Abstract
The reactivity of sulfonyl-substituted closo-decaborate derivatives (the [2-B10H9SH]2– and [2-B10H9S(CН2C(О)NН2)2]– anions) in the complexation of Ag(I) and Pb(II), Pearson soft acids, in the presence of competitive organic ligands has been studied. The substituted derivatives act as bridging ligands to form silver(I) binuclear complexes [(Ag(bipy)2)2(2-B10H9SH)] and [(Ag(bipy)2)2(2-B10H9S(CН2C(О)NН2)2]NO3; the [2-B10H9SH]2– anion is involved in the lead(II) coordination polyhedron in the [Pb(2-B10H9SH)] and [Pb(bipy)2(2-B10H9SH)] complexes; in [Pb(bipy)2][2-B10H9S(CН2C(О)NН2)2]2, the substituted decaborate derivative acts as a counterion. The solubility of the mixed-ligand complexes is different due to the variety of their structures. The synthesized complexes are the first water-soluble lead and silver compounds with the closo-decaborate anion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cluster boron anions with exopolyhedral B–S bond are of great practical importance for boron neutron capture therapy (BNCT) of malignant tumors [1]. The BNCT principle is based on the nuclear reaction of the stable boron-10 isotope with thermal neutrons. The generated alpha particles and recoil nuclei have a high linear energy loss and a small free path commensurate with the diameter of a single cell. In the case of selective boron-10 accumulation in tumor cells, a local radiation effect can be achieved. Therefore, nowadays, the major challenge is to create boron-containing drugs that can selectively deliver a therapeutic amount of boron-10 to malignant tumor cells, ensure its optimal microdistribution, and remain in the cells for the time required for irradiation. Currently, the most well-known and widely used compounds are those of the sulfanyl-substituted closo-dodecaborate anion, [B12H11SH]2–. However, the task of obtaining drugs with high chemical and biological stability, a high boron content in the molecule, low toxicity, and high water solubility has not been completely solved, so research in this area continues and is relevant.
Substituted derivatives of cluster boron anions are of fundamental interest since they form a series of weakly coordinating ligands [2–6]. The formation of substituted derivatives can occur with both persistence of the system charge and its decrease, up to the formation of neutral substituted derivatives. The decrease in the charge of substituted anions significantly suppresses their coordination ability. Synthesis of metal complexes based on sulfanyl derivatives of the [B10H10]2– anion can undoubtedly significantly extend the practical use of such compounds because of the possibility of using their combined properties potentially inherent in the cationic and anionic parts of a compound. It should be noted that the high solubility of such compounds in water is a decisive factor and greatly facilitates the solution of the tasks [7].
The only lead(II) complex with a sulfanyl-substituted derivative—Pb(bipy)2[1-B10Н9SMe2]2—has been described in the literature [8], where its synthesis is reported and its structure is discussed. Single-crystal X-ray diffraction has demonstrated that the Pb(II) coordination polyhedron is formed, in addition to the heterocycle nitrogen atoms, by the BH group of the cluster anion.
The present study deals with the reactivity of the sulfanyl-substituted closo-decaborate anion derivatives in the complexation of Ag(I) and Pb(II), including reactions in the presence of soft bases as competitive ligands. Conditions of formation of intra-sphere complexes have been formulated; the solubility of metal salts and complexes based on sulfanyl-substituted derivatives of the [B10H10]2– anion has been estimated.
The starting reagents were tetrabutylammonium salts ((C4H9)4N)2[2-B10H9SH] and (C4H9)4N[2-B10H9S(CН2C(О)NН2)2], metal salts AgNO3 and Pb(NO3)2, and triphenylphosphine (Ph3P) and 2,2'-bipyridine as competitive ligands.
The reaction of ((C4H9)4N)2[2-B10H9SH] or (C4H9)4N[2-B10H9S(CН2C(О)NН2)2] with AgNO3 in acetonitrile or H2O gives [Ag2(2-B10H9SH)] · nsolv (I) or [Ag(2-B10H9S(CН2C(О)NН2)2)] · nsolv (II). The reaction in the general form is
The resulting complexes are brightly colored and are fairly soluble in water and organic solvents. It should be noted that the solubility of analogous complexes with the [B10H10]2– and [B12H12]2– anions is essentially lower, since the silver compounds of the [Ag2BnHn] composition (n = 10, 12) are the weighing forms (SP ~ 10–24) and are used in analytical determinations.
