Abstract
This work is dedicated to the mechanism of the antitumor action of dinitrosyl iron complexes, which generate nitric oxide NO and nitrosonium cations, NO+. The effect of diethyldithiocarbamate, used in spin trapping of NO radicals, on the antitumor activity of binuclear dinitrosyl iron complexes with glutathione or N-acetyl cysteine has been studied. The effectiveness of these drugs in vivo against solid tumors in mice persists, as expected, or even increases when they are used in combination with diethyldithiocarbamate. It is assumed that the tumor growth inhibitory effect of dinitrosyl iron complexes is due mainly to the presence of nitrosonium cations rather than the nitric oxide molecules released from dinitrosyl iron complexes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recent studies show that the disturbance of physiological processes regulated by nitric oxide, NO, may provoke various morbid conditions, including malignant tumors [1–3].
It has been shown that different NO concentrations can either stimulate tumor growth or cause cell death; therefore, this radical is often called a two-edged sword. Nitric oxide concentrations lower or higher than those optimal for tumor growth can activate the transduction of signals causing tumor growth inhibition and cell death. High NO concentrations can also modulate the antitumor immune defense [1].
Previously, we found that mono- and binuclear forms of dinitrosyl iron complexes with various thiol-containing ligands generating NO, can inhibit some solid tumors in mice, these are: Lewis lung carcinoma, Acatol adenocarcinoma, and Ca-755 adenocarcinoma. Some dinitrosyl iron complexes are cytotoxic to human tumor cells (MCF-7) [4–11].
Professor Vanin analyzed his own experimental results and a great body of data from the literature and proposed a mechanism for the biological action of dinitrosyl iron complexes (DNICs). According to his model, the complexes act as donors of both neutral NO molecules and nitrosonium cations NO+. It was supposed that the antitumor and cytotoxic effect of DNICs with thiol-containing ligands is based on their ability to release both neutral NO and NO+ [2].
In order to find out what component of these DNICs, nitric oxide molecules or nitrosonium cations, plays the key role in their antitumor action, sodium diethyldithiocarbamate (DETC) was employed in experiments as a trap for NO molecules [12–14].
It was found that DETC interacting with a binuclear dinitrosyl iron complex containing glutathione {B-DNIC-GSH) tears a mononitrosyl-iron group away from the complex and forms a stable EPR-active mononitrosyl iron complex with DETC (MNIC-DETC), which releases almost no neutral NO molecules [13, 14].
The second nitrosyl ligand of the dinitrosyl iron group is released as nitrosonium cation NO+, which binds to various thiols to form corresponding S-nitrosothiols. This process may determine the preservation of the antitumor and cytotoxic effects of B-DNIC with the presence of DETC [2, 12].
It was demonstrated in this way that the cytotoxic action of B-DNIC-GSH or B-DNIC with mercaptosuccinate on MCF-7 tumor cells [13] and of B‑DNIC-GSH on Escherichia coli bacteria is determined by the ability of the complexes to act as donors of nitrosonium cations.
This observation brought us to the assumption that NO+ cations can also play the key role in the implementation of the antitumor action of B-DNIC-GSH in vivo [12].
Here we continue the study of the antitumor action of dinitrosyl iron complexes and investigate the influence of various schedules of DETC application on the antitumor activity of B-DNICs with different ligands: glutathione (B-DNIC-GSH) and N-acetylcysteine (B-DNIC-NAC). We also assess the antitumor effect of DETC itself on model murine solid tumors in vivo.
MATERIALS AND METHODS
Materials. Iron sulfate (FeSO4 · 7H2O) was purchased from Fluka (Switzerland). Reduced glutathione, sodium nitrite, N-acetylcysteine, and sodium diethyldithiocarbamate (C2H5)2=N–CS2 were purchased from Sigma (United States).
Gaseous nitric oxide was obtained by the reaction of iron sulfate with sodium nitrite NaNO2 in 0.1 M HCl and purified from nitrogen dioxide by cryosublimaion in vacuo [15].
