon material can be attributed rather to graphite. Analysis of the literature data shows that the available methods [8–17] do not allow obtaining N-graphene with a high specific surface area and a small number of graphene layers (from two to five).
The purpose of this work is to develop a method for the synthesis of nitrogen-doped graphene with a high specific surface area.
EXPERIMENTAL
As a substrate onto which graphene was deposited, magnesium oxide with a specific surface area of 70 m2 g–1 (analytical grade, GOST 4526-75, Vekton, Russia) was used; a 40% NH3–1% C2H2–C2H4 gas mixture was chosen as the source of catalytic carbon.
The structure and microstructure of the samples were studied by high-resolution transmission electron microscopy (HRTEM) on a Thermo Fisher Scientific ThemisZ electron microscope with an accelerating voltage of 200 kV and a limiting lattice resolution of 0.07 nm. Images were recorded using a Thermo Fisher Scientific Ceta 16 CCD matrix.
The specific surface area and porosity of the samples were investigated by the Barrett–Joyner–Halenda method [19, 20] using a Micromeritics ASAP-2400 specific surface analyzer.
A technique for studying samples by X-ray photoelectron spectroscopy (XPS) has been described elsewhere [21]. To determine the qualitative chemical composition and the presence of impurities, survey spectra were recorded in the binding energy range 0–1200 eV and with a step of 1 eV. The quantitative chemical composition and the state of individual elements were determined from the XPS spectra of the elements recorded with a binding energy step of 0.1 eV. The residual gas pressure in the camera during the recording did not exceed 1 × 10–7 Torr. The major background gases were CO, CO2, and H2. The ratio of the concentrations of the individual components in the sample was determined from the corresponding peak areas in the XPS spectra of elements using empirical atomic sensitivity factors (ASF) after recording narrow regions of the corresponding atomic levels [22].
Magnesium oxide was heated in an argon flow to a specified temperature of 650–700°С; then, the argon flow was stopped, and the reaction mixture was supplied. Magnesium oxide was carbonized with a mixture of 40 vol % ammonia, 1 vol % acetylene, and 59 vol % ethylene (40% NH3–1% C2H2–C2H4) in a flow-type quartz reactor with a McBain balance, which made it possible to control the change in the sample wight directly during the experiment. Additionally, the amount of deposited carbon modified by nitrogen atoms was controlled by monitoring its amount remaining after etching of magnesium oxide in a hydrochloric acid solution.
The method for removing magnesium oxide from the N–C/MgO composite using a hydrochloric acid solution is similar to that previously used in [7].
After etching, the obtained sample of N-graphene was filtered off and washed on the filter with distilled water to a neutral pH of the washings. The synthesized sample of N-graphene was dried in a muffle furnace at 150°C for 1 h.
RESULTS AND DISCUSSION
A nitrogen-containing precursor (a 40% NH3–1% C2H2–C2H4 mixture) was chosen as a source of catalytic carbon, since the components of this mixture are prone to the formation of carbon deposits by a consecutive mechanism [18], i.e., through polymerization and condensation reactions. When a gaseous 40% NH3–1% C2H2–C2H4 mixture is used, ammonia can be incorporated in the form of nitrogen-containing groups into the carbon chains of intermediate products during the formation of carbon deposits. Thus, the forming carbon material is modified by nitrogen atoms.
The results of studying the effect of the carbonization reaction time on the amount of nitrogen-modified carbon deposited on magnesium oxide, the specific surface area, and the total pore volume at 650°C are presented in Table 1. As can be seen from Table 1, an increase in the reaction time of the carbonization of magnesium oxide leads to an increase in the amount of deposited carbon modified with nitrogen, which in turn leads to a decrease in the specific surface area of N-graphene samples from 1400 to 1200 m2 g–1.
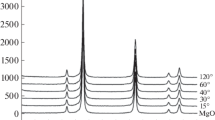
The porous structure of the initial MgO and the N–C/MgO composite has been studied, and Fig. 1 shows the pore size distributions.
It follows from Fig. 1 that after the deposition of nitrogen-modified carbon on magnesium oxide, the pore size distribution changes insignificantly; the peak height only slightly decreases. The data obtained indicate the absence of pore blocking by carbon and its uniform distribution over the magnesium oxide surface. Figure 2 shows the pore size distribution for N-graphene.
