Abstract
The electrophysiological mechanism of the atrial myocardium resistance to the cold-induced arrhythmias was studied in the hibernating ground squirrel Citellus undulatus. The atrial action potentials (APs) and refractoriness were recorded with microelectrodes in isolated multicellular preparations of the atrial myocardium taken from the hibernating and summer active ground squirrels (HS and SAS, respectively) at 37, 27, and 17°С to estimate the AP and refractoriness durations. In both HS and SAS, hypothermia increased the duration of the AP and refractoriness period (APD and RD, respectively), and in both animal groups RD was longer than APD under hypothermia but not at 37°С. This last observation can be a result of the postrepolarization refractoriness (PRR), which seems to contribute substantially to the atrial myocardium tolerance of the hibernating animals to the hypothermia-induced arrhythmias because it prevents afterdepolarizations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The hibernating animal heart is currently considered a natural model that demonstrates myocardium resistance to ischemic disorders and arrhythmias. Yakut ground squirrels (Сitellus undulatus) are the most prominent among the hibernating heterotherm mammals. They are characterized by the most prolonged periods of the hypothermic hypometabolic hibernation when their body temperature can drop from 37°С to –1°С. During hypothermic dormancy, their cardiac activity remains coordinated and rhythmic. In all non-hibernating mammals, a decrease in the body temperature to 30–17°С is accompanied by the so-called hypothermia-induced arrhythmias and both atrial and ventricular fibrillations, which lead to cessation of the organized cardiac activity [1–3].
The hibernating animal heart is well known to differ from that of a non-hibernating animal in numerous parameters at the molecular, cellular. and tissue levels. Nevertheless, despite century-long studying of the hibernating animals and hibernation phenomenon, the electrophysiological mechanisms of the heart tolerance to the rhythm disorders are not still completely understood. The ventricular myocardium was the subject of study in the overwhelming number of reports on electrophysiology of the hibernating animal heart [4–6]. Bioelectrical activity of the atrial myocardium has been little studied under various conditions. Specifically, only two reports characterize bioelectrical activity [7, 8] and one report characterizes contractility [9] of the hibernating animal atria under hypothermia. The purpose of this study was to estimate the action potentials (APs) and refractoriness of the ground squirrel atria during the animal summer activity and under hibernation at moderate hypothermia.
MATERIALS AND METHODS
The isolated multicellular perfused atrium preparations of the winter hibernating (HS) and summer active (SAS) Yakut ground squirrels (Citellus undulatus) were used in our study. The ground squirrels caught in Yakutia were kept in a dark room at a temperature of +2°С, where they fell into hibernation. The animal activity and body temperature were monitored through December–January to determine the moment corresponding to the middle of the hibernation period when the animals were taken into experiments. The active animals were taken in the middle of summer and kept in a vivarium for 12 h at the day light under normal conditions.
The animals were euthanized to isolate atrial myocardium either at 20°С or at 2–4°С from the SAS or HS, respectively. The preparations were placed into a 5-mL perfusion chamber and after their fixation the solution temperature was gradually raised (by 1°С during 3 min) to 37°С. The solution flow rate was 15 mL/min; it was oxygenated with a mixture of 95% of O2 and 5% of CO2 and had the following composition (мМ): NaCl, 130; KCl, 4.7; NaH2PO4 · 2H2O, 1.2; NaHCO3, 18, MgCl2, 1.05; CaCl2, 3; glucose, 11; pH 7.2–7.4. The rhythm of the multicellular preparation activity was set using silver electrodes connected with an ESL-2 electronic stimulator. The stimulus duration was 2 ms at 37°С and 3–4 ms at 27–17°С; the stimulus amplitude was twice higher than the excitation threshold.
The APs were recorded at 37, 27, and 17°С using the standard microelectrode technique, glass microelectrodes (Ω = 10–20 MΩ) filled with 3 М KCl, a WPI701 amplifier (WPInstruments, United States), an ADC L-card E-154 (Russia), and a PC with the Power Graph 3.3 software (Russia). The AP duration was measured at repolarization levels of 50% and 90% (APD50 and APD90) using the MiniAnalysis 6.0.7. software (Synaptosoft, United States) [1, 7].
The refractoriness period (RP) of the papillary muscles was estimated using the standard “extrasystolic” protocol, according to which after 20 “preconditioning” stimuli (S) applied at regular intervals (500, 300, 250, or 200 ms), an extra stimulus (S1) was added. The time interval between the last, 20th preconditioning stimulus and S1 was gradually reduced (at a step of 4–20 ms) until the absence of AP in response to the extra stimulus. The time interval from the preconditioning stimulus to the extra stimulus that failed to induce AP was considered RP duration. The resultant RD was approximately similar to the refractoriness duration in vivo. RD was measured at 37, 27, and 17°С. The RP was normalized to APD90 in order to determine the postrepolarization refractoriness (PRR).