Synthesis of the binuclear dinitrosyl iron complex with glutathione. The binuclear dinitrosyl iron complex with glutathione (B-DNIC-GSH) was prepared by the simplest method for synthesizing DNICs with thiol-containing ligands [16]. To obtain a 5-mM solution, 62 mg (20 mM) of glutathione were added to 10 mL of distilled water in the air. The solution became acidic, pH 4.0. Iron sulfate (28 mg, 10 mM) was added, further acidifying the solution to pH 3.8. Sodium nitrite (6.9 mg, 10 mM) was added, and the solution turned pink owing to S-nitrosoglutathione formation. Judging from the light absorbance at 334 nm, characteristic of S-nitrosoglutathione, the reaction was complete after 1.5 h to yield 10 mM concentration of the compound. The solution was deacidified to pH 7.2, and it turned orange, as the formation of B-DNIC-GSH from S-nitrosoglutathione, Fe2+, and glutathione started [16]. The complete conversion of S-nitrosoglutathione to B-DNIC-GSH took several hours. The precipitated iron(III) hydroxide was filtered out, and the solution was frozen in liquid nitrogen to be thawed immediately before experiments with animals. The prepared B-DNIC-GSH (MW 846 Da) was quantified by light absorbance at the characteristic wavelengths 310 and 360 nm with the molar attenuation coefficients 4600 and 3700 M–1 cm–1, respectively (corrected for one iron atom in B-DNIC) [15]. The B‑DNIC-GSH concentration in the solution proved to be 5 mM.
Synthesis of the binuclear dinitrosyl iron complex with N-acetylcysteine. Iron(II) sulfate dissolved in distilled water (1 mL) and N-acetylcysteine in 15 mM HEPES buffer pH 7.4 were loaded into the upper and lower containers of a Thunberg vial, respectively. Nitric oxide was injected at 150 mmHg, and the solutions were mixed. The final iron sulfate concentration was 5 mM. The mixture was shaken for 5 min, so that all iron(II) was incorporated into DNIC-NAC. Nitric oxide was evacuated, and the solution was frozen in liquid nitrogen to be thawed immediately before use in experiments. The prepared B-DNIC-NAC was quantified by light absorbance at the characteristic wavelength 360 nm.
Antitumor activity in vivo. Experiments were conducted with 80 inbred mice BDF1 (first-generation hybrids f1(C57Bl/6 × DBA2)) and Balb/c, weighing 18–20 g. The mice were from the Stolbovaya vivarium, branch of the Blokhin National Medical Research Center for Oncology, Moscow.
Three murine solid tumors were chosen as models: Lewis lung carcinoma, Acatol adenocarcinoma, and Ca-755 adenocarcinoma. The tumors were grafted subcutaneously by the conventional protocol [17].
B-DNIC-GSH was administered in aqueous solution at the daily dose 2 μmol/kg intravenously (i/v), intraperitoneally (i/p), or subcutaneously (s/c) for 5 to 8 days after tumor grafting.
B-DNIC-NAC was administered s/c in aqueous solution at the daily doses 2 and 10 μmol/kg for 9 days after tumor grafting.
Depending on experimental settings, DETC solutions were administered i/p at daily doses of 10 or 50 mg/kg. Injections were done 1 h or 15 min after the injection of B-DNIC-GSH or B-DNIC-NAC or as a single medication for 5–10 days after tumor grafting.
The antitumor efficacy of the treatments was assessed by comparing the tumor growth kinetics in groups of control and treated animals. Tumor growth inhibition expressed as percentage (TGI%) was calculated as TGI% = (PC – PT) × 100%/PC, where PC and PT are the mean tumor weights (or volumes) in the control and treated animal groups, respectively. Tumor volumes were calculated as for an ellipsoid: V = abc/2, where a is the length; b, the width; and c, the height of the tumor node. Tumor tissue density was taken to be 1 g/cm3 [17].
Each experimental group included six to eight animals, and the control, eight to ten. The animals were monitored during the entire time span of tumor development, until animal death.
Statistical evaluation of the results was done by assessing tumor size (weight) in control and treated animals with the Statistica 6.0 software.
RESULTS AND DISCUSSION
We present the results characterizing the antitumor activity of DETC alone and in combination with dinitrosyl iron complexes with various ligands (B-DNIC-GSH and B-DNIC-NAC) in murine solid tumors as models.
