Abstract
Extracellular heat shock protein 90 (eHsp90) plays an important role in cell motility, invasion, and metastasis of tumor cells. eHsp90 stimulates migration and invasion of cells via interaction with surface receptors, which is accompanied by the activation of multiple cell motility-related signaling pathways. In addition, еHsp90 promotes cell invasion by the activation of extracellular matrix metalloproteinases. The role of different receptors, intracellular signaling pathways, and matrix metalloproteinases in the еHsp90-dependent migration and invasion of different types of cells has been investigated insufficiently. In this study, we demonstrated that HER2 is involved in the еHsp90-mediated stimulation of migration and invasion of human glioblastoma A-172 and fibrosarcoma HT1080 cells in vitro. eHsp90-induced migration and invasion of cells are accompanied by the activation of ERK1/2-, IKK/NF-κB-, FAK-, ROCK1- and Src-mediated signaling pathways and by the limited activation of JNK, while the p38-mediated signaling cascade is not activated. eHsp90 also stimulates PI3K-Akt signaling pathway in А-172 cells, while in НТ1080 cells Akt is activated regardless of PI3K. It has been established that matrix metalloproteinases are involved in the eHsp90-dependent stimulation of invasion of А-172 and НТ1080 cells in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Heat shock protein 90 (Hsp90) is an intracellular molecular chaperone, which is present in the cytosol both under normal conditions and under stress effects on the cell. It participates in the folding of newly synthesized proteins, the refolding of proteins that have lost their native conformation, the prevention of protein aggregation, as well as their maintenance in a native reactive state [1]. There are two Hsp90 isoforms: Hsp90α (inducible form) and Hsp90β (constitutive form) [2]. More than 200 intracellular protein clients have been found for Hsp90, many of which are involved in cell signaling, cell-cycle regulation, and cell proliferation [1, 2]. The diversity of Hsp90 client proteins and their role in key cellular processes (signal transduction, intracellular signaling, transcription, gene expression regulation, etc.) make Hsp90 one of the most important proteins involved in the processes of proliferation and differentiation of cells, cell motility, morphogenesis, development of organisms, and other fundamental biological processes [1–3].
In addition to its intracellular localization, Hsp90 is also secreted by tumor cells; it is also found the surface of normal and tumor cells [4–6]. Membrane-associated and secreted extracellular Hsp90 (eHsp90) activates cellular motility, as well as invasion and metastasis of tumor cells [7–15]. The mechanisms of action of eHsp90 on migration and cell invasion are very diverse. eHsp90 may act as a ligand of cell surface receptors. The identified eHsp90 receptors are LRP1 (protein 1, associated with a low density lipoprotein receptor), which can be detected in almost all cell types [7–13, 16], and HER2 (human epidermal growth factor receptor 2) [17]. The interaction of Hsp90 with cell receptors activates various intracellular signaling pathways, which regulate migration and cell invasion [8, 10, 11, 13–15]. Hsp90-dependent signaling is sometimes complicated by the appearance of feedback loops. As an example, Hsp90-dependent Src activation may potentiate receptor activation. This was shown for integrins [14] and epidermal growth factor receptor (EGFR) [15]. On the other hand, Hsp90-dependent Src activation in glioma cells stimulates Akt-mediated formation of the invasive complex between EphA2 and LRP1 [10].
In addition to functioning as a ligand of cell surface receptors, eHsp90 can act as an extracellular chaperone that regulates several extracellular proteins. By interacting with extracellular proteases, eHsp90 affects migration and invasion by sequentially regulating the proteolytic microenvironment of cells. eHsp90 regulates the activities of metalloproteinases: MMP2 [18–23], MMP-9 [18, 24], and MMP3 [25]. eHsp90 also binds to fibronectin and participates in the assembly and/or maintenance of its structure in the extracellular matrix [26]. It also participates in the regulation of the expression of MMP-3 [27] and integrin [8], in the induction of myofibroblasts and tumor-associated myofibroblast-like cells [27, 28].