The results were processed statistically using the Statistica 6.0 software (StatSoft). The significance of differences between groups were estimated using two-way ANOVA (with subsequent tests for multiple comparisons in groups with repeated or independent post-hoc measurements, as well as using the subsequent Dunnett correction) after preliminary check up by the Shapiro–Wilk test of the distribution normality in groups. The data were considered significant at p < 0.05; they are presented as the mean ± standard deviation.
RESULTS AND DISCUSSION
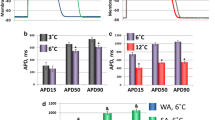
Under the conditions of hypothermia, the AP duration (APD) grows in the atrial myocardium of both HS and SAS. This effect of hypothermia on APs is characteristic of almost all mammalian animals. Figure 1a demonstrates a typical example of AP changes in an HS during a temperature decrease from 37 to 17°С. Both in HS and SAS, AP duration increased mostly due to the increase in APD90, while the AP duration at 50% repolarization varied slightly (Fib. 1b; hereinafter, no data on APD50 are shown). The cold-induced changes in the ground squirrel AP are manifested in retarding of the final repolarization phase, which is probably a result of changes in potassium repolarizing currents. Since APD50 is correlated with the values of calcium transmembrane depolarizing current, calcium currents in the atrial myocardium of HS seem to be less sensitive to temperature than potassium currents.
The effect of hypothermia on the action potentials (APs) of the Yakut ground squirrel atrial myocardium. (a) A representative example of changes in the atrial AP configuration in a winter-hibernating ground squirrel (HS) during a temperature decrease from 37 to 17°С. (b) A temperature decrease little affects the atrial AP duration at the repolarization level of 50% (APD50), but lead to an increase of the same parameter at the repolarization level of 90% (APD90). (c, d) Dependence of AP duration (APD90) on the cycle time (an interval between stimuli) at 37°С and under hypothermia in the atria of the hibernating and summer active ground squirrels (HS and SAS, respectively) * p < 0.05 (from the magnitude at 37°С); #p < 0.05 (from the magnitude at 27°С). n is the number of animals and multicellular preparations.
In both HS and SAS, the atrium AP duration grows with increasing cycle duration (Figs. 1с, 1d). The same dependence is preserved under hypothermia, although in the HS, the APD-rhythm dependence was the most pronounced at the least temperature (17°С) (Fig. 1d): in the HS, APD90 changed from 112 ± 11 to 162 ± 18 ms (n = 6) in response to stimulation at intervals from 200 to 500 ms, respectively. In general, the ground squirrel pattern of the rhythm-dependent changes in APD during cooling was similar to that observed in the non-hibernating small rodents, e.g., in rats.
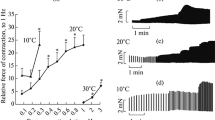
Like the AP duration, the refractoriness duration also increased in the atrial myocardium of both SAS and HS (Figs. 2a, 2b), which is typical of mammals. The RP duration in HS and SAS depended on the excitation rhythm of the atrial myocardium: in general, with increasing intervals between stimuli, the refractoriness duration also increased and this was also characteristic of both ventricular and atrial myocardium of the non-hibernating ground squirrels. In the HS, the RP dependence on intervals between stimuli was the most pronounced at the least temperature tested: RP duration changed from 115 ± 16 to 217 ± 20 ms (n = 6) at stimulation intervals of 200 and 500 ms (Fig. 2c).
The effect of hypothermia on the refractoriness duration in the atrial myocardium of Yakut ground squirrels. (a) Representative examples of the moments preceding the onset of refractoriness (above) and of the refractoriness onset at 37, 27, 17°С in the atrium of a hibernating ground squirrel. (b, c) Dependence of the refractoriness duration in the atria of a winter-hibernating (HS) and summer active SAS) ground squirrels on the cycle duration (interval between stimuli) at 37°С and under hypothermia. * p < 0.05 (from the magnitude at 37°С).