The antitumor activity of diethyldithiocarbamate applied as a single agent was studied on three murine solid tumors: Lewis lung carcinoma, Acatol adenocarcinoma, and Ca-755 adenocarcinoma. Various treatment schedules were tested.
As shown in Table 1, DETC is active against Lewis lung carcinoma. It inhibited tumor growth by 61–76% compared to control. Apparently, the efficacy of DETC administered at the daily dose 50 mg/kg depends on the treatment schedule. The greatest TGI (76%) was observed when the treatment followed an intermittent regimen: five injections on days 1, 4, 7, 10, and 13. Five injections on days 1–5 or eight injections on days 1–4 and 7–10 were less efficient. They inhibited tumor growth by 62 and 61%, respectively (Table 1).
Acatol and Ca-755 adenocarcinomas were not very sensitive to DETC. Their inhibition by the medication administered i/p in five doses of 50 mg/kg and in nine doses of 10 mg/kg, was 24 and 20%, respectively (Table 1).
Thus, our experiments showed the ability of DETC to inhibit Lewis lung carcinoma growth in vivo.
The antitumor activity of the combination of B‑DNIC-GSH and diethyldithiocarbamate on the Lewis lung carcinoma model. The effect of DETC on the antitumor action of B-DNIC-GSH was studied in various treatment schedules with constant daily doses of the medications: 50 and 2 μmol/kg, respectively. We studied the influence of the interval between the injections (1 h or 15 min) and the administration route (i/v, i/p, or s/c) on the effect of DETC, which was always injected i/p after the injection of B-DNIC-GSH.
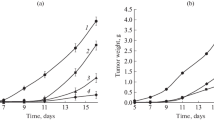
Five-time administration of B-DNIC-GSH(i/v) and DETC (i/p) one hour apart. The efficacy of the combined and monotherapeutic application of the medications with five-time injection of B-DNIC-GSH is illustrated in Fig. 1 and Table 2.
The effect of combined five-time administration of B-DNIC-GSH (i/v) and DETC (i/p) 1 h apart on the growth of Lewis lung carcinoma: (1) control; (2) B‑DNIC-GSH, 2 μmol/kg, i/v; (3) DETC, 50 mg/kg (250 μmol/kg, i/p; (4) B-DNIC-GSH, 2 μmol/kg, i/v + DETC, 50 mg/kg, i/p. Medications were administered on days 1, 4, 7, 10, and 13 after tumor grafting.
The i/v administration of B-DNIC-GSH at the daily dose 2 μmol/kg inhibited tumor growth by 48%, and the five-time i/p administration of DETC, by 76% compared to control. The combined administration of B-DNIC-GSH and DETC (the latter one hour after the former) caused almost 57% tumor growth inhibition compared to control (Fig. 1, Table 2).
Thus, combined treatment with DETC and B‑DNIC-GSH (i/v) 1 h apart only insignificantly increased the antitumor action of B-DNIC-GSH: from 50 to 60%. The use of DETC alone was more efficient than other combinations, 76%. In all cases, tumor growth inhibition was observed within one week after the end of the treatment (day 13 after tumor grafting), and then the tumor began to grow.
Eight-time administration of B-DNIC-GSH (i/p) and DETC (i/p) one hour apart. The efficacy of the combined and monotherapeutic application of the medications with the eight-time (days 1–4 and 7–10 after tumor grafting) regimen is demonstrated in Fig. 2 and Table 3.
The effect of combined eight-time administration of B-DNIC-GSH and DETC i/p 1 h apart on the growth of Lewis lung carcinoma: (1) control; (2) B-DNIC-GSH, 2 μmol/kg, i/p; (3) DETC, 50 mg/kg, i/p; (4) B-DNIC-GSH, 2 μmol/kg, i/p + DETC, 50 mg/kg, i/p. Medications were administered on days 1–4 and 7–10 after tumor grafting.
We see that eight B-DNIC-GSH injections, both alone and in combination with DETC inhibited tumor growth by 52%. DETC alone inhibited tumor growth by 62% compared to control (Fig. 2, Table 3).
Thus, the experiments with i/p B-DNIC-GSH administration, as with the i/v route, show that the addition of DETC does not affect the antitumor activity of B-DNIC-GSH compared to its monotherapeutic use (Tables 2 and 3).
