Abstract
This paper reports that pre-incubation of a neutrophil suspension in the presence of a near-null magnetic field produced using a system of magnetic shields (a residual constant magnetic field not greater than 20 nT) results in a considerable decrease in the intensity of neutrophil lucigenin-dependent chemiluminescence. The addition of the NADPH oxidase inhibitor diphenyliodonium to the incubation medium reduced the chemiluminescence intensity in both the experimental and the control samples (geomagnetic field). It should be noted that the differences observed between the groups, which were caused by the exposure to a near-null magnetic field, are almost the same both at lower (2.5, 5, and 10 μM) and higher (50 and 100 μM) diphenyliodonium concentrations. In contrast, the addition of 2,4-dinitrophenol, an uncoupler of oxidative phosphorylation in mitochondria, in concentrations starting from 5 μM and up to 200 μM almost completely eliminated the difference between the control and experimental samples, which was observed at low inhibitor concentrations, or in its absence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The literature data indicate that production of reactive oxygen species (ROS) is reduced in various types of cells exposed to hypomagnetic field [1–4]. We have previously demonstrated that the exposure of peritoneal mouse neutrophils to the hypomagnetic field environment created by magnetic shielding caused a decrease in the intracelullar production of reactive oxygen species detected by the change in the intensity of the fluorescence produced by the 2,7-dichlorodihydrofluorescein and dihydrorhodamine 123 oxidation products [5–7]. Considering the fact that this effect of a hypomagnetic field was observed in experiments in which neutrophils were not subjected to additional stimulation by any chemical respiratory burst activator, and thus, was not a consequence of impaired neutrophil response to these stimulators, we performed a specially designed complex study using nonactivated neutrophils in order to elucidate the possible molecular mechanisms that underlie the effects of a near-zero magnetic field [6]. It has been demonstrated that the reduced intensity of 2,7-dichlorodihydrofluorescein oxidation in nonactivated neutrophils under hypomagnetic conditions is not associated with calcium-dependent regulatory mechanisms, which was supported by the fact that the intracellular calcium ions chelator (1,2-bis(2-aminophenoxy)ethane-N,N, N',N;-tetraacetic acid acetoxymethyl ester) had no effect on the intensity of this process [6]. The observed reduction is also not likely to be a result of a hypomagnetic field affecting the phosphorylation of NADFH oxidase components, since the addition of the protein kinase C inhibitor (Ro 31-6233) had almost no impact on the fluorescence intensity of the intracellular dichlorodihydrofluorescein [6]. The addition of the phospholipase C inhibitor (U73122) led to a slight, and almost similar, decrease in the ROS production both in the control and in the experiment [6]. The possible involvement of the mitochondrial electron transport chain in the mechanism underlying the near-zero magnetic field effect is indicated by the decrease in the ROS production in the presence of rotenon, which was more profound in the experimental samples exposed to a hypomagnetic field [6].
All the above-mentioned results were obtained using fluorescence spectroscopy with fluorescent probes (2,7-dichlorodihydrofluorescein and dihydrorhodamine 123) that respond intensely to ROS, but are not selective towards particular ROS types [8–10]. In the present work, we employed another technique to assess the radical-producing ability of neutrophils after exposure to a near-zero magnetic field, namely, activated chemiluminescence using lucigenin, a selective probe for the superoxide anion [11, 12]. Using this experimental model we also performed inhibition analysis using diphenyliodonium, which is an NADPH oxidase inhibitor [13, 14], and 2,4-dinitrophenol, an uncoupler of oxidative phosphorylation in mitochondria [15, 16], in order to find potential sources of superoxide production that would respond to a hypomagnetic field.
