Abstract—Spin labeling EPR spectroscopy was used to study the structural and conformational characteristics of human serum albumin and human erythrocyte membranes under normal conditions and with symptoms of heart failure. 5-Doxyl stearic acid and 16-doxyl stearic acid were used as spin labels, whose paramagnetic NO fragments are bound to different sites of the hydrocarbon chain. The EPR spectra of 16-doxyl stearic acid indicate that in the physiological temperature range serum albumin molecules are characterized by several types of fatty acid binding sites, which differ in parameters of spin-label rotational diffusion. This distribution of fatty-acid binding sites was typical for the blood serum of all patients who participated in our study, regardless of deviations from the normal blood parameters. The microviscosity of erythrocyte membranes from patient blood was measured using both 5-doxyl stearic and 16-doxyl stearic spin labels, whose paramagnetic fragments are located at different depths inside the lipid bilayer. It was found that in patients with an increased erythrocyte distribution width, the membrane lipid microviscosity is statistically significantly higher than under normal conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Serum albumin is the most common human plasma protein; it is present in concentrations of ~40 mg/mL (~0.6 mM) [1–3]. This protein, with a molecular weight of 66.5 kDa, is synthesized in the liver, from which it is secreted as a single non-glycosylated polypeptide chain. Human serum albumin has many functions under physiological conditions. It has a remarkable reversible binding ability to various ligands and can carry many endogenous and exogenous compounds, such as fatty acids, bilirubin, hemin, hormones, polypeptides, and free metal ions. It also binds a wide range of medications, including aspirin, ibuprofen, diazepam, and warfarin [3]. The total surface area of relatively small molecules of serum albumin is large, which makes it very effective for binding and transfer of various compounds.
The seven major fatty acid binding sites are in different parts of the protein and show different relative affinities for fatty acids [1–3]. In addition, medium chain fatty acids bind to additional albumin sites, forming a total of up to eleven different binding loci. The main centers that link fatty acids have topological differences, are usually contained inside one of the subdomains of serum albumin, and overlap with nearby domains or subdomains. Such a specific localization of fatty acid binding sites can lead to an allosteric effect on the interaction of albumin with other ligands and with the fatty acids themselves. In a liquid medium, a serum albumin molecule exhibits conformational flexibility and undergoes several transitions that depend on the pH of the medium.
Biomarkers associated with various diseases that affect serum albumin can cause allosteric modifications of this protein and lead to changes in its binding and transport properties. One of the modifications of the albumin molecule is a change in its ability to bind and transport fatty acids. Many studies [4–10] have shown a change in the structural and conformational characteristics of serum albumin molecules in various types of oncologic diseases, such as lymphomas (Hodgkin’s disease) and leukemia.
Structural and functional changes that occur with albumin can be detected using various physicochemical methods, including spin labeling EPR spectroscopy.
The erythrocyte membrane is a flexible structure of lipid sites, proteins, lipoproteins, and glycoproteins; the cell membrane thickness is ~10 nm [4]. The erythrocyte plasma membrane can be divided into three layers. The outer layer is formed by glycoproteins and contains complexes of the terminal parts of antigens. The surface of the erythrocyte membrane is a complex multidimensional structure. This structure is determined by the heterogeneity of the membrane itself and the wide spectrum of its protein composition.
Changes in the membrane properties and in the general physical and mechanical parameters of erythrocytes are typical for many human diseases. These changes can be characterized by such general parameters of erythrocytes as their distribution width (RDW—red blood cell distribution width): the average cell volume variability, or the average cell volume itself. These changes are not as pronounced in the pathogenesis of the cardiovascular system diseases; however, several works show very significant deviations of the erythrocyte parameters from the norm [12–15]. As an example, significant differences in the physico-chemical characteristics of erythrocytes were revealed for normotensive and hypotensive patients [16]; a significant increase in the microviscosity of erythrocyte membranes in acute attacks of unstable angina was demonstrated [17].
