Abstract
A model and the principle of constructing a corresponding computer program are proposed for the electronic synthesis of polymer networks with a specified range of glass transition temperatures have been proposed for the first time. A repeating fragment of the network is synthesized from the smallest basic fragments, which are connected to each other using a control matrix of interactions. As an example, there are presented 27 chemical structures of the cross-linked points, 26 basic fragments for constructing polymer chains connecting the cross-linked points, and five repeating fragments of polymer networks with glass transition temperatures falling within the range 450–480 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The problem of predicting the glass transition temperature Tg and other physical properties of network polymers from their chemical structure has been considered in detail in monographs [1–4]. In monographs [5, 6], such an approach is absent, and only corrections are made to the methods for calculating linear polymers, which make it possible to evaluate the Tg values of network polymers.
Earlier [1–4], a model and a computer program have been developed that allow the computer synthesis of linear polymers. The synthesis is performed from the smallest basic fragments that cannot be “cut” along the axis of the macromolecule. As an example, Table 1 shows a number of these fragments.
Labels define the possibility of chemical bonding of atoms to the basic fragment structure. Among the atoms are carbon, hydrogen, oxygen, nitrogen, sulfur, etc. The left label means that this atom is attached to the left side of the basic fragment, and the right label means that this atom is attached to the right side of the basic fragment. The attached atoms can be different; for example, oxygen is attached to the left and carbon is attached to the right.
In the present work devoted to the computer synthesis of network polymers, linear chains are structural elements of a repeating fragment of a network connecting cross-linked points. For highly cross-linked polymers, the variety of repeating fragments of the network during its computer synthesis is achieved by specifying a relatively large number of basic fragments. In [1–4], 96 basic fragments have been used, some of which are presented in Table 1. Experiments performed earlier for linear polymers show that an increase in the number of basic fragments by one (for example, from 3 to 4, from 4 to 5, from 5 to 6, etc.) increases the number of synthesized structures by an order of magnitude.
The possibility of addition of an atom to the basic fragment in the course of computer synthesis is determined by the so-called connectivity matrix presented in Table 2.
If there is 0 at the intersection of the horizontal and vertical lines, it means that the structures cannot be chemically bonded; if there is 1 at such an intersection, it means that the structures can be chemically bonded.
In addition to the above, the influence of the homogeneous and inhomogeneous distribution of the number of repeating units in linear chains connecting the cross-linked points has been analyzed [2, 4]. We have also analyzed the influence of inhomogeneities in the network structure (dangling chains and rings) on the Tg value.
Let us proceed directly to the consideration of the principles of computer synthesis of networked polymers.
RESULTS AND DISCUSSION
Previously [2, 4], a model and a calculation scheme have described for a quantitative assessment of the glass transition temperature Tg, for which the van der Waals volume of a repeating network fragment, atomic constants for linear chains connecting the cross-linked points, and atomic constants for cross-linked points have been taken into account in the calculations.
From the point of view of chemical structure, a cross-linked point consists of an atom from which chain branching begins and neighboring atoms chemically bonded to it. The latter contain chemical substituents, which are also included in the cross-linked point.
Table 3 shows, for example, a number of atoms and atomic structures from which chain branching occurs. In total, in [2–4], there are 78 such structures that form cross-linked points of polymer networks.
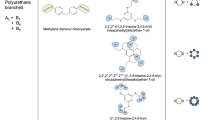
As an example, let us choose the range of glass transition temperatures of network polymers from 450 to 480 K. The program synthesizes many repeating fragments of polymer networks, of which, for example, we select only five structures (Table 4).
All Tg values fall within the specified glass transition temperature range.
Thus, the fundamental possibility of carrying out electronic synthesis of not only linear polymers, which has been developed by us earlier [1–4], but also of network polymers. Further work in this direction is related to taking into account the network topology. Indeed, with the same chemical structure of the basic fragments and their amount, as well as with the same cross-linked points, polymer networks can have different chemical structures. It depends on where the chain crosslinks are located, not to mention structural defects (the formation of cycles, the presence of branched chains one end of which is not linked to the network structure, etc.). Let’s give an example of this influence. For radiation cross-linked polymer chains of polyethylene, the following structure is possible:

This network is four-functional; i.e., four chains come out of its cross-linked point. For such a network, the glass transition temperature is calculated by the relationship
where m is the number of repeating units in linear chains connecting the cross-linked points; ai are atomic constants related to the energy of the intermolecular bond of the ith atom with neighboring atoms; bj are the increments related to the energies of dipole-dipole interactions and hydrogen bonds; \(\left( {\sum\nolimits_i {{{a}_{i}}\Delta {{V}_{i}}} } \right.\) + \({{\left. {\sum\nolimits_j {{{b}_{j}}} } \right)}_{l}}\) is the set of atomic constants and increments for the repeating unit of the chains connecting cross-linked points; \(\left( {\sum\nolimits_i {{{a}_{i}}\Delta {{V}_{i}}} } \right.\) + \(\left. {\sum\nolimits_j {{{b}_{j}}} } \right)_{l}^{ * }\) is the same for the end units directly bonded to the cross-linked points; \({{\left( {\sum\nolimits_i {{{K}_{i}}\Delta {{V}_{i}}} } \right)}_{{{\text{cr}}{\text{.p}}{\text{.}}}}}\) is the set of atomic constants for cross-linked points.
CONCLUSIONS
The proposed approach allows the computer synthesis of network polymers. Using this approach and the corresponding equations, it is possible to construct polymer networks of the most diverse topology with constant chemical structure.
REFERENCES
Askadskii, A.A., Physical Properties of Polymers. Prediction and Control, Amsterdam: Gordon and Breach, 1996.
Askadskii, A.A., Computational Materials Science of Polymers, Cambridge: Cambridge Int. Sci. Publ., 2003.
Askadskii, A.A., Analysis of the Structure and Properties of High-Cross-linked Polymer Networks, Paris: Harwood Acad. Publ., 1992.
Askadskii A.A. and Kondrashchenko, V.I., Komp’yuternoe materialovedenie polimerov (Computer Materials Science of Polymers), vol. 1: Atomno-molekulyarnyi uroven’ (Atomic and Molecular Level), Moscow: Nauchnyi Mir, 1999.
Van Krevelen, D.W., Properties of Polymers, 3d ed., Amsterdam: Elsevier, 1990.
Bicerano, J., Prediction of Polymer Properties, New York: Marcel Dekker, 2002.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (project “Theoretical and experimental design of new composite materials to ensure safety during the operation of buildings and structures under conditions of man-made and biogenic threats” no. FSWG-2020-0007 and the subject of the State Assignment of INEOS RAS no. 0085-2019-0004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Askadskii, A.A., Matseevich, T.A. Computer Synthesis of Network Polymers. Dokl Phys Chem 494, 151–158 (2020). https://doi.org/10.1134/S0012501620100012
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012501620100012