It is seen that N-graphene contains micropores with a size of 2–3 nm and mesopores with a size of 20–50 nm. An estimate of the number of micropores demonstrates that their contribution to the specific surface area of N-graphene can be as large as 25%. Since the specific surface area of an ideal monolayer of carbon atoms can reach 2675 m2 g–1 [23], the specific surface area without micropores is at most 975 and 1050 m2 g–1 for N-graphene 650-90 and N-graphene 650-60 samples, respectively; the thickness of N-graphene is about three monolayers.
An increase in the carbonization reaction temperature to 700°C leads to an increase in the deposition rate of nitrogen-modified carbon on magnesium oxide from a 40% NH3–1% C2H2–C2H4 mixture. At this temperature, a second series of N–C/MgO materials was obtained. Varying the reaction time from 30 to 120 min afforded the following samples: N–C/MgO 700-30, N–C/MgO 700-60, N–C/MgO 700-90, and N–C/MgO 700-120. After removing magnesium oxide from the above composite N–C/MgO materials, a series of N-graphene 700 samples was obtained.
It was found that an increase in the reaction temperature leads to a decrease in the specific surface area of N-graphene (Table 2), which is probably due to a less uniform deposition of nitrogen-modified carbon on the surface of magnesium oxide.
Phase Composition of N–C/MgO Composites and N-Graphene
The X-ray diffraction pattern of a N–C/MgO composite sample is shown in Fig. 3. The X-ray diffraction pattern shows peaks corresponding to magnesium oxide (no. ICSD 26958, cubic system, a = 4.22 Å). The size of the coherent scattering region (CSR) of magnesium oxide is 30–50 nm in different directions. The magnified X-ray diffraction pattern (see the inset in Fig. 3) shows a broad maximum at 2θ = 25°, which most likely belongs to turbostratic carbon.
After the removal of magnesium oxide from the N–C/MgO composite, the remaining carbon material modified with nitrogen was also studied by X-ray diffraction (Fig. 4).
The graphite-like phase in the etched sample is represented by wide halos in the 2θ ranges 15°–30° and 40°–48°. The degree of disorder of N-graphene 650-90 is high. The CSR size of turbostratic carbon in the N-graphene 650-90 sample is 1.4 nm (Fig. 4).
XPS Study of N-Graphene Samples
The XPS method was used to study two series of N-graphene samples obtained after treatment in a 40% NH3–1% C2H2–C2H4 mixture at 650 and 700°C. For all synthesized N-graphene samples, survey spectra were recorded. An analysis of the lines in the survey spectra made it possible to establish the presence of carbon (C1s), oxygen (O1s), nitrogen (N1s), and chlorine (Cl2p) on the surface of all samples. The presence of chlorine in the composition of all samples can be explained by the residual amounts after etching in a hydrochloric acid solution.
A quantitative analysis of the elements found on the surface of synthesized N-graphene is presented in Tables 3 and 4. Note that the nitrogen concentration on the surface was higher for the samples obtained at 650°C as compared to the growth temperature 700°C. The maximum nitrogen concentration was found on the surface of the N-graphene 650-90 sample. The same dependences were observed for oxygen.
The normalized spectral C1s and N1s regions for the studied carbon samples are shown in Fig. 5.
The major C1s peak for all samples is characterized by a typical position for sp2-hybridized carbon at 284.6 eV, which was additionally confirmed by a characteristic satellite in the region of ~291 eV caused by the π → π* transition. On the whole, the N1s spectral line shapes for all samples were similar, which indicates a close set of nitrogen states on their surface.
Figure 6 shows the N1s spectra curve-fitted into components for all studied N-graphene samples. For the curve-fitting of these spectral regions, five components with peak binding energies of ~398.4 (N1), ~400.2 (N2), ~401.1 (N3), ~402.2–402.7 (N4), and 403.7–404.5 (N5) eV were used.
In accordance with the literature data [21, 24], the N1 and N3 components can be assigned to pyridinic and graphitic nitrogen forms. The N2 component, which is characterized by a binding energy intermediate between N1 and N3, is assigned to pyrrole or amino groups on the surface of carbon materials. Component N4 typically refers to weakly oxidized nitrogen forms, for example, pyridine oxide. Deeply oxidized NOx forms (for example, nitrites) can be attributed to the N5 component with a binding energy >403 eV. Quantitative data on the distribution of various forms of nitrogen on the surface of all samples are presented in Table 5.
It should be noted that the major forms of nitrogen in N–C/MgO carbon materials are the N1–N4 components, while the N5 component with a concentration of no higher than 4% is an impurity.