EPR spectroscopy is the most important method for studying the physico-chemical and functional characteristics of various biological objects at the molecular and cellular level [18–21]. In this work, using ESR spectroscopy of spin labels, we studied the structural and conformational characteristics of serum albumin and human blood erythrocyte membranes in normal conditions and with signs of various pathologies associated with cardiovascular failure.
MATERIALS AND METHODS
Preparation of human blood serum and erythrocytes. Serum and erythrocytes samples of 57 patients were obtained in the clinical diagnostic laboratory of the National Medical Research Center for Cardiology, Ministry of Health of the Russian Federation. Biochemical and hematological blood tests were also carried out there. For the study, samples were taken with different blood characteristics: with hypercholesterolemia, with hypertriglyceridemia, increased anisotropy of red blood cells, and with normal indicators according to the corresponding parameters. Standard clinical laboratory techniques were used to obtain samples of blood serum and erythrocyte mass and to determine the main biochemical and hematological parameters.
Conducting experiments on EPR spectroscopy with 5DS and 16DS spin labels. Nitroxyl free radicals spin-labeled derivatives of stearic acid: 5-doxyl stearic acid (5DS) and 16-doxyl stearic acid (16DS) were used at concentrations from 0.4 to 1.6 mM to study the structural and dynamic characteristics of erythrocyte membranes and serum albumin. The resulting samples were placed in glass capillaries with an inner diameter of 0.8 mm.
EPR spectra were recorded on a Varian E-109E spectrometer (United States) equipped with an E-254 temperature block, as well as on a small ESR 70-03 XD/2 UE automated spectrometer (KBST BSU, Belarus). Spectra recorded on a Varian E-109E spectrometer were processed using special software created at the Chair of Biophysics of the Physics Department of Moscow State University; they were recorded on the ESR 70-03 XD/2 spectrometer using original software from the manufacturer of the spectrometer.
EPR spectra were recorded at a microwave power of 10 mW (E-109E) or 5 dB (ESR 70-03 XD/2), a high-frequency modulation frequency of 100 kHz, and a high-frequency modulation amplitude of 0.2 mT (in the case of 5DS) or 0.1 mT (in the case of 16DS); the scan of the magnetic field was 10 mT. Temperature dependences were recorded on an E-109E spectrometer using an E-254 block in the temperature range from 0 to 50°C.
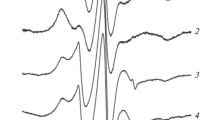
Figure 1 shows the characteristic EPR spectra of 5DS and 16DS spin labels in a suspension of erythrocytes isolated from normal donor blood. These spin-labeled derivatives of stearic acid are characterized by different immersion depths of their paramagnetic NO fragment in the lipid bilayer of erythrocyte membranes and, accordingly, by different rates of rotational diffusion. Figure 1 also shows the parameters that are necessary for analyzing the EPR spectra of 5DS and 16DS [18–20]:
(1) The order parameter S, determined by the formula
where \(A_{\parallel }^{'} = {1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0em} 2}\Delta {{H}_{\parallel }}\) and \(A_{ \bot }^{'} \approx {1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0em} 2}\Delta {{H}_{ \bot }}\), a' = \({1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-0em} 3}(A_{\parallel }^{'} + 2A_{ \bot }^{'})\), a = \({1 \mathord{\left/ {\vphantom {1 3}} \right. \kern-0em} 3}({{A}_{{zz}}} + {{A}_{{xx}}} + {{A}_{{yy}}})\).
(2) The correlation time of rotational diffusion, as estimated by the formulas
(3) The p/h parameter, which allows one to estimate the ratio of the label dissolved in the polar medium to the label embedded in the erythrocyte membranes or bound to various centers for fatty acids on the serum albumin molecule.
The obtained values of the spectral parameters for 5DS and 16DS spin labels were compared for groups of patients with different blood characteristics.
