Abstract
Biological effects produced by low-energy electromagnetic radiation were often explained by transcriptional induction of the Hsp70 gene. In this study, we investigated a series of important adaptation traits developed in Drosophila melanogaster strains with different copy numbers of Hsp70 genes when subjected to microwave irradiation. In our experiments, we used mutant strains with gene deletion in all or several Hsp70 copies. The wild-type strain (Canton-S containing the full set of Hsp70 genes in its genome) was used as a control. Electromagnetic radiation (power density10 μW/cm2, frequency 37.7 GHz and 65.0 GHz, exposure duration 5 min) was used for the irradiation of adult flies (imago). The experimental results showed that exposure to microwave radiation produced no effect on the number of the wild-type offspring (Canton-S with the full set of Hsp70 genes) by the pupal stage and imago but was accompanied by increased embryonic mortality and an increased median lifespan. In most cases, exposure to microwave radiation led to adverse effects on the viability of strains without all copies or with the presence of one copy of Hsp70 genes. In these strains, the external influence resulted in a lower number of offspring by the imago, an increased number of dead individuals during the pupal and early stages of imago development, and a decrease in the median and maximum lifespan of the imago. Interestingly, when the strain containing four copies of Hsp70 was exposed to microwave radiation it was found that individuals tend to show sexual dimorphism in response to such an external influence: a decrease in the median and maximum lifespan of the female imago and an increase of the lifespan of the male flies. The results of this study demonstrate the importance of the presence of the full set of Hsp70 genes in the Drosophila genome to adapt to microwave radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Electromagnetic radiation (EMR) as a new anthropogenic environmental factor can cause various genetic effects, thereby modifying the adaptive abilities of organisms. The Hsp70 heat shock proteins play an important role in the development of resistance to unfavorable environmental factors. Their role in the formation of fitness under exposure to low-intensity EMR remains obscure.
The series of works by M. Blank, R. Goodman, et al. in experiments on human cell culture showed the induction of Hsp70 transcription in response to exposure to low-intensity EMR (8 μT, 60 Hz) was shown [1]. In addition, this treatment led to transient activation of genes that encode the heat shock transcription factor 1 (HSF-1) [2]. In human cell cultures exposed to EMR for 3 h (8 μT, 60 Hz), Hsp70 levels increased after 20 min of exposure and gradually decreased, returning to control values 2 h later [3]. Exposure to EMR (8 μT, 60 Hz, 20 min exposure) induced the activation and DNA binding of Hsp70 transcription factors such as HIF, AP-1, and SP-1 [4]. These results allowed the authors to propose the concept of a domain that is specifically sensitive to the electromagnetic field, which is located in the Hsp70 promoter [5, 6].
Many contradictory results were obtained in studies of the regulation of Hsp70 activity by electromagnetic irradiation of varying intensity on model objects. As an example, it was shown that the response of a cell culture to magnetic field and microwaves is determined by Hsp70 gene activation [7–9]. However, no change in Hsp70 gene transcription in cells exposed to electromagnetic fields has been detected. It was shown in [10] that although EMR does not affect Hsp70 transcription it can significantly increase the constitutive level of the Hsp70 protein, probably by increasing its stability. On the other hand, a sinusoidal magnetic field with a frequency of 50 Hz increases the level of Hsp70 mRNA in porcine aortic endothelial cells but does not affect the Hsp70 protein content [11].
The effect of EMR on Hsp70 expression described above was studied primarily on various human and animal cell lines, while only very few experiments have been performed on model objects. In experiments on Drosophila, Hsp70 expression levels under exposure to EMF were assessed. The incorporation of labeled uridine, which was assessed autoradiographically, showed EMT-activated transcription at 87 loci containing six Hsp70 genes [12]. Cell phone radiation also caused a 3.6-fold increase in the Hsp70 protein level in Drosophila larvae exposed for 60 min twice a day for 10 days [13].
New opportunities in studying the mechanisms of action of EMR in experiments on Drosophila were opened up after Gong and Golic [14] obtained strains with deleted Hsp70 genes (Hsp70-null). It was found that Hsp70 genes are essential for survival under fairly severe heat shock but are not necessary under milder heat exposure. This fact indicated that a significant degree of heat resistance is retained at a reduced number of Hsp70 copies. In addition, several pleiotropic effects induced by Hsp70 deletion were demonstrated [15]. Heat shock exposure in experiments on Drosophila melanogaster strain Hsp70-null showed the activation of the synthesis of several inducible and constitutive Hsp. Heat shock caused both the expression of new gene patterns and an almost complete mortality of individuals of the Hsp70-null strain [16].
