Abstract
Mouse monoclonal antibodies to Hsp90β (β isoform of heat shock protein 90) have been shown to bind specifically to Hsp90β localized on the surface of tumor and nontransformed cells. After binding to the membrane-associated Hsp90β, the antibodies actively dissociated into the culture medium and were also internalized by the cells. An immunoconjugate based on the Hsp90β-specific antibody and the cytotoxic agent mertansine did not have high cytotoxic activity for tumor cells in vitro. Administration of Hsp90β-specific antibodies in mice did not affect the growth of the primary Lewis lung carcinoma, while tumor metastasis to the lungs decreased and the average lifespan of mice increased. The results indicate a certain therapeutic potential of antibodies to Hsp90β for the treatment of tumor diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Heat shock protein 90 (Hsp90) is a chaperone protein that participates in folding of newly synthesized proteins, assembly of multi-molecular complexes, protein degradation, and prevents aggregation and denaturation of proteins under various types of stress [1]. More than 200 intracellular Hsp90 client proteins have been identified that are involved in signal transduction, intracellular signaling, transcription, regulation of gene expression, etc. [2, 3]. There are two isoforms of Hsp90, namely Hsp90α (inducible form) and Hsp90β (constitutive form). The homology level of the two Hsp90 isoforms is 86%, while there are significant differences in their biochemical properties, expression, and function [4].
In addition to performing intracellular functions, Hsp90 was found outside the cell (extracellular Hsp90, eHsp90) in free form in the extracellular space and in the form of Hsp90 associated with the plasma cell membrane [5–7]. EHsp90 plays an important role in providing migration and invasion of normal and tumor cells, as well as in the processes of metastasis of tumor cells [5–15]. The membrane expression and secretion of Hsp90 are significantly increased in tumor cells in comparison with normal cells; the level of Hsp90 on the plasma membrane correlates with the metastatic potential of cells [11, 16]. Surface-associated Hsp90 has been shown to be actively internalized by tumor cells in vitro and in animals [17].
The important role of eHsp90 in providing invasion and metastasis of tumor cells [5–15], as well as the pronounced expression of Hsp90 on the membrane of tumor cells [11, 16] make it a promising molecular target for the development of antitumor drugs. It has been shown that inhibition of eHsp90 by specific antibodies, as well as by nonpenetrating low-molecular-mass inhibitors of Hsp90, resulted in reduced migration of tumor cells in vitro and inhibition of tumor metastasis in animals [6, 18–22]. It is obvious that eHsp90 inhibitors, including Hsp90-specific antibodies, have a significant potential for creating anti-cancer drugs with anti-metastatic action on their basis. In addition, since increased membrane expression and internalization of eHsp90 is a phenomenon characteristic of tumor cells in vitro and in vivo [11, 16, 17], eHsp90 can be considered as a tumor-specific target for immunotoxic drugs that selectively bind to and affect tumor cells. The activity of Hsp90α-specific antibodies in inhibiting tumor metastasis in mice has been demonstrated [20, 21]. The antitumor activity of antibodies directed to the Hsp90β isoform has not yet been studied. As well, the potential of Hsp90-specific antibodies for creating cytotoxic antitumor drugs has not been studied. In this study, we have shown that immunotoxins based on Hsp90β-specific antibodies and mertansin (DM1) have weak cytotoxic activity in vitro against tumor cells, which is probably due to active dissociation of antibodies from the cell surface and slow internalization of antibodies by cells. Antitumor activity of Hsp90β-specific antibodies was demonstrated in a model of mouse Lewis carcinoma in terms of reducing tumor metastasis and increasing the lifespan of mice with an implanted tumor.
MATERIALS AND METHODS
Materials and Reagents
Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum (FBS) (GE Healthcare, United States), solutions of versen and trypsin (Biolot LLC, St. Petersburg, Russia), antibodies to mouse IgG (Imtek, Moscow, Russia), and peroxidase conjugates of antibodies against mouse IgG (Biorad, United States) were used in the study. General purpose laboratory plastic labware and plastic labware for cell culture were from Greiner and Corning (United States). All other chemical reagents were purchased from Sigma (United Stated).