MATERIALS AND METHODS
Preparation of neutrophil suspensions. The work was performed using mouse peritoneal neutrophils. Neutrophils were obtained from laboratory-kept CD‑1 line male mice with a weight of 24–26 g provided by the Laboratory Animal Incubator of the Puschino Branch of the Institute of Bioorganic Chemistry of the Russian Academy of Sciences (Puschino, Moscow oblast). Mice were injected with 150 μL of an opsonized zymosan suspension at a concentration of 5 mg/mL (Zymozan A from Saccharomyces cerevisiae, Sigma, United States) into the peritoneal cavity. Twelve hours after the injection, the mice were euthanized by cervical dislocation and their abdominal cavity was washed with 4 mL of cooled Hank’s solution without calcium. Peritoneal exudate was collected using a pipette and centrifuged at 600 g for 5 min. Supernatant was decanted and the pellet was resuspended in 4 mL of Hank’s solution without calcium and left to stay for 1 h at 4°C. The number of isolated cells was calculated using a Goryaev chamber. Cell viability was assessed using the trypan blue vital stain. The portion of living cells was not less than 98%. Experimental samples were prepared by diluting the neutrophil suspension with the standard Hank’s medium (138 mM NaCl, 6 mM KCl, 1 mM MgSO4, 1 mM Na2HPO4, 5 mM NaHCO3, 5.5 mM glucose, 1 mM CaCl2, and 10 mM HEPES, pH 7.4; Sigma, United States) to a concentration of 1 million cells/mL.
Exposure of the neutrophil suspension to near-zero and weak constant magnetic fields. Neutrophils at a concentration of 1 million/mL were incubated in a volume of 0.25 mL in round-bottom polystyrene cuvettes (1.2 cm in diameter and 5.5 cm in length), in which chemiluminescence was further measured, at 37.0 ± 0.2°C. The typical incubation time was 40 min. The required temperature was maintained using a circulating constant-temperature bath.
The control group samples were exposed to a local geomagnetic field with a constant component of ~44 μT and a 50 Hz component of the magnetic background of 15–50 nT at the same temperature as the experimental samples and simultaneously with them. The experimental samples were placed into the hypomagnetic-environment simulator.
A specially designed experimental installation that allows one to create a hypomargentic environment was used, which made it possible to significantly weaken the geomagnetic field, up to a reduction by 10 000 times (the residual constant field did not exceed 20 nT), and to substantially reduce the variable technogenic noise (to several nT). This installation was described in detail previously [7, 17]. It consists of three coaxially nested cylindrical permalloy magnetic shields (1-mm thick). The residual magnetic fields inside the installation were determined by direct measurement using a Mag-03 MS 100 flux gate magnetometer (Bartington, Great Britain). To produce an experimental weak uniform constant magnetic field (MF), a specially designed inducing coil (solenoid) connected to a constant current supply was placed inside the installation, in order to produce a weak constant MF of different intensities (2.5, 7.0, and 44 μT) used in a number of experiments. The size of the experimental plot inside the shield system (a plot diameter of 20 cm and a plot length of 40 cm) allowed a sufficient number of experimental samples (not less than six) to be simultaneously located in the uniform weak magnetic field area. All experiments were performed no less than three times.
Prior to exposure, a number of chemical compounds, namely, diphenyliodonium chloride, which is an inhibitor of NADPH oxidase (Sigma, United States), in varying concentrations (2.5, 5.0, 10, 20, 50, and 100 μM), which was dissolved in dimethylsulfoxide (Sigma, United States) prior to the experiment, and 2,4-dinitrophenol, an uncoupler of oxidative phosphorylation (Sigma, United States), in varying concentrations (1.0, 4.0, 5.0, 10, 20, 200 μM, and 2 mM), also pre-dissolved in dimethylsulfoxide, were added individually to certain samples. The individual effects of dimethylsulfoxide in the final concentration of 1 mM, which corresponded to its level in the sample containing 10 μM diphenyliodonium, or 20 μM 2,4-dinitrophenol, were estimated as well. In some experiments, these inhibitors were added to the samples immediately after the exposure was finished, rather than prior to its start, but before the introduction of lucigenin and chemiluminescence measurements.
Chemiluminescence measurement. When the exposure ended, the intensity of the chemiluminescence produced by neutrophil suspension samples in the experimental and control cases was measured after the addition of the lucigenin (Enzo Life Science, United States) solution at a final concentration of 0.35 mM. The Lum-1200 (OOO DISoft, Russia) chemiluminometer was used to perform the measurements. Power-Graph software was used to analyze the chemiluminescence data. Some results were provided in percent relative to the chemiluminescence response amplitude in the control, which was assumed to be 100%. The statistical processing of the measurement results was performed using the Student’s t-test.