The aim of this study was to investigate some components of the fitness of D. melanogaster strains containing different numbers of Hsp70 genes under microwave irradiation.
MATERIALS AND METHODS
This study was performed on Drosophila melanogaster mutant strains containing different copy numbers of Hsp70 genes: 8841 (Hsp70-null), 8842, and 1♀41♂. Flies of strain 8841 lack both loci containing Hsp70 copies (87A and 87B). In flies of strain 8842 the 87A locus is removed, while the 87B locus containing four Hsp70 copies is present [14]. The 1♀41♂ transgenic strain, which was derived from the 8841 strain by P-mediated transformation in the laboratory by M. Evgen’ev [17], contains one Hsp70 copy. The wild-type Canton-S strain was used as a control because it was shown earlier [18] that the genome of the wild-type flies contained six almost identical copies of Hsp70 genes.
The flies were grown on a standard sugar-yeast medium at 23.0 ± 0.5°С. In the experiments, 2-day-old virgin imagoes were subjected to external influences. On the third day, the flies were crossed and the indices that characterize fecundity (embryonic mortality and the number of offspring at the pupal and imago stages) and the lifespan of the parental individuals were assessed.
The characteristics of the external influence. Two-day-old imagoes were irradiated in test tubes filled with a temporary medium through cotton wool. Irradiation was performed using measuring instruments included in the Secondary Standard for the Power Units of Electromagnetic Oscillations in waveguides in the frequency range of 37.50–78.33 GHz. This standard is annually subjected to metrological certification at the National Standard for the Power Units of Electromagnetic Oscillations in Waveguides (by calibrating the standards of the absorbed power unit carriers) at the National Research Center Institute of Metrology (Kharkov, Ukraine) [19].
The capacities of the standard allow working with EMP powers ranging from 1 × 10–3 to 1 × 10–2 W at a total relative error of 0.2 × 10–2–0.5 × 10–2. Rectangular waveguides of 5.2 × 2.6 mm and 3.6 × 1.8 mm were used to generate radiation at frequencies up to 37.5 GHz and up to 78.33 GHz, respectively. The open end of the waveguide was used as an emitter; the test tube with Drosophila melanogaster imago was placed (put on the waveguide) in such a way that the far zone condition was met.
The radiation power at the waveguide exit was measured with an M1-25-type feed-through power meter. To compensate for the influence of EMP attenuation in cotton wool at the irradiation frequency, the absorption coefficient of cotton wool (3 × 2 cm) was determined immediately before the experiment using an M3-22A-type power meter and a thermistor head, which was 1.25 at a frequency of 65.0 GHz and 1.12 at a frequency of 37.5 GHz. The power fed to the open end of the waveguide was set for an energy flux with a density of W = 10 μW/cm2 (0.1 W/m2), taking this coefficient into account using the expression
where D is the EMF energy flux density, W/m2; Ps is the radiation power from the source, W; Cd is the coefficient of directional action of the emitter; and r is the distance to the radiation source, m.
The final parameters of the microwave effect on imagoes were as follows: a power flux density W = 10 μW/cm2, frequency F = 37.7 and 65.0 GHz, and an exposure duration of t = 5 min. During irradiation, each tube contained 15–20 imagoes. Depending on the type of experiment, it was necessary to obtain 70 to 200 irradiated flies of each sex of each strain.
Incorporation of 35S-labeled methionine into D. melanogaster salivary gland proteins. Third-instar larvae were subjected to heat shock (37.5°С) for 30 min. Their salivary glands were then isolated and incubated at 25°С for 40 min in 20 μL of methionine-free Schneider’s insect medium (Sigma, United States) supplemented with 1 μL (1 μCi) of 35S-labeled methionine (GE Healthcare, Great Britain). The salivary glands of the larvae stored at 25°С were used as a control. The labeled salivary glands were lysed in 20 μL of Laemmli buffer. Protein extracts were separated by SDS-PAGE in 10% polyacrylamide gel. Equal amounts of each sample were used. The incorporation of the radioactive label was assessed autoradiographically.