Cell Lines
Cell lines of human HT1080 fibrosarcoma and human A-172 glioblastoma from the collection of cell cultures of the Institute of Cytology RAS (St. Petersburg) were used. Baby hamster kidney cells (BHK-21), green monkey kidney cells Vero, and Lewis carcinoma cells (LLC) were obtained from the collection of cell cultures of the ICB RAS (Pushchino, Moscow oblast). The cells were grown in DMEM medium containing 10% fetal bovine serum and antibiotics (40 units of penicillin, streptomycin, and gentamicin) (DMEM-FBS).
Antibody Purification and Conjugation with DM1
Mouse monoclonal antibodies to Hsp90 (clone 1D5/A7, materials are being prepared for publication) and control antibodies against gB of Aujeszky’s disease virus (clone 34/2) obtained from the laboratory of cell culture and cell engineering of the ICB RAS [23] were purified from the ascitic fluid using ion-exchange chromatography, as described earlier [24]. The purified 1D5/A7 and 34/2 antibodies were conjugated with the cytotoxic compound mertansine (DM1) using the heterobifunctional agent Sulfo-SMCC [25]. For experiments on cell cultures, preparations of purified antibodies and antibody conjugates with mertansine were dialized against a large volume of DMEM medium for two days (three changes of DMEM medium) and sterilized by filtration through Durapor filters (Millipore, United Stated) with a pore diameter of 0.22 μm.
Evaluation of Antibody Binding to the Cells, Their Internalization by the Cells, and Dissociation into the Culture Medium
To assess the binding of antibodies to cells, the cells were grown to a monolayer state, washed with a cold phosphate-buffered saline (PBS) containing sodium azide (PBS–NaN3), and incubated with antibodies (1D5/A7 or 34/2) at a concentration of 5 μg/mL for 1 h at 4°C. After incubation, the cells were thoroughly washed with cold PBS–NaN3, lysed in 0.5% Triton X-100 solution, and the antibody content in the lysates was determined using the enzyme-linked immunosorbent assay (ELISA), as described below. To assess the dissociation of antibodies bound to the cell surface, the cells were treated with antibodies (5 μg/mL) in a DMEM medium at 4°C for 1 h. After thorough washing with cold medium, DMEM-FBS culture medium was added to the cells and incubated at 4 or 37°C for 2 h, followed by an assessment of the antibody content in the culture medium (dissociated antibodies) and in cell lysates (cell-associated antibodies) using ELISA. To assess the internalization of antibodies by cells, the cells were incubated for 4 h at 37°C in the presence of antibodies in the culture medium (5 μg/mL), washed, and the amount of cell-associated antibodies was determined using ELISA. To determine the amount of cell-internalized antibodies, the membrane-associated antibodies were removed by treatment with a 0.1 M sodium citrate solution (pH 3.0) for 2 min at room temperature, followed by analysis of intracellular antibodies using ELISA.
Determination of 1D5/A7 Antibody Content in Cell Lysates and Culture Medium
To determine the content of 1D5/A7 antibodies in cell lysates and culture medium, 96-well microplates were sensitized with affinity-purified goat antibodies against mouse IgG (Imtek) and nonspecific sorption was blocked with PBS containing 0.1% Tween-20 and 5% bovine serum (PBS-T-BS). The studied cell lysates and culture medium were titrated in PBS-T-BS and incubated in the wells for 1 h at 37°C. A purified 1D5/A7 antibody preparation with a known concentration was used to construct a calibration graph. After washing, mouse peroxidase conjugate against IgG adsorbed by human immunoglobulins was added to the wells and the plates were incubated for 1 h at 37°C. The wells were washed, the reaction was developed using ortho-phenylenediamine, the optical density at 490 nm (OD490) was measured, and the concentration of antibodies in the samples was calculated.
Determination of the Cytotoxicity of Antibody Conjugates with DM1
Sterile dialyzed preparations of 1D5/A7 and 34/2 antibody immunoconjugates with DM1 were diluted to the required concentrations in DMEM-FBS medium. The cytotoxicity of antibody preparations and immunotoxic conjugates was determined on A-172, HT1080, BHK-21, and Vero cells using the MTT method as described earlier [26]. IC50 was calculated as the minimum concentration of the immunoconjugate causing the death of 50% of cells in 72 h.