Reagents. We used reagents from Sigma (United States), Aldrich (United States), ICN (United States), Serva (Germany), and other companies.
Statistical analysis. Results are presented as the mean ± standard error. Statistical processing of the results was carried out according to the t-test and the ANOVA test, using applications of the Origin 8 program from Origin Lab Corporation (United States). Differences between experimental data were considered significant at P ≤ 0.05.
RESULTS AND DISCUSSION
EPR spectra of 5DS and 16DS labels in human blood.Figure 2 shows the EPR spectra of the 5DS and 16DS labels, spin-labeled derivatives of stearic acid, embedded in serum and in the membranes of human erythrocytes. The spin labels we used (5-doxyl stearic acid and 16-doxyl stearic acid) differ in the places of attachment of the paramagnetic fragment to the hydrocarbon chain and, accordingly, the speed and nature of its rotational diffusion [5]. The EPR spectrum of the 5DS label in erythrocyte membranes is typical for slow rotational diffusion in the presence of faster anisotropic rotation around the long axis of the stearic acid molecule. Such spectral parameters indicate the localization of the NO fragment near the surface of the lipid bilayer of the erythrocyte membrane. The EPR spectrum of the 5DS label localized in hydrophobic pockets on a serum albumin molecule in blood serum also indicates a slowing of the rotational diffusion of the paramagnetic label fragment, which, however, is more isotropic.
The EPR spectrum of the 16DS label in erythrocytes is typical for a spin label with localization of a paramagnetic fragment deep in the lipid bilayer of the membrane and indicates a slow and practically isotropic rotational diffusion. However, in the case of the 16DS label a more complex EPR spectrum is observed in serum due to the existence of several types of hydrophobic pockets for the binding of fatty acids on the surface of serum albumin, which is the main blood transport protein, with different possibilities for rotational diffusion of the paramagnetic fragment of this spin label.
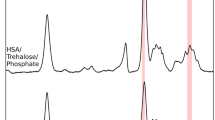
The characteristics of the binding of 16DS tags to human serum albumin. 16-Doxylstearic acid has been widely used as a very informative marker of human serum albumin for many biophysical and biomedical studies. It was shown that deviations of blood parameters from normal during the pathogenesis of several oncological diseases are accompanied by significant structural conformational changes in serum albumin molecules, leading to a change in the degree of fatty acid binding and their distribution in hydrophobic pockets with different characteristics (see [4]). In addition to oncological diseases, the effect of deviation of blood indices from the norm on the physicochemical characteristics of serum albumin was also detected for other diseases, such as heart failure and hypertension. Various researchers have shown that the recorded total EPR spectrum of human blood serum is a superposition of the spectra from the highly immobilized 16DS label (τ ≥ 10–8 s), the average immobilized 16DS label (τ ≈ 10–9 s), the free 16DS label in blood plasma (τ < 10–10 s), and 16DS labels in micromicelles (a wide singlet without the allowed hyperfine structure). The contribution of each of these spectra is determined by the impact of the pathogenesis of the disease on the structural and functional characteristics of blood components.
Figure 3 shows the EPR spectra of 16DS labels in blood serum recorded at different temperatures (0, 12.5, 27, and 37°C) and 16DS concentrations in the incubation medium (0.4 mM on Fig. 3a, 1.6 mM on Fig. 3b). The EPR spectra presented in this figure demonstrate that 16-doxylstearic acid binds to serum albumin in several hydrophobic pockets with different conditions for rotational diffusion of the paramagnetic radical fragment. At a 0.4 mM 16DS label concentration, almost the entire spin label binds to serum albumin; at a concentration of 1.6 mM, a significant portion of the label remains in blood serum that is not bound to protein.