Consideration of indices that characterize the reproductive value of imago. The average number of offspring at the imago stage from two parental pairs was determined. To do this, two pairs of 3-day-old virgin imagoes were placed in test tubes filled with 5 mL of nutrient medium for 5 days and all offspring were counted. In each experimental variant, offspring from 24–30 pairs of individuals were assessed. Simultaneously, the number of individuals that died at the pupal stage was counted.
To determine the stage of embryonic death, undeveloped Drosophila eggs were analyzed as described in [20]. We distinguished the initial stages of cleavage and formation of blastoderm (the death of individuals occurred before 5.5 h of embryonic development (I)); the stages of gastrulation and segmentation (the death of embryos occurred 5.5 to 17 h of development (II)); and the stage of organogenesis (death occurred 17 to 22 h of embryonic development (III)). In total, egg clutches of approximately 1200 Drosophila females were analyzed in the experiments.
For each experimental variant, when analyzing the number of offspring at the imago stage, we calculated the arithmetic mean value and the standard error of the mean. The results of the analysis of the indices of mortality of individuals at the pupal stage and the embryonic mortality are presented as proportions (%) with a 95% confidence interval. The confidence interval was calculated according to Jeffreys [21]. The statistical significance of the differences between the control and experimental values was estimated using the Fisher’s test. The null hypotheses were tested at a significance level of 0.05.
Analysis of the lifespan of the imagos. The average and median lifespans of Drosophila imagos were calculated. Virgin individuals (females and males separately) were kept on a temporary medium in glass test tubes (25 individuals in each). In each variant of the experiment the lifespan of 100–120 imagoes of each sex was analyzed. The dead individuals were counted and flies were transferred to a fresh medium every 2 days. The differences in the median lifespan were compared using the Wilcoxon–Breslow–Gehan test. The significance of differences in the maximum lifespan (the last 10% of deaths from the sample) was assessed using the Wang–Ellison test with the Bonferroni correction [22].
RESULTS AND DISCUSSION
To determine the number of gene copies of heat shock proteins in strains 8841 and 1♀41♂, we analyzed the incorporation of 35S-labeled methionine into the salivary glands of Drosophila larvae of three strains with different Hsp70 copy numbers. The results corroborated the presence of one copy (strain 1♀41♂) and the absence of Hsp70 genes in the genome of individuals of the mutant strain 8841 (Fig. 1).
Incorporation of methionine S35 into the salivary glands of Drosophila larvae under normal conditions and after heat shock (37°С, 30 min): (1) control (strain w1188 containing all six Hsp70 copies), development at normal temperature; (2) strain w1188 after heat shock; (3) strain 1♀41♂ containing one Hsp70 copy after heat shock; (4) strain 8841 with deletion of all Hsp70 copies after heat shock. The positions of Hsp70 and Hsp68 are indicated with arrows.
The results of the viability studies showed that in the control the greatest number of offspring at the imago stage is characteristic of the wild-type strain Canton-S and the mutant strain 8842, which contains four Hsp70 copies (Figs. 2, 3). For the strains with the deletion of all Hsp70 copies (8841) and with only one copy of this gene (1♀41♂), the fecundity decreased by almost two times. The exposure had no significant effect on the number of offspring at the imago stage in the wild-type strain but increased the fecundity in the strains with different Hsp70 copy numbers. As an example, irradiation at a power of 37.7 GHz increased the fecundity of strain 8842 by 15.84% relative to the control (Figs. 4a, 5a), and irradiation at a power of 65 GHz increased the fecundity in strains 8841 and 1♀41♂ by 48.19 and 29.59%, respectively (Figs. 4b, 5b).
The observed effect of microwave irradiation was mediated by changes in the number of offspring at the pupal stage. As an example, the exposure of parental individuals of the strains with the deletion of all Hsp70 copies (8841) and with one copy of this gene (1♀41♂) at a frequency of 65 GHz increased the number of pupae by 1.7 and 1.3 times, respectively (Fig. 2). However, the number of individuals that died at the pupal stage in the same strains increased, on average, by 1.5 times compared to the control (Table 1).
The results obtained in this study that show an increase in the fecundity of Drosophila imago under the influence of microwave irradiation are in good agreement with our previous results [23]. However, it should be noted that the literature data are fairly contradictory: there is information about both an increase in the effect [13] and its absence [24], as well as a decrease in fecundity [25–27]. The differences in the obtained results are, apparently, associated with the differences in the experimental procedures, the parameters (frequency, power, and duration of exposure) of microwave radiation, the stage of ontogenesis at which exposure was performed, and the characteristics of Drosophila strains.