RESULTS AND DISCUSSION
Pre-incubation of the neutrophil suspension in the near-zero magnetic field environment led to a substantial decrease in the intensity of the lucigenin-dependent chemiluminescence (approximately by 30%) (Figs. 1 and 2). When the constant field magnitude was increased to 2.5 μT this effect disappeared; it re-appeared again at 7 μT and disappeared again at 44 μT (this value corresponds to the constant MF magnitude in the control) (Fig. 1). We also observed the same multiple-peaked (polyextreme) pattern of the response to a constant weak MF in previous experiments using neutrophils where ROS production was assessed by fluorescence measurement [7], as well as when using other biological objects [18, 19].
The effects of a constant MF on the intensity of the lucigenin-dependent chemiluminescence in the neutrophil suspension. X axis, constant MF magnitude in μT, Y axis, maximum chemiluminescence intensity in percent relative to the control (mean values and standard deviations, n = 8). Hypomagnetic field (HypoMF) corresponds to the constant MF with the magnitude not greater than 0.02 μT; (1) control and (2) experiment. Statistically significant differences from the control are indicated with an asterisk (P < 0.05).
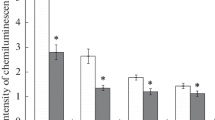
The effects of diphenyliodonium on the intensity of the lucigenin-dependent chemiluminescence in a neutrophil suspension after exposure to a near-zero magnetic field. X axis, diphenyliodonium concentration in μM, Y axis, the maximum chemiluminescence intensity in percent relative to the control (mean values and standard deviations, n = 6). (1) Control, diphenyliodonium addition prior to the incubation; (2) experiment, diphenyliodonium addition prior to the incubation; (3) control, diphenyliodonium addition after the incubation, and (4) experiment, diphenyliodonium addition after the incubation. Statistically significant differences from the control are indicated with an asterisk (P < 0.05).
The addition of diphenyliodonium to the incubation medium led to a decrease in the chemiluminescence intensity in both the experimental and control samples (Figs. 2 and 3). The diphenyliodonium effect showed a nearly linear dependence on its dose (it increased with an increase in the concentration) in both the control and experimental cases (Fig. 2). At the same time, the difference between the two groups caused by the effect of a near-zero magnetic field was almost the same at both lower (2.5, 5.0, and 10 μM) and higher (50 and 100 μM) diphenyliodonium concentrations. The absence of any significant impact of dimethylsulfoxide (the difference between the samples with the addition of 1 mM dimethylsulfoxide and the corresponding control samples did not exceed 5%), which is used as a solvent to prepare diphenyliodonium solutions, allowed us to link the observed effects with the activity of the studied inhibitor.
The kinetics of the chemiluminescence response of the neutrophil suspension to lucigenin after exposure to a near-zero MF in the absence, as well as in the presence, of diphenyliodonium: (1) control; (2) experiment; (3) control, with the addition of 10 μM diphenyliodonium; and (4) experiment, with the addition of 10 μM diphenyliodonium.
When diphenyliodonium was added after the incubation immediately before the chemiluminescence measurements, the direction of its effect exerted on the experimental and control samples remained the same, although the size of the effect relative to the inhibitor dose appeared to decrease (Fig. 2).
A substantially different result was obtained in the experiments where 2,4-dinitrophenol, which uncouples oxidation and phosphorylation, was used. In particular, the addition of this inhibitor in concentrations starting from 5 μM and up to 200 μM almost completely eliminated the differences between the control and experimental samples (Figs. 4 and 5). At the same time, the intensity of the lucigenin-dependent chemiluminescence produced by the control neutrophil suspension decreased proportionally to the 2,4-dinitrophenol concentration. On the basis of these data, the conclusion may be drawn that dinitrophenol in certain concentrations is able to completely eliminate the effect of a near-zero MF. It may be suggested that the most probable explanation for the observed dinitrophenol effects may be a certain similarity between its mechanism of action and the action of a near-zero magnetic field. In fact, in this case, as a result of substrate competence, the chemical agent may be able to suppress the effect of the factor of a physical nature. An alternative hypothesis to explain this dinitrophenol effect is that coupling of oxidation and phosphorylation in the neutrophil mitochondria is required to enable the near-zero magnetic field effect, in other words, certain ATP production levels are required. However, the experiments where dinitrophenol was added after the end of incubation in the near-zero field, but not at the start of the experiment, which demonstrated that the dinitrophenol effect can still be observed in this case and showed no qualitative changes (Fig. 4), provide evidence that supports the first hypothesis.