Thus, the complex pattern of Hsp90 functioning includes the direct interaction of Hsp90 with various surface cellular receptors and proteolytic enzymes, signal transduction using different signaling pathways, interaction of the participants of these signaling pathways between themselves, with receptors, and adapter molecules. The roles of individual receptors, signaling pathways, and matrix metalloproteinases in Hsp90-induced migration and invasion of various cell types have not been adequately studied. In this work, we have demonstrated the participation of the HER2 receptor, as well as Akt-, ERK1/2-, IKK/NF-κB, FAK-, ROCK1- and Src-dependent signaling pathways and matrix metalloproteinases in Hsp90-induced migration and invasion of human tumor cells of fibrosarcoma (HT1080) and glioblastoma (A-172) cell lines in vitro.
MATERIALS AND METHODS
Materials, reagents, and antibodies. DMEM and fetal bovine serum (FBS) manufactured by HyClone (United States) were used in this research. Versene and trypsin solutions manufactured by Biolot (Russia) were also used. Antibodies to beta-actin, Akt, JNK, p38, and their phosphorylated forms, as well as peroxidase conjugates against mouse and rabbit IgG manufactured by Abcam (United States), Enzo (United States), Millipore (United States), SantaCruz (United States), and Serotec (United States) were used in the study. Rat collagen VI was were purchased from Trevigen (United States). General laboratory plastic plastic labware and plastic labware for cell culture (culture flasks, 24-well plates, Petri dishes, pipettes) were manufactured by Greiner (Austria) and Corning (United States). The 24-well plate inserts with polyethylene terephthalate (PET, 8 μm) membrane were produced by Greiner (Austria). The polyvinylidenfluorid (PVDF) immunoblotting membrane (0.45 μm) was manufactured by Millipore (United States). The Lab-Tek II chambers for confocal microscopy were produced by ThermoFisher (United States). The inhibitors of protein kinases and matrix metalloproteinases used in the work (Table 1) were purchased from Sellekchem (United States). All other chemical reagents were purchased from Sigma (United States).
Cell lines. The human fibrosarcoma (HT1080) and glioblastoma (A-172) cell lines from the cell culture collection of the Institute of Cytology of the Russian Academy of Sciences were used in the work. Cells were grown in DMEM containing 10% FBS and antibiotics (penicillin, streptomycin, and gentamicin, 40 units of each) (DMEM–10% FBS).
Purification of Hsp90 from the brain of mice. Purification of native Hsp90 from the mouse brain was carried out using a previously developed protocol, including differential precipitation with ammonium sulfate, chromatography on a thiophilic gel, and ion-exchange chromatography on DEAE-sepharose [29].
The determination of migration and cell invasion in vitro. The experiments were performed using 24-well plate PET membrane inserts (pore size 8 μm, Geriner, Austria). To assess cell invasion, PET membrane inserts were treated with collagen VI in accordance with the manufacturer’s recommendations (Trevigen, United States). Prior to the experiment, the cells were kept in DMEM containing 0.2% bovine serum albumin (BSA) for 20 h at 37°C. Cells were removed from the substrate with trypsin and washed in DMEM–BSA. To conduct analysis of basal (non-stimulated) migration/invasion, the cells were placed in inserts in DMEM–BSA in the presence or in the absence of various inhibitors in the concentrations shown in Table 1. As chemoattractant, DMEM–5% FBS was used in the lower chamber. Migration and cell invasion were estimated after 6 and 24 h, respectively. The cells that passed through the PET membrane were fixed with methanol, stained with crystal violet, lysed, transferred to the wells of a 96-well plate, and the optical density was measured at a wavelength of 595 nm (OD595). Basal migration/invasion was assessed using the OD595 of cells that migrated through the membrane, with the substraction of OD595 cells that passed through the membrane in the absence of a chemotactic gradient (spontaneous migration/invasion). The effect of inhibitors was evaluated relative to the control cells and expressed in percentages. Basal migration of control cells was taken as 100%.
To analyze Hsp90-stimulated migration/invasion, cells were prepared as described above. Cells were placed in prepared inserts into the DMEM-BSA, containing purified Hsp90 (50 μg/mL) to stimulate migration/invasion in the presence or absence of inhibitors. Cell migration and invasion were assessed after 6 and 24 h respectively. The OD595 of cells that passed through the membrane was determined as described above. To calculate the Hsp90-induced migration stimulation/invasion, the OD595 values of the spontaneously migrated cells were subtracted from the values of the OD595 of the cells that passed through the membrane along the chemotactic gradient. After this, OD595 values of non-stimulated cells was subtracted from OD595 values of Hsp90-stimulated cells; the difference was expressed in percentages relative to OD595 of non-stimulated cells. The estimation of the effect of inhibitors on Hsp90-dependent stimulation of migration/invasion was calculated in comparison with control cells without inhibitors. Hsp90-dependent stimulation of migration/invasion of control cells was taken as 100%.