TEM Study of N-Graphene
The structure of the synthesized N-graphene samples was studied using electron microscopy. Figure 7 shows a TEM image of N-graphene produced at 650°C.
It is seen that the N-graphene 650-90 particles are spherical. The sphere size of 20–100 nm corresponds to the size of magnesium oxide crystals in the N–C/MgO composite. The resulting structure reflects the template method for the synthesis of N-graphene. Figure 8 shows an TEM image of N-graphene 650-90, obtained with a higher magnification.
In Fig. 8, separate layers of N-graphene are visible. The wall thickness of N-graphene spheres is 2–4 monolayers, which is in agreement with the graphene thickness estimate obtained on the basis of measuring the specific surface area and porosity.
CONCLUSIONS
A method for producing graphene modified with nitrogen atoms has been developed. The method consists of the synthesis of an N–C/MgO composite by treating magnesium oxide in a 40% NH3–1% C2H2–C2H4 mixture at 650 and 700°C. The amount of deposited carbon and, accordingly, the thickness of the N-graphene layer can be controlled by the time of magnesium oxide carbonization. Magnesium oxide was removed from the N–C/MgO composite by treatment with a hydrochloric acid solution.
For the first time, N-graphene with a specific surface area of 1400 m2 g–1 and a thickness of 2–4 monolayers has been obtained. The maximum content of atomic nitrogen in N-graphene was 5 wt %. An increase in the treatment temperature of magnesium oxide in a 40% NH3–1% C2H2–C2H4 mixture from 650 to 700°C leads to a decrease in both the specific surface area of N-graphene and the nitrogen content in it.
An increase in the nitrogen content and a high specific surface area of the N-graphene material will make it possible to increase the number of metal centers (Pt, Pd) on the graphene surface, which in turn should lead to an increase in the activity of catalysts in the reactions of selective hydrogenation of organic compounds with a triple bond.
Thus, the N-graphene synthesized in this work is significantly superior in quality to the available materials [8–15].
REFERENCES
Novoselov, K.S., Geim, A.K., Morozov, S.V., Jiang, D., Zhang, Y., Dubonos, S.V., Grigorieva, I.V., and Firsov, A.A., Science, 2004, vol. 306, no. 5696, pp. 666–669. https://doi.org/10.1126/science.1102896
Ning, G., Fan, Z., Wang, G., Gao, J., Qian, W., and Wei, F., Chem. Commun., 2011, vol. 47, no. 21, pp. 5976–5978. https://doi.org/10.1039/c1cc11159k
Bulushev, D.A., Zacharska, M., Shlyakhova, E.V., Chuvilin, A.L., Guo, Y., Beloshapkin, S., Okotrub, A.V., and Bulusheva, L.G., ACS Catal., 2016, vol. 6, no. 2, pp. 681–691. https://doi.org/10.1021/acscatal.5b02381
Chesnokov, V.V., Kriventsov, V.V., Malykhin, S.E., Chichkan, A.S., and Podyacheva, O.Yu., J. Struct. Chem., 2018, vol. 59, no. 4, pp. 876–882. https://doi.org/10.1134/S0022476618040133
Chesnokov, V.V., Kriventsov, V.V., Malykhin, S.E., Svintsitskiy, D.A., Podyacheva, O.Y., Lisitsyn, A.S., and Richards, R.M., Diamond Relat. Mater., 2018, vol. 89, pp. 67–73. https://doi.org/10.1016/j.diamond.2018.08.007
Chesnokov, V.V., Chichkan, A.S., Bedilo, A.F., and Shuvarakova, E.I., Fullerenes Nanotubes Carbon Nanostruct., 2020, vol. 28, no. 5, pp. 402–406. https://doi.org/10.1080/1536383x.2019.1695249
Chesnokov, V.V., Chichkan, A.S., Bedilo, A.F., Shuvarakova, E.I., and Parmon, V.N., Dokl. Phys. Chem., 2019, vol. 488, pp. 154–157. https://doi.org/10.1134/S0012501619100038
Qu, L., Liu, Y., Baek, J.-B., and Dai, L., ACS Nano, 2010, vol. 4, no. 3, pp. 1321–1326. https://doi.org/10.1021/nn901850u
Zhao, L., He, R., Rim, K.T., Schiros, T., Kim, K.S., Zhou, H., Gutierrez, Ch., Chockalingam, S.P., Arguello, C.J., Palova, L., and Nordlund, D., Science, 2011, vol. 333, no. 6045, pp. 999–1003. https://doi.org/10.1126/science.1208759.