Determination of the Antitumor Activity of 1D5/A7 Antibody Preparations in Animals
A suspension of the LLC cells grown in vitro was centrifuged, diluted in DMEM to a concentration of 1 × 107 cells/mL, and implanted to syngeneic mice of the C57BL/6 line (6 to 8 weeks, females weighing 18–20 g). The C57BL/6 mice obtained from the animal Nursery of the IBCh RAS (Pushchino, Moscow oblast) were used. The tumor cells were implanted in a volume of 100 μL (1 × 106 cells/mouse) subcutaneously in the lateral area of the animal. Sterile antibodies dialyzed against PBS were administered intraperitoneally for 11 days, starting from the first day after implanting tumor cells. The following antibody doses were used: first injection was 2 mg/mouse and subsequent injections were 1 mg/mouse. The control group of experimental animals was injected with control 34/2 antibodies according to the same scheme. The experimental and control groups consisted of 15 mice when metastasis was evaluated and of 20 mice when the lifespan of mice and the size of the primary tumor were evaluated. Measurements of the length (D) and width (W) of the tumor were performed using a caliper every day, starting from the ninth day. The volume of the tumor was calculated using the formula: V = (1/2) × D × W2. The effect of antibodies on the average lifespan of animals was determined in the same experiments. In the experiments to assess metastasis, the animals were killed by cervical dislocation on the 21st day of carcinoma growth, the lungs were removed, and the number of colored metastases on the lung surface was determined.
Statistical Processing
Each experiment was performed at least three times. Each point represents the arithmetic mean of the repeats ± standard deviation. Statistical processing of the results was performed using the student’s t-test.
RESULTS
Monoclonal mouse 1D5/A7 antibodies directed to the Hsp90β-isoform obtained at the ICB RAS were studied (materials are being prepared for publication). Quantitative assessment of the binding of the 1D5/A7 antibody to the cell surface was performed using ELISA, which determines the content of antibodies in the samples. Under conditions where the process of antibody internalization by the cells was blocked at 4°C, 1D5/A7 antibodies effectively and specifically bound to the cells of the A-172 and HT1080 tumor lines, as well as to the cells of untransformed BHK-21 and Vero lines (Fig. 1).
To assess internalization of antibodies by the cells in the continuous presence of antibody in the medium, the cells were incubated with antibodies in DMEM-FBS medium at 37°C for 4 h, after which the amount of antibodies associated with the cells and internalized antibodies was evaluated (treatment of the cells with acidic buffer solution resulted in the removal antibodies from the cell surface; this procedure enabled us to differentiate membrane-associated and internalized antibodies). The results are shown in Fig. 2. Under the conditions of the constant presence of antibodies in the medium, 1D5/A7 antibodies were internalized by the A-172 and HT1080 tumor cells and the internalization of the antibody by the BHK-21 and Vero nontumor cells was less by two–four times in comparison with the tumor cells. It should be noted that the negative 34/2 antibody was also effectively internalized by the cells: the level of internalization of the negative antibody was only one and a half times lower compared to the 1D5/A7 antibodies.
To evaluate dissociation of membrane-associated 1D5/A7 antibodies into the culture medium, the cells were incubated with antibodies at 4°C for 1 h under conditions when endocytosis/pinocytosis processes were blocked. The cells were washed with a medium and incubated without antibodies at 4°C or 37°C for 2 h, and the amounts of cell-associated antibodies and antibodies dissociated in the medium from the cell surface were evaluated. A significant fraction of the antibodies associated with Hsp90β (more than 80%) dissociated from the cell surface and passed into the medium during their incubation for 2 h at 37°C (Fig. 3). Dissociation of antibodies (antibody-Hsp90β complexes) into the medium during incubation at 4°C was also observed, although to a lesser extent (Fig. 3).
Evaluation of the dissociation of 1D5/A7 antibodies bound to the cell surface into the culture medium. The antibodies were incubated with the cells for 1 h at 4°C. After thorough washing, the cells were incubated in the medium for 2 h at 4°C or 37°C. The number of cell-associated (Ka-1D5/A7) and dissociated from the cell surface (D-1D5/A7) antibodies was evaluated.