The refractoriness duration normalized for APD at 37°С proved to be significantly less than unity in the active ground squirrels at the cycle range of 200–500 ms (Fig. 3a). This ratio is characteristic of the non-hibernating animals, which were shown to have an effective refractoriness magnitude of 66–85% of the AP duration. Normalized refractoriness of HS ground squirrels also approaches the unity or exceeds this magnitude insignificantly at 37°С (Fig. 3b). However, under hypothermia (27 and 17°С), and at the cycle durations of 500 and 200 ms in SA and 500 and 300 ms in HS animals, the magnitude of normalized refractoriness of atrial myocardium is significantly greater than at 37°С and substantially greater than unity (Fig. 3). Thus, hypothermia causes in the ground squirrel atrial myocardium the postrepolarization refractoriness, i.e., an atypical state when the refractoriness duration (the time required to restore excitability) was significantly greater (by values close to 30% in our experiments) than the action potential duration and repolarization time.
Normalized refractoriness duration in the atrial myocardium of a hibernating and active ground squirrels (HS and SAS, respectively) at 37°С and under hypothermia. RP, period of refractoriness. * p < 0.05 (from the magnitude at 37°С). The values above the dotted line indicate the postrepolarization refractoriness.
Thus, our study is the first to demonstrate the phenomenon of PRR in the atrial myocardium of the HS. We have earlier reported that under cooling to 17°С the duration of refractoriness in the ground squirrel ventricular myocardium reaches virtually the AP duration [1]. Unlike the ventricular myocardium, in the atrial myocardium, PRR is observed already under a light hypothermia (27°С) in both HS and SAS, and it reaches a greater magnitude than in the ventricular myocardium. In the ground squirrel atrium myocardium, PRR is little dependent on rhythm, because similar values were recorded at stimulation with 200- and 500-ms intervals.
Postrepolarization refractoriness can be observed not only in hibernating animals, but also in myocardium of the non-hibernating animals under normothermia. In non-hibernating animals, PRR can play a dual role under different conditions. PRR is formed in the cardiac ventricles in the central areas of ischemic foci [10]. In this case, PRR is a negative physiological phenomenon that promotes re-entry and the fibrillation arising because of reducing the speed of the extra stimulus conduction. The mechanisms of the ischemic PRR are still not completely elucidated. Nevertheless, potassium accumulation in the extracellular medium and a significant decrease in AP duration are believed to be responsible for PRR arising in the ischemized myocardium [10].
Under normal physiological conditions, PRR develops in response to a series of antiarrhythmic pharmacological preparations (AAP) of the first and third classes. Moreover, PRR is considered an important mechanism of the antiarrhythmic AAP effect. For instance, class I AAP, such as propafenone, procainamide, ranolazine and class III AAP such as amiodarone, dronedarone, and vernakalant, cause PRR [11–13]. AAP-induced antiarrhythmic PRR effect is believed to be involved in suppression of postdepolarizations, which are triggers of the extra stimuli; this effect is also responsible for an increase of the excitation wave length in myocardium and re-entry prevention.
“Cold postdepolarizations” appear in non-hibernating animals under hypothermia because the depolarizing and repolarizing ionic currents differ in the degree of cold inhibition. Note that, in non-hibernating animals, rhythm disorders often arise under moderate hypothermia. The hibernating animals under moderate hypothermia that develops, e.g., in vivo when they are going into or out of hibernation never have rhythm disorders. PRR that suppress postdepolarizations seems to be a mechanism underlying the atrium myocard tolerance to arrhythmias.
Under the action of class I AAP, PRRs seem to appear because they influence the kinetics of the sodium potential-sensitive Nav1.5 ionic channels, in particular, because of an increase in the time required for the ionic channel recovery after inactivation. The class III AAP effect on the mechanism of PRR induction is also assumed to depend on AAP effect on the sodium apart from potassium channels. The mechanism of PRR development in the atrial myocardium of the hibernating animals may be similar.
During hibernation, numerous changes are known to occur in the hibernating animal heart, such as an increased expression of gap junctions Сх43, Сх45 [2] and Na, K ATPase resistant to hypothermia [14]. In addition, expression of the cold-tolerant isoform of the sarcoplasmic Ca ATPase increases, while the incoming calcium current ICa,L is reduced [3, 15]. One would assume that when hibernating animals fall into hibernation some specific forms of the potential-sensitive sodium or other channels are produced. However, PRRs in our experiments were recorded in both hibernating and active ground squirrels in the middle of summer under the acute cooling of the atrial preparations. This means that PRR development is probably not related to changes in expression of the ionic channel genes. In the Yakut ground squirrels, either the cardiac Nav1.5 channels have a specific sensitivity to temperature or hypothermia activates some intracellular regulatory mechanisms that prolongate Nav1.5 inactivation.