The effects of dinitrophenol on the intensity of the lucigenin-dependent chemiluminescence in a neutrophil suspension after exposure to a near-zero MF. X axis, dinitrophenol concentration in μM, Y axis, maximum chemiluminescence intensity in percent relative to the control (mean values and standard deviations, n = 6). (1) Control, dinitrophenol addition prior to the incubation; (2) experiment, dinitrophenol addition prior to the incubation; (3) control, dinitrophenol addition after the incubation, and (4) experiment, dinitrophenol addition after the incubation. Statistically significant differences from the control are indicated with an asterisk (P < 0.05).
The kinetics of the chemiluminescence response of the neutrophil suspension to lucigenin after exposure to a near-zero MF in the presence of dinitrophenol: (1) control, (2) experiment, (3) control, with the addition of 10 μM dinitrophenol, and (4) experiment, with the addition of 10 μM dinitrophenol.
It is well-known that the mechanism of action of dinitrophenol, which is a well-characterized uncoupler of mitochondrial respiration, is based on its ability to break the proton gradient formed as a result of electron transport [15, 16]. Disruption of the proton gradient at the inner mitochondrial membrane does not interfere with respiration, but it does inhibit ATP synthesis, which is normally fueled by the proton gradient. This uncoupling abolishes the membrane potential across the inner mitochondrial membrane [15].
Previously, it was commonly thought that mature neutrophils contain few functional mitochondria, if they contain them at all [20]. This suggestion was based on the fact that electron microscopy of fixed cells is usually unable to detect intact mitochondria, while those that can be observed are small with poorly developed cristae and inner mitochondrial membrane [20]. Moreover, it has been demonstrated that neutrophils mainly utilize glycolysis for energy production [21], while the intensity of mitochondrial respiration appears to be very low. This O2-independent energy production mechanism is advantageous for neutrophils, since it enables their functioning in inflammation foci, or infection sites, where the O2 partial pressure may be rather low [22]. During phagocytosis neutrophils utilize large amounts of molecular O2 for the generation of superoxide anion and other ROS during the respiratory burst catalyzed by NADPH oxidase rather than for the mitochondrial respiration [23]. Thus, considering these morphological and biochemical properties it seemed that neutrophils do not have functionally active mitochondria and do not even need them.
However, recently, new evidence for the important role that functional mitochondria may play in the control of neutrophil apoptosis is emerging [24]. Using the fluorescent indicators of mitochondrial function in living cells it has been demonstrated that neutrophils possess a well-developed mitochondrial network [25]. The membrane potential in these mitochondria can be disrupted using the chemical uncouplers of electron transport. It has been shown that mitochondria in neutrophils do not participate in the rapid activation of the respiratory burst or phagocytosis; however, the intensity of these processes appeared to be decreased in neutrophils pre-treated with the mitochondria inhibitors [25]. All these data, along with our findings, indicate that mitochondria in neutrophils may be investigated as potential targets towards which the effects of a near-zero magnetic field may be directed.
In neutrophils, there are several major systems in which free radicals are formed as the main or a side product. First, this regards NADPH oxidases, membrane enzymes that produce the superoxide anion radical (SAR) by one-electron reduction [26]. Apart from them, mitochondria may be an important SAR production system. It is well known that SAR leakage occurs at 11 sites of the mitochondrial inner membrane, mostly in complexes I, II, and III, with SAR being released both into the matrix and the intermembrane space [27].
Lucigenin is considered to be a selective probe for SAR [11] and is thus intensively used to study ROS production by both NADPH oxidase and mitochondria [12]. The characteristics of the inhibitory effect of diphenyliodonium (a non-specific NADPH oxidase inhibitor) revealed in the present work using neutrophils exposed to a near-zero magnetic field put the suggestion that NADPH oxidase is the main source of SAR in response to a hypomagnetic field in doubt.