Analysis of the stages of embryonic death showed that the number of dead embryos of the wild-type strain was at a maximum at the initial stages of blastoderm fragmentation (the first 5.5 h of development) and the stage of organogenesis (the last 17–22 h of development) (Table 2). A decrease in the number of Hsp70 copies in the genome of the mutant strains 8841, 8842, and 1♀41♂ was accompanied by a decrease in the viability of embryos at the stage of gastrulation and the initial stages of segmentation (5.5–17 h of development).
Microwave irradiation adversely affects the processes of early ontogenesis in Drosophila, increasing embryonic mortality primarily at the stages of segmentation and organogenesis. In the wild-type strain, the number of embryos that died at the stage of gastrulation and the initial stages of segmentation increased by 2.29 times (p < 0.05). Similar changes were observed in strain 8841 (p < 0.05). In individuals of strain 1♀41♂, an increase in the mortality of embryos at the initial stages of cleavage was detected (p <0.05).
Thus, exposure to microwave radiation increased the number of dead embryos in strains 8841 and 1♀41♂ mainly due to the death of individuals at the later stages of embryonic development. Obviously, this is one of the factors that lead to a decrease in the number of offspring in individuals of these strains after irradiation.
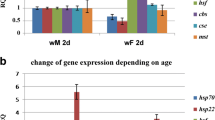
In the wild-type Canton-S strain, the exposure to microwave radiation increased the median lifespan of imagoes of both sexes and increased the lifespan of the longest-lived individuals (Fig. 3, Table 3). In flies of all mutant strains that differed in the Hsp70 copy number irradiation decreased the lifespan. However, the features of this process are determined by the specific set of heat shock genes in the Drosophila genome. As an example, in the Hsp70-null strain (8841) irradiation led to a decrease in both the median and maximum lifespan of imagoes of both sexes in all variants of the experiments (p ≤ 0.05).
In the strain with one Hsp70 copy (1♀41♂), a decrease in the average lifespan was shown only for males. The long-lived part of the sample (both females and males) was found to be sensitive to microwave exposure and the maximum lifespan decreased in all experimental variants (p ≤ 0.05).
If microwaves are considered as a stressor the decrease in the median lifespan of females and males of strain 8841 in our experiments can be explained by a reduced stress resistance. Obviously, one Hsp70 copy in males of strain 1♀41♂ was not insufficient to neutralize the effects of extremely high frequencies. Females of this strain are more resistant to stress and the median lifespan values in the experiment correspond to the control (Fig. 3).
Strain 8842, which contains four Hsp70 copies, shows sexual dimorphism in response to microwave exposure. In females, both the median and maximum lifespan decreased after irradiation (p ≤ 0.05). The opposite effect was observed in males: after irradiation at a frequency of 37.7 GHz a very significant increase in both the median and maximum lifespan was detected (p ≤ 0.05) (Fig. 3, Table 3).
The synthesis of heat shock proteins in Drosophila is not only a universal response to stress but also a parameter that characterize the age-related changes in metabolism [28]. As an example, the dose-dependent effect of Hsp70 on the viability of Drosophila during aging at normal temperature was shown [29]. The authors of that study used a transgenic strain with twelve additional copies of the heat shock protein genes and a strain with a reduced Hsp copy number that carried only a residual P-element construct at the same integration site where additional Hsp70s were inserted. Experiments with these strains showed that an increase in the copy number of the Hsp70 genes improved survival after heat shock. On the other hand, flies of the mutant strain devoid of Hsp70 copies showed a shorter lifespan compared to the original strain and the strain containing additional gene copies. This difference became more pronounced at elevated temperatures (29°С) [30]. Thus, the results obtained in this work that demonstrate a decrease in the lifespan of the Drosophila Hsh70-null strain (8841) are in good agreement with the results of our previous studies.