The ability of Hsp90β-specific 1D5/A7 antibodies to create immunotoxins was evaluated. The antibodies were conjugated with the widely used toxic agent mertansine (DM1). The spectra of the obtained 1D5A7 conjugate are shown in Fig. 4. The presence of an additional absorption peak at 250 nm in the spectrum of conjugates indicated that DM1 was attached to the antibody.
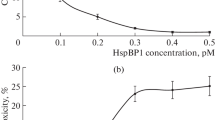
The obtained 1D5/A7-DM1 immunoconjugate interacted with native bovine Hsp90 in ELISA, which indicated that the activity of antibodies in the immunoconjugate was preserved. The 1D5/A7-DM1 conjugate was toxic to the cells, while the concentration that caused 50% cell death (IC50) for the A-172 and HT1080 tumor cell lines was quite high and was 1–4 μ/mL (Fig. 5). The IC50 for the control 34/2 antibody immunoconjugate was comparable to the 1D5/A7 antibody immunoconjugate, which was consistent with the data on the comparable effectiveness of internalization of 1D5/A7 antibody and the control 34/2 antibody. The cytotoxicity of 1D5/A7-DM1 immunoconjugate for tumor cells was two to five times higher than for cells of nontransformed lines; the death of HT1080 and A-172 tumor cells was observed at lower concentrations of immunoconjugates than for BHK-21 and Vero cells (Fig. 5).
In general, the data obtained indicated the low effectiveness of immunotoxins based on the Hsp90β-specific 1D5/A7 antibody. Based on the results, we considered it impractical to conduct experiments on animals to assess the activity of the immunotoxin 1D5/A7-DM1, since the need to maintain high concentrations of the conjugate inevitably leads to a high overall toxicity of the drug to animals.
Binding of antibodies to tumor cells can trigger innate immune effector mechanisms [27–30] that lead to elimination or inhibition of tumor cell development. With this in mind, we evaluated the effect of the 1D5/A7 antibody on primary tumor growth, metastasis, and mouse survival on a model of Lewis metastatic lung carcinoma. The administration of the 1D5/A7 antibody to animals did not affect the development of the primary tumor, but it reduced the metastasis of the tumor in the lungs by about half and increased the average lifespan of animals after the tumor implantation by 16% (Fig. 6).
DISCUSSION
Hsp90 is expressed on the membrane of tumor cells and plays an important role in the invasion and metastasis of tumor cells [8–13, 16]. Inhibition of eHsp90 using Hsp90α-specific antibodies resulted in slowing the migration of tumor cells in vitro and reducing tumor metastasis in animals [19–21], which indicated the potential of Hsp90-specific antibodies as anti-tumor drugs with anti-metastatic action. In this study, we investigated the antitumor potential of Hsp90β-specific antibodies (clone 1D5/A7) obtained at the ICB RAS. The antitumor activity of 1D5/A7 was evaluated both in the form of a purified antibody and in the form of an immunotoxin.
The cytotoxic effect of immunotoxins is based on their specific binding to the cell surface and internalization by cells, which leads to the release of cytotoxin inside the cell and its death. We found that the 1D5/A7 antibody specifically bound to A-172 and HT1080 tumor cells, as well as to the untransformed BHK-21 and Vero cells; the antibody was internalized by the cells. At the same time, the effectiveness of antibody internalization by the tumor A-172 and HT1080 cells was two to four times higher in comparison with the untransformed BHK-21 and Vero cells. It should be noted that the internalization of the control 34/2 antibodies that do not bind to cells was comparable to Hsp90β-specific 1D5/A7 antibodies. This suggested that the receptor-dependent internalization of antibody–Hsp90β complexes by cells was slow and slightly prevailed over the nonspecific capture of antibodies by cells via pinocytosis. Previously, it was also demonstrated that Hsp90α-specific 4C5 antibodies were practically not internalized by cells after binding to the surface Hsp90 [21], which is consistent with our data. On the other hand, surface-associated Hsp90 is known to be actively internalized by tumor cells [17]. It is possible that binding of the antibody to surface-associated Hsp90 slows down its internalization. We found that, in addition to the slow internalization of Hsp90β-specific antibodies after their binding to the membrane-associated Hsp90β, a significant part of the antibodies quickly dissociated from the cell surface into the culture medium; the rate of dissociation of antibodies significantly exceeded the rate of their internalization by the cells.