Five-time administration of B-DNIC-GSH (s/c) and DETC (i/p) 15 min apart. The results of the antitumor action of the five-time combined and monotherapeutic application of the medications with s/c injection of B-DNIC-GSH and i/p injection of DETC 15 min thereafter are demonstrated in Fig. 3 and Table 4.
The effect of combined five-time administration of B-DNIC-GSH (s/c) and DETC (i/p) 15 min apart on the growth of Lewis lung carcinoma: (1) control; (2) B‑DNIC-GSH, 2 μmol/kg, s/c; (3) DETC, 50 mg/kg, i/p; (4) B-DNIC-GSH, 2 μmol/kg, s/c + DETC, 50 mg/kg, i/p. Medications were administered on days 1–5 after tumor grafting.
As expected, s/c injection of B-DNIC-GSH exerted weaker antitumor action than the i/v or i/p routes: TGI% was 34, 48, and 52, respectively, compared to control (Tables 2–4, Figs. 1–3).
DETC injections 15 min after B-DNIC-GSH increased the tumor-inhibiting capacity of the dinitrosyl complex in s/c administration from 34 to 51% (Fig. 3, Table 4).
We see that in experiments with s/c injection of B-DNIC-GSH, as with the i/v or i/p routes, the combination with DETC, trapping NO molecules, shows the preservation and even a slight increase (in the i/v and s/c administration routes of B-DNIC-GSH) of the antitumor activity compared to B-DNIC-GSH alone. The most pronounced effect of DETC on the B-DNIC-GSH activity was observed with the 15-min interval (compared to injections 1 h apart).
This observation may be interpreted as support for the hypothesis that the tumor growth inhibition by B‑DNIC-GSH is determined by the formation of nitrosonium cations, NO+, rather than nitric oxide molecules, NO.
The hypothesis is indirectly supported by experiments on the effect of DETC on the activity of the gold-based medication aurumacryl against Acatol adenocarcinoma, whose mechanism is not associated with the generation of nitric oxide nor nitrosonium cations. It was shown there that the combined application of their half-doses dramatically reduced aurumacryl activity compared to the full dose of the latter applied alone (unpublished results).
The antitumor activity of the combination of B-DNIC-NAC and DETC on Ca-755 adenocarcinoma. In order to investigate the effect of the ligand nature on the sensitivity of a dinitrosyl complex to DETC action, we studied the combination of DETC and the dinitrosyl iron complex with N-acetylcysteine on the Ca-755 adenocarcinoma model.
Previously, we found that B-DNIC-NAC applied i/v or i/p ten times at 10 μmol/kg daily doses inhibited Acatol adenocarcinoma growth by 60% compared to control [11].
The experiments reported here followed another schedule of treatment with B-DNIC-NAC than that used in [11]. The medication dose was 2 μmol/kg s/c instead of the previously applied 10 μmol/kg i/v or i/p. DETC was administered at the dose 10 μmol/kg i/p, that is five times lower than in [11] (50 μmol/kg).
The results are presented in Fig. 4 and Table 5. They indicate that, as expected, the monotherapeutic use of fivefold lower doses of the medications resulted in almost complete disappearance of the antitumor effect observed with the previously used doses. As already mentioned, B-DNIC-NAC applied at the dose 10 μmol/kg inhibited tumor growth by 60% compared to control but exerted no action at 2 μmol/kg. The effect of DETC, which inhibited Lewis carcinoma growth by 61–76% compared to control at 50 mg/kg i/p, fell to 20% at 10 mg/kg i/p.
The effect of combined administration of B‑DNIC-NAC (s/c) and DETC (i/p) 1 h apart on the growth of Ca-755 adenocarcinoma: (1) control; (2) B‑DNIC-NAC, 2 μmol/kg, s/c; (3) DETC, 10 mg/kg (50 μmol/kg), i/p; (4) B-DNIC-NAC, 2 μmol/kg, s/c + DETC, 10 mg/kg, i/p. Medications were administered on days 1–9 after tumor grafting.