Western-blot analysis. Cells were grown to 50–70% of confluency and were treated with inhibitors of intracellular signaling pathways for 2 h at 37°C. Cells were washed with phosphate-buffered saline (PBS, 150 mM NaCl, 12 mM Na-phosphate buffer solution, pH 7.2). Cells were then lysed in PBS, containing 1% Triton X-100, 0.3% sodium dodecyl sulfate, protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 50 μM leupeptin, 200 nM aprotinin, and 10 μM pepstatin A), and phosphatase inhibitors (a cocktail of phosphatase inhibitors 3, Sigma, United States). Electrophoresis was carried out according to Laemmli protocol in polyacrylamide gels of various concentrations (7.5 and 10% acrylamide) in the presence of sodium dodecyl sulfate [30]. Proteins from polyacrylamide gels were transferred to polyvinylidene fluoride membranes. Nonspecific sorption of membranes was blocked by incubation in PBS containing 0.05% Tween-20 and 1% BSA (PBS–Tween–BSA). The membranes were incubated for 2 h at room temperature with primary antibodies to native and phosphorylated forms of proteins of the intracellular signaling pathways. After washing the membranes in PBS containing 0.05% Tween-20, the membranes were incubated for 1 h at room temperature with peroxidase conjugates specific to mouse or rabbit IgG, which were diluted in PBS–Tween–BSA. The visualization of membrane bands was performed with a diaminobenzidine solution (0.7 mg/mL, 0.02% H2O2, 0.05 M tris-HCl buffer, pH 7.5). Samples were standardized relative to beta-actin. After antibody staining, the membranes were scanned and processed with Total Lab v. 2.01 software for the quantitative assessment of the cell content of specific proteins or their phosphorylated forms.
Confocal microscopy. Cells were grown in Lab-Tek II chambers for confocal microcopy (ThermoFisher, United States) until they reached 50–70% confluency. Cells were washed with cold PBS–0.01% NaN3 and incubated with mouse antibodies to LRP1, rabbit antibodies to HER2, negative control mouse antibodies (34/2), or with immunoglobulins from non-immune rabbits (rIgG). After washing, the cells were incubated with Alexa 488-labeled secondary antibodies against the IgG of mice or rabbits. All incubations with the antibodies were carried out for 1 h at 4°C in PBS–NaN3−1% BSA. After washing, the cells were fixed with 0.5% formaldehyde for 15 min at 4°C and analyzed using a Leica TCS SP5 laser microscope (Leica Microsystems, Germany) with x63 objective.
Statistical analysis. Each cell migration/invasion experiment was performed at least five times. Each point represents an arithmetic mean of three to five repetitions ± standard deviation. The statistical processing of the obtained results was performed using the Student’s t-test (significance level P < 0.05).
RESULTS AND DISCUSSION
This study evaluated the mechanisms of activation of the migration and invasion of human glioblastoma A-172 and fibrosarcoma HT1080 cells in vitro in response to stimulation with extracellular Hsp90. Native Hsp90 purified from the mouse brain was used to stimulate the cell migration/invasion. The purity of the Hsp90 preparations was 95–97%. Hsp90 at concentrations of up to 1.0 mg/mL was not cytotoxic and did not affect the proliferation of A-172 and HT1080 cells. The effect of various inhibitors on basal (non-induced) and Hsp90-induced migration and cell invasion was determined in the study. Basal migration/invasion was considered as the cell migration in vitro through a semi-permeable PET membrane (in the case of an invasion, through a collagen barrier membrane) along the chemotactic gradient in the absence of growth factors (in DMEM-BSA). Hsp90-induced migration/invasion was considered as the migration of cells stimulated with eHsp90 along the chemotatic gradient through the semi-permeable PET membrane.