To assess the possibility of using the 1D5/A7 antibody to create immunotoxins, the antibodies were conjugated with the known toxic agent mertansine (DM1). Mertansine interacts with tubulin at the site of rhizoxin binding; it thus inhibits microtubule assembly, which leads to degradation of the latter and mitosis disruption [31]. We showed that the obtained 1D5/A7–DM1 conjugate had low toxicity to tumor cells: IC50 for the A-172 and HT1080 cell lines was of 1–4 μg/mL. At the same time, IC50 for the control 34/2 antibody immunoconjugate and the 1D5/A7 antibody immunoconjugate were comparable; this was consistent with the data on the comparable internalization efficiency of the 1D5/A7 antibody and the control 34/2 antibody. In general, the data indicated a low efficiency of immunotoxins based on Hsp90β-specific 1D5/A7 antibody: to achieve a cytotoxic effect, high concentrations of immunoconjugates are required, which is probably due to active dissociation of antibody complexes with Hsp90β from the cell surface and insufficiently high rate of internalization of antibodies bound to membrane-associated Hsp90β. Taking this into account, we did not study 1D5/A7-DM1 immunoconjugate with animals, since high concentrations of 1D5/A7-DM1 immunoconjugate will inevitably lead to high toxicity of the drug when administered to animals.
It is known that binding of antibodies to proteins expressed on the surface of tumor cells can trigger innate immune effector mechanisms [27–30]. Class G antibodies can activate antibody-dependent cellular cytotoxicity by binding immune effector cells (NK cells, etc.) via Fc-gamma receptors located on the surface of effector cells to the Fc fragment of tumor-specific antibodies; after binding, the effector cells cause lysis of tumor cells using various mechanisms [32]. Cell-associated antibodies are also able to activate the classical complement activation pathway by binding the complement protein complex to the Fc fragment of the antibody; the formation of such a complex ultimately leads to cell lysis and phagocytosis [33, 34]. With this in mind, we evaluated the antitumor activity of the Hsp90β-specific antibody 1D5/A7 on a model of Lewis carcinoma, a relatively rapid mouse tumor that produces pronounced metastases to the lungs of animals [35]. The 1D5/A7 antibody did not affect the development of the primary tumor when repeatedly administered to mice, which was consistent with data obtained with other extracellular Hsp90 inhibitors [6, 18–22]. On the other hand, administration of the Hsp90β-specific antibody to mice reduced tumor metastasis to the lungs and increased the average lifespan of the animals. It was shown previously that antibodies to the Hsp90α isoform significantly inhibited tumor metastasis in mice [20, 21], which is consistent with our data. The inhibitory effect of Hsp90β-specific 1D5/A7 antibody is most likely associated with inhibition of migration and invasion of tumor cells, which leads to a decrease in cell metastasis. Further studies of the antimetastatic activity of Hsp90α and Hsp90β-specific antibodies in various animal and human tumor models are needed.
CONCLUSIONS
1D5/A7 monoclonal antibodies directed to the heat shock protein Hsp90β effectively bound to Hsp90β associated with the cell surface of tumor and nontransformed cultures. The antibodies bound to the cells actively dissociated into the culture medium and were internalized by the cells. The conjugate based on the Hsp90β-specific antibody and the cytotoxic agent mertansine did not have a high level of cytotoxic activity. Repeated administration of the 1D5/A7 antibody in C57BL/6 mice with an implanted Lewis carcinoma did not slow the growth of the primary tumor, while the metastasis of tumor cells to the lungs decreased and the average lifespan of the mice increased. The results indicate the therapeutic potential of Hsp90β-specific antibodies for the treatment of tumor diseases.