Moon, J., An, J., Sim, U., Cho, S.-P., Kang, J.H., Chung, C., Seo, J.-H., Lee, J., Nam, K.T., and Hong, B.H., Adv. Mater., 2014, vol. 26, pp. 3501–3505. https://doi.org/10.1002/adma.201306287
Rybin, M., Pereyaslavtsev, A., Vasilieva, T., Myasnikov, V., Sokolov, I., Pavlova, A., Obraztsova, E., Khomich, A., Ralchenko, V., and Obraztsova, E., Carbon, 2016, vol. 96, pp. 196–202. https://doi.org/10.1016/j.carbon.2015.09.056
Deng, D., Pan, X., Yu, L., Cui, Y., Jiang, Y., Qi, J., Li, W.-X., Fu, Q., Ma, X., Xue, Q., Sun, G., and Bao, X., Chem. Mater., 2011, vol. 23, no. 5, pp. 1188–1193. https://doi.org/10.1021/cm102666r
Reddy, A.L.M., Srivastava, A., Gowda, S.R., Gullapalli, H., Dubey, M., and Ajayan, P.M., ACS Nano, 2010, vol. 4, no. 11, pp. 6337–6342. https://doi.org/10.1021/nn101926g
Zhao, J., Zhu, G., Huang, W., He, Z., Feng, X., Ma, Y., Dong, X., Fan, Q., Wang, L., Hu, Z., Lu, Y., and Huang, W., J. Mater. Chem., 2012, vol. 22, no. 37, pp. 19679–19683. https://doi.org/10.1039/c2jm33209d
Wang, H., Maiyalagan, T., and Wang, X., ACS Catal., 2012, vol. 2, no. 5, pp. 781–794. https://doi.org/10.1021/cs200652y
Jin, Z., Yao, J., Kittrell, C., and Tour, J.M., ACS Na, vol. 5, no. 5, pp. 4112–4117. https://doi.org/10.1021/nn200766e
Pan, W., He, M., Zhang, L., Hou, Y., and Chen, C., Nanomaterials, 2019, vol. 9, no. 5, p. 798. https://doi.org/10.3390/nano9050798
Buyanov, R.A. Zakoksovanie katalizatorov (Carbonization of Catalysts). Moscow: Nauka, 1983.
Gor, G.Yu., Thommes, M., Cychosz, K.A., and Neimark, A.V., Carbon, 2012, vol. 50, pp. 1583–1590. https://doi.org/10.1016/j.carbon.2011.11.037
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., and Sing, K.S.W., Pure Appl. Chem., 2015, vol. 87, pp. 1051–1069. https://doi.org/10.1515/pac-2014-1117
Chesnokov, V.V., Chichkan, A.S., Paukshtis, E.A., Svintsitskii, D.A., Ismagilov, Z.R., and Parmon, V.N., Dokl. Phys. Chem., 2017, vol. 476, part 2, pp. 186–189. https://doi.org/10.7868/S0869565217290126
Moulder, J.F., Stickle, W.F., Sobol, P.E., and Bomben, K.D., Handbook of X-ray Photoelectron Spectroscopy, Perkin-Elmer Corp., Eden Prairie, Minnesota, USA, 1992.
Yang, H., Kannappan, S., Pandian, A.S., Jang, J-H., Lee, Y.S., and Lu, W., J. Power Sources, 2015, vol. 284, pp. 146–153. https://doi.org/10.1016/j.jpowsour.2015.03.015
Arrigo, R., Schuster, M.E., Xie, Z., Yi, Y., Wowsnick, G., Sun, L.L., Hermann, K.E., Friedrich, M., Kast, P., Havecker, M., Knop-Gericke, A., and Schlogl, R., ACS Catal., 2015, vol. 5, pp. 2740–2753. https://doi.org/10.1021/acscatal.5b00094
ACKNOWLEDGMENTS
Electron microscopic studies were performed using the equipment of the Shared Use Center “National Center for Catalyst Research,” Boreskov Institute of Catalysis, SB RAS, Novosibirsk.
Funding
The work was supported by the Russian Science Foundation (project no.17-73-30032).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Chesnokov, V.V., Chichkan, A.S., Svintsitskiy, D.A. et al. A Method for Synthesis of Nitrogen-Doped Graphene with High Specific Surface Area. Dokl Phys Chem 495, 159–165 (2020). https://doi.org/10.1134/S0012501620110019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012501620110019