On the contrary, the experiments using dinitrophenol, an uncoupler of oxidative phosphorylation, which demonstrated almost complete elimination of the effects of a near-zero magnetic field in the presence of this compound, support the idea that it is mitochondria as SAR producers that are the main target of this physical factor. Certainly, this hypothesis requires further study.
References
H. Zhang, Z. Zhang, W. Mo, et al., Prot. Cell 8 (7), 527 (2017).
C. F. Martino and P. R. Castello, PLoS One 6 (8), e22753 (2011).
P. Politanski, E. Rajkowska, M. Brodecki, et al., Bioelectromagnetics 34, 333 (2013).
V. N. Binhi and F. S. Prato, PLoS One 12 (6), e0179340 (2017).
V. V. Novikov, E. V. Yablokova, and E. E. Fesenko, Biophysics (Moscow) 63 (3), 365 (2018).
V. V. Novikov, E. V. Yablokova, E. R. Valeeva, and E. E. Fesenko, Biophysics (Moscow) 64 (4), 571 (2019).
V. V. Novikov, E. V. Yablokova, I. A. Shaev, and E. E. Fesenko Biophysics (Moscow) 65 (3), 524 (2020).
J. P. Crow, Nitric Oxide Biol. Chem. 1 (2), 145 (1997).
S. L. Hempel, G. R. Buettner, Y. Q. O’Malley, et al., Free Radic. Biol. Med. 27 (1–2), 146 (1999).
G. Bartosz, Clin. Chim. Acta 368, 53 (2006).
T. B. Aasen, B. Bolann, J. Glette, et al., Scand. J. Clin. Lab. Invest. 47, 673 (1987).
A. A. Dzhatdoeva, E. V. Proskurnina, A. M. Nesterova, et al., Biol. Membrany 34 (6), 116 (2017).
A. R. Cross and O. T. Jones, Biochem. J. 237, 111 (1986).
Y. Li and M. A. Trush, Biochim. Biophys. Acta 253, 295 (1998).
S. Matsuyama, J. L. Lopis, Q. L. Deveraux, et al., Nat. Cell. Biol. 2, 318 (2000).
M. M. El-Guindy, A. C. Neder, and C. B. Gomes, Cell Mol Biol. 27 (5), 399–402 (1981).
V. V. Novikov, E. V. Yablokova, and E. E. Fesenko, Biophysics (Moscow) 65 (1), 82 (2020).
V. V. Novikov, I. M. Sheiman, and E. E. Fesenko, Biophysics (Moscow) 52 (5), 498 (2007).
V. V. Novikov, I. M. Sheiman, and E. E. Fesenko, Bioelectromagnetics 29, 387 (2008).
S. W. Edwards, Biochemistry and Physiology of the Neutrophil (Cambridge Univ. Press, New York, 1994).
M. L. Karnovsky, Semin. Hematol. 5, 156 (1968).
S. W. Edwards, M. B. Hallett, and A. K. Campbell, Biochem. J. 217, 851 (1984).
A. W. Segal and A. Abo, Trends Biochem. Sci. 18, 43 (1993).
J. G. Pryde, A. Walker, A. G. Rossi, et al., J. Biol. Chem. 275, 33574 (2000).
G. Fossati, D. A. Moulding, D. G. Spiller, et al., J. Immunol. 170, 1964 (2003).
A. Panday, M. K. Sahoo, D. Osorio, and S. Batra, Cell Mol. Immunol. 12, 5 (2015).
V. Kozjak-Pavlovic, Cell Tissue Res. 367 (1), 83 (2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest. Authors declare no conflict of interest.
Additional information
Translated by E. Martynova
Abbreviations: ROS, reactive oxygen species; MF, magnetic field; SAR, superoxide anion radical.
Rights and permissions
About this article
Cite this article
Novikov, V.V., Yablokova, E.V., Shaev, I.A. et al. Decreased Production of the Superoxide Anion Radical in Neutrophils Exposed to a Near-Null Magnetic Field. BIOPHYSICS 65, 625–630 (2020). https://doi.org/10.1134/S0006350920040120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350920040120