In this study, we investigated the delayed effects of low-intensity electromagnetic radiation, which were assessed by the viability of Drosophila imagoes. We used strains that contain different copy numbers of heat shock genes. It was shown that the exposure to EMR of extremely high frequencies enhanced the mutational process, leading to an increase in the number of embryos that died primarily at the stages of segmentation and organogenesis. This pattern does not depend on the number of Hsp70 genes in the Drosophila genome. However, an increase in the number of offspring at the imago stage in the mutant strains was also observed. Apparently, external influence stimulates oviposition in the strains with the reduced number of heat shock genes, which may be mediated by changes in the hormonal balance of imago, in particular, a decrease in the ecdysone titer in the hemolymph [31].
If we compare the effects of irradiation of imagoes with EMR of extremely high frequencies with different wavelengths, the same tendency of changes in viability indices was observed. Differences were found only when the median lifespan was analyzed. As an example, in strain 8841, this index decreased in both experimental variants, while the effect was more pronounced under the exposure to microwave irradiation at a wavelength of 37.7 GHz. In strain 1♀41♂ with one Hsp70 copy, the decrease in the median lifespan in the experiment was more pronounced at 65 GHz. The opposite effect was also shown for strain 8842: an increase in the median lifespan in males under irradiation at 37.7 GHz (similar results were obtained for the wild-type strain) and a decrease in this index in females under irradiation at 65 GHz.
Summarizing the results obtained in this study, it can be seen that in the wild-type Canton-S strain with a normal set of heat shock genes, reproductive indices almost do not change under the influence of microwave irradiation; however, the median lifespan increases. Strains without Hsp genes (8841) or that contain one copy of heat shock genes (1♀41♂) were susceptible to microwave irradiation. The delayed changes in the viability indices were mostly negative. As an example, the exposure led to a decrease in the number of offspring at the imago stage, an increase in the number of dead individuals at the pupal and early ontogeny stages (mainly at the late stages of embryonic development), and a decrease in the median and maximum lifespan. Interesting opposite results were obtained for strain 8842. Individuals of this strain, which has four Hsp70 copies, showed sexual dimorphism in response to microwave irradiation: a decrease in the median and maximum lifespan of females and an increase in these parameters in males. Undoubtedly, experimental manipulations with the ancient and highly balanced system of Hsp70 genes [30] led to disturbances in the development of normal stress response of the organism and to the observed changes in viability of imagoes after irradiation. The planned proteomic and transcriptomic methods will provide insight into the mechanisms that underlie the role of Hsp70 in the response to microwave irradiation.
REFERENCES
R. Goodman, M. Blank, H. Lin, et al., Bioelectrochem. Bioenerg. 33 (2), 115 (1994). https://doi.org/10.1016/0302-4598(95)05040-X
H. Lin, M. Opler, M. Head, et al., J. Cell. Biochem. 66 (4), 482, (1997). https://doi.org/10.1002/(sici)1097-4644(19970915)66:4<482::aid-jcb7>3.0.co;2-h
L. Han, H. Lin, M. Head, et al., J. Cell. Biochem. 71 (4), 577 (1998). https://doi.org/10.1002/(sici)1097-4644(19981215)71:4<577::aid-jcb12>3.0.co;2-v
H. Lin, L. Han, M. Blank, et al., J. Cell. Biochem. 70 (3), 297 (1998). https://doi.org/10.1002/(SICI)1097-4644(19980901)70:3<297::AID-JCB2>3.0.CO;2-I
H. Lin, M. Blank, and R. Goodman, J. Cell. Biochem. 75 (1), 170 (1999). https://doi.org/10.1002/(SICI)1097-4644(19991001)75:1<170::AID-JCB17>3.0.CO;2-5