Of interest is that a series of AAP (ranolazin, vernakalant) are capable of PRR inducing only in the atrial myocardium. The atrial Nav1.5 isoforms and/or some additional regulatory subunits of the channel are more prone to the formation of PRR than the ventricular ones, which is especially characteristic of HS.
Thus, a number of reports suggest that a series of adaptations have been developed in hibernating animals to support cardiac activity under hypothermia. These adaptations include the capabilities of preventing excessive AP increase under hypothermia, supporting calcium cycle at the required level and preventing cardiomyocyte overload with calcium, as well as maintaining the excitability and conductance of the cardiac myocardium. Another important feature of the HS found in our experiments is the fact of the refractoriness “balance” is maintained in them: the refractoriness duration is long enough to prevent premature myocardium excitation, but it is not too long, so that the cardiac activity is not suppressed under low temperatures.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interest. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
Kuz’min, V.S., Abramov, A.A., Egorov, Yu.V., and Rozenshtraukh, L.V., Ross. Fiziol. Zh. im. I.M. Sechenova, 2014, vol. 100, no. 12, pp. 1399–1408.
Fedorov, V.V., Glukhov, A.V., Sudharshan, S., Egorov, Y., Rosenshtraukh, L.V., and Efimov, I.R., Heart Rhythm, 2008, vol. 5, no. 11, pp. 1587–1596.
Yatani, A., Kim, S.J., Kudej, R.K., Wang, Q., Depre, C., Irie, K., Kranias, E.G., Vatner, S.F., and Vatner, D.E., Am. J. Physiol. Heart Circ. Physiol., 2004, vol. 286, pp. 2219–2228.
Averin, A.S., Kosarskii, L.S., Tarlachkov, S.V., Vekhnik, V.A., Averina, I.V., Alekseev, A.E., Fesenko, E.E., and Nakipova, O.V., Biofizika, 2017, vol. 62, pp. 127–133.
Zakharova, N.M., Nakipova, O.V., Averin, A.S., Ti-khonov, K.G., Solomonov, N.G., Dokl. Biol. Sci., 2009, vol. 424, no. 5, pp. 21–24.
Nakipova, O.V., Averin, A.S., Evdokimovskii, E.V., Pimenov, O.Y., Kosarski, L., Ignat’ev, D., Anufriev, A., Kokoz, Y.M., Reyes, S., Terzic, A., and Alekseev, A.E., PLoS One, 2017, vol. 12, no. 5, pp. 1–20.
Kuz'min, V.S., Abramochkin, D.V., Sukhova, G.S., and Rozenshtraukh, L.V., Kardiologiya, 2008, vol. 48, no. 11, pp. 53–60.
Marshall, J.M. and Willis, J.S., J. Physiol., 1962, vol. 164, no. 1, pp. 64–76.
Charnock, J.S., Dryden, W.F., and Marshall, R.J., Br. J. Pharmacol., 1983, vol. 78, no. 1, pp. 151–158.
Coronel, R., Janse, M.J., Opthof, T., Wilde, A.A., and Taggart, P., Heart Rhythm, 2012, vol. 9, no. 6, pp. 977–982.
Kirchhof, P., Degen, H., Franz, M.R., Eckardt, L., Fabritz, L., Milberg, P., Laer, S., Neumann, J., Breithardt, G., and Haverkamp, W., J. Pharmacol. Exp. Ther., 2003, vol. 305, no. 1, pp. 257–263.
van Hunnik, A., Lau, D.H., Zeemering, S., Kuiper, M., Verheule, S., and Schotten, U., Heart Rhythm, 2016, vol. 13, no. 4, pp. 964–972.
Frommeyer, G., Rajamani, S., Grundmann, F., Stypmann, J., Osada, N., Breithardt, G., Belardinelli, L., Eckardt, L., and Milberg, P., J. Card. Fail., 2012, vol. 18, no. 12, pp. 939–949.
Liu, B., Arlock, P., Wohlfart, B., and Johansson, B.W., Cryobiology, 1991, vol. 28, pp. 96–104.
Wang, S.Q., Lakatta, E.G., Cheng, H., and Zhou, Z.Q., J. Exp. Biol., 2002, vol. 205, pp. 2957–2962.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Nikolaeva
Rights and permissions
About this article
Cite this article
Kuzmin, V.S., Abramov, A.A., Egorov, Y.V. et al. Hypothermia-Induced Postrepolarization Refractoriness Is the Reason of the Atrial Myocardium Tolerance to the Bioelectrical Activity Disorders in the Hibernating and Active Ground Squirrel Citellus undulatus. Dokl Biol Sci 486, 63–68 (2019). https://doi.org/10.1134/S0012496619030050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012496619030050