For the total EPR spectrum of the 16DS label in blood serum, it was possible to calculate the distance between the extremes ΔHII that characterize the rotational diffusion of the part of the labels that are strongly immobilized in their hydrophobic pockets (see Figs. 1, 2, and 3). It was found that ΔHII = 6.004 ± 0.013 mT with a median 6.016 mT (n = 42); almost the same distance between the extremes of the EPR spectrum of spin-labeled serum was observed for the 5DS label, which is a spin label with a different localization of the paramagnetic fragment on the stearic acid molecule (ΔHII = 6.134 ± 0.045 mT, median 6.190 mT). However, unlike the 16DS label, the EPR spectrum of the 5DS label in all hydrophobic pockets of serum albumin was typical for strongly immobilized spin labels only.
Small differences in ΔHII values were observed in our experiments for groups of patients with altered blood parameters; however, these differences were not statistically significant.
Even the early works that used the 16DS label to study the physicochemical parameters of blood serum components revealed the dependence of the degree of this spin label binding to serum albumin on the development of various diseases, primarily oncological ones [4, 5]. The p/h parameter turned out to be very informative (see Fig. 1); it determines the proportion of 16DS label that is not bound with blood serum in a nonviscous medium. In our experiments, we used the p/h parameter to compare groups of patients with normal and elevated blood cholesterol and triglycerides (i.e., in the norm and under conditions of hypercholesterolemia and/or hypertriglyceridemia). The results are presented in Table 1.
Comparison of patient groups by these parameters did not reveal significant differences. The mean values for the group with high blood cholesterol did not significantly differ from the mean of the total sample.
Physicochemical properties of erythrocyte membranes.Figure 4 shows the ESR spectra of the 5DS label in erythrocyte membranes recorded at temperatures of 0, 12.5, 27, and 37°C. The EPR spectra shown in this figure are typical for slow rotational diffusion of the spin label in the presence of faster anisotropic rotation around the long axis of the stearic acid molecule. The spectral parameters of the 5DS label also indicate the localization of the NO fragment of the label near the surface of the lipid bilayer of the erythrocyte membrane.
The rotational diffusion of the 5DS label in the erythrocyte membrane at different temperatures can be characterized by the distance between the extremes ΔHII and/or the order parameter S (see the Materials and Methods section). Figure 5 shows a typical dependence of the ΔHII parameter on temperature, which is characterized by a bend at 20°С.
The temperature dependence of the spectral parameter ΔHII (see Fig. 1) for the 5DS label in the membranes of erythrocyte from donor blood with normal values.
For patients with insignificant deviations of blood indices from the norm (n = 24), it was found that ΔHII = 5.781 ± 0.029 mT with a median 5.751 mT (at room temperature). For groups of patients with more significant deviations of blood indices from the norm, significant differences were observed in ΔHII values, which, however, were not statistically significant.
The rotational diffusion of 16DS in the erythrocyte membrane was characterized at room temperature by the correlation times τ+ 1 = (1.46 ± 0.19) × 10–9 s and τ0 = (1.89 ± 0.21) × 10–9 s, i.e., label rotation ellipticity coefficient ε = τ0/τ+1 = 1.3.
We performed a comparative analysis of the parameters of the EPR spectra of the 5DS label in a group of patients with an increased erythrocyte distribution width (RDW > 16%) and a group with normal indicators of erythrocyte anisotropy (11.5 ≤ RDW ≤ 14%). The average values of the order parameter S for these groups of patients are shown in Fig. 6. This figure demonstrates that the increased anisotropy of erythrocytes compared with the norm is accompanied by a statistically significant increase in the parameter S, i.e., increase in microviscosity of lipids of their membranes.
CONCLUSIONS
Our studies have shown that the method of EPR spectroscopy of spin labels can provide significant information on the physicochemical properties of serum albumin and membranes of human erythrocytes. Using the 16DS label, a complex EPR spectrum of blood serum was observed, indicating the existence of at least two types of fatty-acid binding sites. Several parameters of the EPR spectra of both spin labels (5DS and 16DS) associated with serum albumin and erythrocyte membranes, with relatively small deviations of blood parameters, revealed no statistically significant differences between groups of patients with hypercholesterolemia and hypertriglyceridemia and patients with normal parameters.