The introduction of 2,2'-bipyridine into the reaction solution containing ((C4H9)4N)2[B10H9SH] or (C4H9)4N[2-B10H9S(CН2C(О)NН2)2] and AgNO3 in acetonitrile leads to the formation of fine crystalline precipitates of [(Ag(bipy)2)2(2-B10H9SH)] (III) or [(Ag(bipy)2)2(2-B10H9S(CН2C(О)NН2)2]NO3 (IV). The reactions are described by schemes (2) and (3):
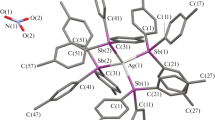
The reaction of (Bu4N)2[2-B10H9SH] with AgNO3 and Ph3P in a CH3CN/C6H6 mixture leads to the anionic complex [Ag(PPh3)4]{[Ag(PPh3)3]2(2-B10H9SH)}(NO3) · nCH3CN (V) irrespective of the reagent ratio (4):
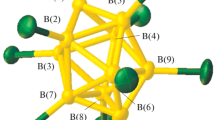
The reaction of ((C4H9)4N)2[2-B10H9SH] or (C4H9)4N[2-B10H9S(CН2C(О)NН2)2] with Pb(NO3)2 in acetonitrile or H2O gives [Pb(2-B10H9SH)] (VI) (bright yellow) or [Pb(solv)6][2-B10H9S(CН2C(О)NН2)2]2. The scheme of complexation is shown below:
The introduction of 2,2'-bipyridine (bipy) into the reaction solution containing ((C4H9)4N)2[2-B10H9SH] or (C4H9)4N[2-B10H9S(CН2C(О)NН2)2] and Pb(NO3)2 in acetonitrile leads to the [Pb(bipy)2(2-B10H9SH)] (VIII) or [Pb(bipy)2][2-B10H9S(CН2C(О)NН2)2]2 (IX) complexes; complex VIII is rich colored.
Complexes I–IX were identified using elemental analysisFootnote 1 and IR spectroscopy dataFootnote 2 (Table 1). The IR spectra provide much information, which makes it possible to determine, at the first stage of studies, the participation of sulfanyl-substituted derivatives in the formation of metal coordination cores. In particular, for the complexes with the substituent group coordinated to the metal atom, the B–S stretching vibration band ν(ВS) at 960 cm–1 is informative; for coordination by BH groups of the polyhedron, the B–H stretching vibration band ν(ВН) at 2340 cm–1 is informative (Table 1).
The IR spectra of compounds I–IV show, in the BH stretching vibrations, two broad overlapping bands with maxima at about 2460 and 2320 cm–1, due to the ν(BH) and ν(BH)MHB vibrations. The presence of the low-frequency band at 2320 cm–1 points to the coordination of sulfanyl-substituted derivatives through the ВН groups of the polyhedron to the Ag(I) or Pb(II) atoms. The spectrum of VII shows no low-frequency band at 2320 cm–1, which is evidence of the outer-sphere position of the [2-B10H9S(CН2C(О)NН2)2]– anion in the aqua complex. The coordinated water molecules in VII give rise to the ν(ОН) stretching vibration band in the range 3600–3400 cm–1 and δ(ОН) bending vibration band at 1640 cm–1. The IR spectra of I, VI, and VIII allow us to conclude that the sulfur atom is involved in contacts with metal atoms, since the spectra show characteristic changes in the range of ν(BS) vibrations 700–900 cm–1, which are absent in the spectra of the initial sulfanyl-substituted derivatives.
In the range of BH stretching vibrations, the IR spectrum of V shows the broadened strong ν(BH) band of “free” BH groups at 2460 cm–1 and the moderate ν(BH)MHB band at about 2330 cm–1, which indicates the coordination of the substituted derivative [2-B10H9SH]2– through ВН groups of the polyhedron to form three-center two-electron (3c2e) metal–H–B (MHB) bonds. The spectrum of this complex also shows changes in the range of B–S bond vibrations: instead of one moderate ν(BS) band at 954 cm–1, the spectrum displays a broadened and very weak band with maxima at 972, 945, and 915 cm–1, which can reflect the participation of the thio group of the sulfanyl-substituted derivative in contacts with metal atoms. The presence of the broadened strong ν(NO) band at about 1350 cm–1 corresponds to the presence of the NO3 group in the complex. The spectrum also shows a full set of vibrations of coordinated triphenylphosphine molecules in the range 1500–700 cm–1.
The IR spectra of complexes III, VII, VIII, and IX show, in addition to ν(ВН) and ν(ВН)МНВ stretching vibration bands (III and VII), a full set of absorption bands of coordinated 2,2'-bipyridine molecules in the range 1600–700 cm–1.
It should be noted that the high solubility of the initial alkylammonium salts of sulfanyl-substituted [B10H10]2– derivatives is inherent in the synthesized Ag(I) and Pb(II) complexes with the corresponding substituted derivatives.