REFERENCES
J. Li and J. Buchner, Biomed. J. 36, 106 (2013).
P. C. Echeverria, A. Bernthaler, P. Dupuis, et al., PLoS One 6, 26044 (2011).
D. Picard, Cell. Mol. Life Sci. 59 (10), 1640 (2002).
A. S. Sreedhar, E. Kalmar, P. Csermely, and Y. F. Shen, FEBS Lett. 562, 11 (2004).
W. Li, Y. Li, S. Guan, et al., EMBO J. 26, 1221 (2007).
S. Tsutsumi, K. Beebe, and L. Neckers, Future Oncol. 5, 679 (2009).
X. Wang, X. Song, W. Zhuo, et al., Proc. Natl. Acad. Sci. U. S. A. 106, 21288 (2009).
J. S. Chen, Y. M. Hsu, C. C. Chen, et al., J. Biol. Chem. 285, 25458 (2010).
C. F. Cheng, J. Fan, M. Fedesco, et al., Mol. Cell. Biol. 28, 3344 (2008).
U. Gopal, J. E. Bohonowych, C. Lema-Tome, et al., PLoS One 6, 17649 (2011).
M. W. Hance, K. Dole, U. Gopal, et al., J. Biol. Chem. 287 (45), 37732 (2012).
P. Jayaprakash, H. Dong, M. Zou, et al., J. Cell Sci. 128, 1475 (2015).
F. Tsen, A. Bhatia, K. O’Brien, et al., Mol. Cell Biol. 33, 4947 (2013).
S. Tsutsumi, B. Scroggins, F. Koga, et al., Oncogene 27, 2478 (2008).
D. Thuringer, A. Hammann, N. Benikhlef, et al., J. Biol. Chem. 286, 3418 (2011).
B. Becker, G. Multhoff, B. Farkas, et al., Exp. Dermatol. 13, 27 (2004).
L. B. Crowe, P. F. Hughes, D. A. Alcorta, et al., ACS Chem. Biol. 12, 1047 (2017).
B. K. Eustace, T. Sakurai, J. K. Stewart, et al., Nat. Cell Biol. 6, 507 (2004).
K. Sidera, M. Gaitanou, D. Stellas, et al., J. Biol. Chem. 283, 2031 (2008).
D. Stellas, A. El Hamidieh, and E. Patsavoudi, BMC Cell Biol. 11, 51 (2010).
D. Stellas, A. Karameris, and E. Patsavoudi, Clin. Cancer Res. 13 (6), 1831 (2007).
J. McCready, D. S. Wong, J.A. Burlison, et al., Cancers (Basel) 6, 1031 (2014).
M. M. Zaripov, O. S. Morenkov, B. Siklodi, et al., Res. Virol. 149, 29 (1998).
M. Oppermann, in Monoclonal Antibodies, Ed. By J. P. Peters and H. Baumgarten (Springer, Berlin, 1992), pp. 271–275.
A. G. Polson, S.-F. Yu, K. Elkins, et al., Blood 110 (2), 616 (2007).
A. Lisov, V. Vrublevskaya, Z. Lisova, et al., Viruses 7 (10), 5343 (2015).
T. Ben-Kasus, B. Schechter, M. Sela, and Y. Yarden, Mol. Oncol. 1, 42 (2007).
A. M. Scott, J. D. Wolchok, and L. J. Old, Nat. Rev. Cancer 12, 278 (2012).
C. W. Shuptrine, R. Surana, and L. M. Weiner, Semin. Cancer Biol. 22, 3 (2012).
L. M. Weiner, J. C. Murray, and C. W. Shuptrine, Cell 148, 1081 (2012).
M. Lopus, Cancer Lett. 307, (2011).
T. Kubota, R. Niwa, M. Satoh, et al., Cancer Sci. 100, 1566 (2009).
J. R. Dunkelberger and W. C. Song, Cell. Res. 20, 34 (2010).
K. A. Stoermer and T. E. Morrison, Virology 411, 362 (2011).
J. S. Bertram and P. Janik, Cancer Lett. 11, 63 (1980).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors state that there is no conflict of interest.
Statement of the Welfare of Animals
All applicable international, national and institutional principles for the care and use of animals in the performance of the work have been observed.
Additional information
Translated by E. Puchkov
Abbreviations: Hsp90, heat shock protein 90; eHsp90, extracellular Hsp90; DMEM, Dulbecco’s Modified Eagle Medium; FBS, fetal bovine serum; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate-buffered saline.
Rights and permissions
About this article
Cite this article
Zhmurina, M.A., Vrublevskaya, V.V., Skarga, Y.Y. et al. Internalization by Cells and Antitumor Activity of Antibodies and Immunotoxins Specific for the Heat Shock Protein 90 β Isoform. BIOPHYSICS 65, 951–957 (2020). https://doi.org/10.1134/S0006350920060238
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920060238