M. Blank and R. Goodman, Pathophysiology 16 (2–3), 71 (2009). https://doi.org/10.1016/j.pathophys.2009.01.006
A. O. Rodriguez de la Fuente, J. M. Alcocer-Gonzalez, A. J. Heredia-Rojas, et al., Cell Biol. Int. 33 (3), 419 (2009). https://doi.org/10.1016/j.cellbi.2008.09.014
A. Garip and Z. Akan, Acta Biol. Hung. 61 (2), 158 (2010). https://doi.org/10.1556/ABiol.61.2010.2.4
A. C. Mannerling, M. Simko, K. H. Mild, and M. O. Mattsson, Radiat. Environ. Biophys. 49 (4), 731 (2010). https://doi.org/10.1007/s00411-010-0306-0
R. Alfieri, M. Bonelli, G. Pedrazzi, et al., Radiat. Res. 165 (1), 95 (2006). https://doi.org/10.1667/rr3487.1
C. Bernardini, A. Zannoni, M. E. Turba, et al., Bioelectromagnetics 28 (3), 231 (2007). https://doi.org/10.1002/bem.20299
R. Goodman, D. Weisbrot, A. Uluc, and A. Henderson, Bioelectromagnetics 13 (2), 111 (1992). https://doi.org/10.1002/bem.2250130205
D. Weisbrot, H. Lin, L. Ye, et al., J. Cell. Biochem. 89 (1), 48 (2003). https://doi.org/10.1002/jcb.10480
W. J. Gong and K. G. Golic, Genetics 168 (3), 1467 (2004). https://doi.org/10.1534/genetics.104.030874
W. J. Gong and K. G. Golic, Genetics 172 (1), 275 (2006). https://doi.org/10.1534/genetics.105.048793
B. R. Bettencourt, C. C. Hogan, M. Nimali, and B. W. Drohan, BMC Biol. 6 (1), 5 (2008). https://doi.org/10.1186/1741-7007-6-5
V. Shilova, O. G. Zatsepina, D. G. Garbuz, et al., Insect Mol. Biol. 27 (1), 61 (2018). https://doi.org/10.1111/imb.12339
M. E. Feder and R. A. Krebs, Am. Zool. 38, 503 (1998). https://doi.org/10.1093/icb/38.3.503
. P. Serednii, V. I. Ogar, T. M. Golyakova, et al., Ukr. Metrol. Zh., No. 4, 50 (2009).
D. L. Hill, Drosophila Inform. Service 19, 62 (1945).
L. D. Brown, T. T. Cai, and A. DasGupta, Stat. Sci. 16 (2), 101 (2001).
S. K. Han, D. Lee, H. Lee, et al., Oncotarget 7, 56147 (2016). https://doi.org/10.18632/oncotarget.11269
O. V. Gorenskaya, D. V. Rybak, N. V. Rybak, et al., Visn. Kharkiv. Nats. Univ. im. V. N. Karazina, Ser. Bi-ol. 32, 52 (2020). https://doi.org/10.26565/2075-5457-2020-34-6
T. L. Poy, E. C. Beyer, and C. F. Reichelderfer, J. Microwave Power 7 (2), 75 (1972). https://doi.org/10.1080/00222739.1972.11688836
E. Atli and H. Unlu, Turk. J. Biol. 31 (1), 1 (2007). https://doi.org/10.1080/09553000600798849
L. H. Margaritis, A. K. Manta, K. D. Kokkaliaris, et al., Electromagn. Biol. Med. 33 (3), 165 (2014). https://doi.org/10.3109/15368378.2013.800102
N. E. Sagioglou, A. K. Manta, I. K. Giannarakis, et al., Electromagn. Biol. Med. 35 (1), 40 (2016). https://doi.org/10.3109/15368378.2014.971959
R. I. Morimoto, Cold Spring Harb. Symp. 76, 91 (2011). https://doi.org/10.1101/sqb.2012.76.010637
M. Tatar, A. A. Khazaeli, and J. W. Curtsinger, Nature 390 (6655), 30 (1997). https://doi.org/10.1038/36237
M. B. Evgen’ev, D. G. Garbuz, and O. G. Zatsepina, in Heat Shock Proteins and Whole Body Adaptation to Extreme Environments (Springer, Dordrecht, 2014), pp. 59–115. https://doi.org/10.1007/978-94-017-9235-6
M. R. Meiselman, T. G. Kingan, and M. E. Adams, BMC Biol. 16, 18 (2018). https://doi.org/10.1186/s12915-018-0484-9
Funding
This work was supported by the Ministry of Education and Science of Ukraine (project no. 0119U002549, O. Gorenskaya) and the Russian Science Foundation (project no. 17-74-30030, M. Evgen’ev).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This work does not contain a description of research using humans and animals as objects.
Additional information
Translated by M. Batrukova
Abbreviations: EMR, electromagnetic radiation.
Rights and permissions
About this article
Cite this article
Gorenskaya, O.V., Gavrilov, A.B., Zatsepina, O.G. et al. The Role of Hsp70 Genes in Promoting Control of Viability in Drosophila melanogaster Subjected to Microwave Irradiation. BIOPHYSICS 66, 541–549 (2021). https://doi.org/10.1134/S0006350921040059
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350921040059