Thus, the synthesized Ag(I) and Pb(II) complexes with sulfanyl derivatives of the closo-decaborate anions—[2-B10H9SH]2– and [2-B10H9S(CН2C(О)NН2)2]–-and competitive soft bases are the first examples of water-soluble complexes with cluster boron anions and their substituted derivatives. The high solubility of the [Ag(2-B10H9R)]n (n = 1, 2), Pb[2-B10H9SH], and [Pb(solv)6][2-B10H9S(CН2C(О)NН2)2]2 complexes, comparable with the solubility of alkali metal and alkylammonium salts with the corresponding anions, makes them good candidates for using in BNCT. The solubility of the synthesized mixed-ligand complexes is different and is likely caused by the diversity of their structures. In particular, in the binuclear [(Ag(bipy)2)2(2-B10H9SH)] and [(Ag(bipy)2)2(2-B10H9S(CН2C(О)NН2)2]NO3 complexes, the substituted derivatives act as bridging ligands; in the [Pb(2-B10H9SH)] and [Pb(bipy)2(2-B10H9SH)] complexes, the [2-B10H9SH]2– anion is involved in the formation of the Pb(II) coordination polyhedron (the coordination occurs only through the SH group of the substituted derivative); in [Pb(bipy)2][2-B10H9S(CН2C(О)NН2)2]2, the sulfanyl-substituted derivative acts as a counterion.
Notes
Elemental analysis on carbon, hydrogen, and nitrogen was performed on a Carbo Erba CHNS-3 FA 1108 Elemental Analyzer. Boron was quantified by atomic absorption spectroscopy on a Perkin-Elmer Model 2100 spectrometer with an HGA-700 electrothermal atomizer [9]; Ag was determined on an AAS-303 in an acetylene–air flame.
IR spectra were recorded on a Lumex Infralum FT-02 spectrophotometer as Nujol mulls, NaCl plates, 4000–400 cm–1, resolution 1 cm–1.
REFERENCES
Sivaev, I.B. and Bregadze, V.V., Eur. J. Inorg. Chem., 2009, vol. 11, p. 1433.
Ivanov, S.V., Ivanova, S.M., Miller, S.M., Anderson, O.P., Solntsev, K.A., and Strauss, S.H., Inorg. Chem., 1996, vol. 35, p. 6914.
Avdeeva, V.V., Polyakova, I.N., Vologzhanina, A.V., Malinina, E.A., Zhizhin, K.Yu., and Kuznetsov, N.T., Polyhedron, 2017, vol. 123, p. 396.
Zhizhin, K.Yu., Vovk, O.O., Malinina, E.A., Mustyatsa, L.V. Goeva, L.V., Polyakova, I.N., and Kuznetsov, N.T., Russ. J. Coord. Chem., 2011, vol. 27, no. 9, pp. 653–658.
Zhizhin, K.Yu., Malinina, E.A., Polyakova, I.N., Lisovskii, M.V., et al., Russ. J. Inorg. Chem., 2002, vol. 47, no. 8, pp. 1158–1167.
Zhizhin, K.Yu., Mustyatsa, V.N., Malinina, E.A., Matveev, E.Yu., et al., Russ. J. Inorg. Chem., 2005, vol. 50, no. 2, pp. 203—209.
Zhizhin, K.Yu., Malinina, E.A., Goeva, L.V., Chernyavskii, S.A., Ivanov, S.V., Luk’yanets, E.A., Solntsev, K.A., and Kuznetsov, N.T., Dokl. Akad. Nauk, 1997, vol. 357, no. 2, p. 206.
Orlova, A.M., Sivaev, I.B., Lagun, V.L., Solntsev, K.A., and Kuznetsov, N.T., Koord. Khim., 1993, vol. 19, p. 116.
Ochertyanova, L.I., Mustyatsa, V.N., Belousova, O.N., Zhizhin, K.Yu., and Kuznetsov, N.T., Inorg. Mater., 2004, vol. 40, no. 2, pp. 144—146.
ACKNOWLEDGMENTS
This work was supported by the Council of the President of the Russian Federation for State Support of Leading Scientific Schools (project no. NSh-2845.2018.3).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Malinina, E.A., Korolenko, S.E., Goeva, L.V. et al. Synthesis and Structure of New Water-Soluble Ag(I) and Pb(II) Complexes with Sulfonyl-Substituted Derivatives of the closo-Decaborate Anion. Dokl Chem 483, 297–300 (2018). https://doi.org/10.1134/S0012500818120042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012500818120042