Nevertheless, the combined application of the medications at small doses definitely inefficient in monotherapy, significantly inhibited Ca-755 adenocarcinoma growth by 43% compared to control (Fig. 4, Table 5).
It is reasonable to assume that the observed tumor growth inhibition in combined therapy even at very low doses of B-DNIC-NAC and DETC is determined by the presence of nitrosonium cations, NO+, whereas the NO produced by B-DNIC-NAC is inactivated by DETC.
To sum up, the study of the effect of DETC on the antitumor activity of B-DNICs with various ligands (GSH or NAC) showed that their efficacy with the presence of DETC remains at the same level as in monotherapy or even increases. Obviously, the effect of the DETC + DNIC interaction depends significantly on the nature of the ligand in the DNIC, administration schedule, and the nature of the tumor.
Our data favor the earlier assumption that the effect is determined by the ability of DETC to degrade DNICs in tumors so that they release NO+ cations. This process results in the preservation or even enhancement of the antitumor action of the medications in combined use.
It is conjecturable that the reversal of the order of B-DNIC and DETC administration (that is, DETC + B-DNIC instead of B-DNIC + DETC) may augment their antitumor effect. This conjecture stems from the results reported in [2], concerning the formation of MNIC-DETC from DNIC in animal tissues in vivo depending on the order of DNIC and DETC administration. It follows therefrom that the efficacy of NO+ release from DNIC in animal tissues should increase in DNIC administration after DETC. We plan to test this soon.
REFERENCES
D. B. Korman, L. A. Ostrovskaya, and A. F. Vanin, Biofizika 66, 259 (2021). https://doi.org/10.31857/S000630292102006X
A. F. Vanin, Biokhimiya 87, 1739 (2022).
A. F. Vanin, Dinitrosyl Iron Complexes as a “Working Form” of Nitric Oxide in Living Organisms (Cambridge Scholars Publishing, Cambridge, 2019).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 59, 508 (2014).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 60, 152 (2015).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 60, 1157 (2015).
A. F. Vanin, L. A. Ostrovskaya, and D. B. Korman, Austin J. Reprod. Med. Infertil. 2, 1109 (2015).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 62, 591 (2017).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 64 (6), 1216 (2019).
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 65 (5), 1009 (2020). https://doi.org/10.31857/S0006302920050191
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 66 (6), 1223 (2021). https://doi.org/10.31857/S0006302921060193
A. F. Vanin, L. A. Ostrovskaya, D. B. Korman, et al., Biofizika 66 (6), 1217 (2021). https://doi.org/10.31857/S0006302921060181
A. F. Vanin, V. A. Tronov, and R. R. Borodulin, Cell Biochem. Biophys. 79 (1), 93 (2021).
A. F. Vanin, D. I. Telegina, V. D. Mikoyan, et al., Biofizika 67, 942 (2022).
A. F. Vanin, A. P. Poltorakov, V. D. Mikoyan, et al., Nitric Oxide Biol. Chem. 23, 136 (2010).
R. R. Borodulin, L. N. Kubrina, V. O. Shvydkiy, et al., Nitric Oxide Biol. Chem. 35, 110 (2013).
E. M. Treshchalina, O. S. Zhukova, G. K. Gerasimova, et al., A guide to Preclinical Drug Research, Ed. by A. N. Mironova, et al. (Grif i K, Moscow, 2012), Part 1, pp. 642–657.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by V. Gulevich
Abbreviations: DNIC, dinitrosyl iron complex; DETC, sodium diethyldithiocarbamate; B-DNIC-GSH, binuclear dinitrosyl iron complex with glutathione; MNIC-DETC, mononitrosyl iron complex with diethyldithiocarbamate; B-DNIC-NAC, binuclear dinitrosyl iron complex with N-acetylcysteine; i/v, intravenous(ly); i/p, intraperitoneal(ly); s/c, subcutaneous(ly).
Rights and permissions
About this article
Cite this article
Vanin, A.F., Ostrovskaya, L.A., Korman, D.B. et al. Role of the Nitrosonium Cation in the Mechanism Underlying the Antitumor Effects of Drugs in Combination with Dinitrosyl Iron Complexes. BIOPHYSICS 67, 796–801 (2022). https://doi.org/10.1134/S0006350922050219
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350922050219