The physicochemical characteristics of the erythrocyte membranes changed only in the group of patients with an increased erythrocyte distribution width (RDW). A significant difference in the parameter of order S was revealed in comparison with the control group, indicating an increase in microviscosity.
Future studies in this area should determine whether changes in the EPR spectra of spin labels associated with serum albumin and erythrocyte membranes occur in more significant pathological abnormalities (severe hypercholesterolemia, atherosclerosis, angina pectoris, and the post-infarction state).
REFERENCES
N. N. Pshenkina, Farmakologiya 12, 1067 (2011).
J. R. Simard, Anal. Biochem. 347, 97 (2006).
A A. Pavićević, A. D. Popović-Bijelić, M. D. Mojović, et al., J. Phys. Chem. 118, 10898 (2014).
S. C. Kazmierczak, A. Gurachevsky, G. Matthes, et al., Clin. Chem. 52, 2131 (2006).
A. Gurachevsky, E. Muravskaya, T. Gurachevskaya, et al., Cancer Invest. 25 (6), 378 (2007).
Yu. M. Petrusevich, O. P. Revokatov, and A. N. Ti-khonov, USSR Inventor’s Certificate No. 1319705 (1984).
M. Moergel, P. W. Kammerer, K. Schnurr, et al., Clin. Oral Invest. 16, 1529 (2012).
Y. Akdogan, M. Emrullahoglu, D. Tatlidil, et al., Phys. Chem. Chem. Phys. 18, 22531 (2016).
A. Gurachevsky, E. Shimanovitch, T. Gurachevskaya, et al., Biochem. Biophys. Res. Commun. 360, 852 (2007).
M. Gelos, D. Hinderberger, E. Welsing, et al., Int. J. Colorectal Dis. 25, 119 (2010).
V. V. Moroz, A. M. Golubev, A. V. Afanas’ev, et al., Obshch. Reanimatol. 8, 1 (2012).
M. Minetti, J. Cell. Biochem. 25, 73 (1984).
P. S. C. Prete, C. C. Domingues, N. C. Meirelles, et al., Biochim. Biophys. Acta 1808 (1), 164 (2011).
M. Montagnana, G. Cervellin, T. Meschi, et al., Clin. Chem. Lab. Med. 50, 635 (2011).
E. Danese, G. Lippi, and M. Montagnana, J. Thoracic Disease 7, 402 (2015).
K. Tsuda, Int. Heart J. 54, 154 (2013).
E. K. Ruuge, E. A. Noeva., T. Sh. Sharifov, et al., Eur. Heart J. 16 (Suppl.), 473 (1995).
H. M. McConnell and B. G. McFarland, Quart. Rev. Biophys. 3, 91 (1970).
B. J. Gaffney and H. M. McConnell, J. Magn. Reson. 16, 1 (1974).
M. A. Hemminga, Chem. Phys. Lipids 32, 323 (1983).
T. Pohl, T. Spatzal, M. Aksoyoglu, et al., Biochim. Biophys. Acta 1797, 1894 (2010).
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-015-00125.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement of compliance with standards of research involving humans as subjects. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants involved in the study.
Additional information
Translated by I. Shipounova
Abbreviations: 5DS, 5-doxyl stearic acid; 16DS, 16-doxyl stearic acid.
Rights and permissions
About this article
Cite this article
Grachev, D.I., Dudylina, A.L., Titov, V.N. et al. The Physicochemical Characteristics of Serum Albumin and Erythrocyte Cell Membranes under Normal and Heart Failure Symptom Conditions. BIOPHYSICS 64, 721–728 (2019). https://doi.org/10.1134/S0006350919050051
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350919050051