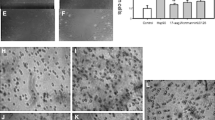
It is generally accepted that the main eHsp90 cellular receptor is the LRP1 protein [7–13]; however, in some works, the HER2 protein was identified as a receptor for eHsp90 [17]. The participation of HER2 in eHsp90-dependent migration stimulation is less studied in comparison with LRP1. HER-2 is a membrane tyrosine protein kinase of the EGFR family, a ligand-free receptor that generates heterodimers with other members of the EGFR family, HER-1, HER-3, and HER-4, bound with ligands [31]. Dimerization leads to autophosphorylation of HER proteins and initiates multiple signaling pathways, including MAPK and PI3K/Akt signaling pathways, which leads to various biological effects [32]. As was shown in various studies, A-172 and HT1080 cells used in our experiments, express on the surface HER2 along with LRP1 [31–35]. Using the immunofluorescent staining with LRP1- and HER2-specific antibodies we confirmed that A-172 and HT1080 cells expressed both eHsp90 receptors on the surface (Fig. 1). We next assessed whether HER2 is involved in Hsp90-dependent activation of migration and invasion of A-172 and HT1080 cells. The HER2 inhibitor mubritinib (TAK 165) did not affect the basal migration and invasion of A-172 cells, as well as the basal migration of HT1080 cells, and slightly reduced the basal invasion of latter (Table 2). In contrast, HER2 inhibition led to a two-fold decrease in Hsp90-induced migration and to three to ten-fold reduction in eHsp90-induced invasion of A-172 and HT1080 cells (Table 3). This indicated that HER2 takes part in Hsp90-induced migration and invasion of HT1080 and A-172 cells. The relative contribution of HER2 and LRP1 to eHsp90-stimulated migration/invasion is difficult to determine based on the obtained data. However, it appears that the contribution of HER2 to these processes is essential, especially for A-172 cells.
Expression of LRP1 and HER2 on the plasma membrane of HT1080 cells (top row) and A-172 (bottom row): a-LRP1, mouse monoclonal LRP1-antibody (sc-57353); 34/2, negative mouse antibody to glycoprotein gB of the Auecki disease virus; a‑HER2, a rabbit polyclonal polyclonal antibody to HER2 (sc-284); rIgG, antibodies from non-immune rabbits. Scale: 20 μm.
It is known that the interaction of LRP1 with different ligands leads to the activation of different signaling pathways, including ERK- and JNK-mediated cascades [36–38], FAK-mediated signal pathway [39, 40], as well as to the Src-mediated activation of cytoplasmic kinases Akt and ERK [41]. HER2-dependent signaling also initiates multiple signal pathways, the main ones of which are PI3K/Akt-, MAPK-, and STAT-kinase pathways [42]. It is not surprising that the interaction of eHsp90 with cellular receptors activates a multitude of signaling pathways in various cell types that regulate the migration and invasion of cells [8, 10, 11, 13–15]. The role of the Akt-mediated signaling pathway in eHsp90-dependent activation of cellular motility was demonstrated earlier in different cell types [10, 11, 13]. We showed that during the treatment of A-172 and HT1080 cells with eHsp90 for 2 h, the level of phosphorylated Akt (pAkt) increased by two to four times (Fig. 2). The protein kinases B inhibitor (Akt1/2/3) slightly affected the basal migration and cell invasion, but dramatically inhibited Hsp90-induced migration and cell invasion (Tables 2 and 3). The data indicate that eHsp90 activated Akt-mediated signaling in A-172 and HT1080 cells.
Phosphatidylinositol-3-kinase (PI3K), the most important regulator protein located at the convergence of various signaling pathways and controlling the key functions of the cell, is the main Akt activator. It turned out that PI3Kα/δ/β inhibitor LY294002 acted differently for different cell cultures. LY294002 inhibited the basal and, in particular, Hsp90-induced migration and invasion of A-172 cells (Tables 2 and 3), thus indicating the activation of the PI3K-Akt signaling pathway in these cells in response to eHsp90 treatment. This agrees with the earlier published data [8], where the PI3K role in the Hsp90-dependent activation of the signaling via Akt was established. On the other hand, LY294002 did not actually affect the basal and Hsp90-induced migration and invasion of HT1080 cells, testifying against PI3K-dependent activation of Akt in this cell culture (Tables 2 and 3). It is possible that in HT1080, the previously described PI3K-independent activation of Akt is observed, for example, through the non-receptor tyrosine kinases of the Scr family [43, 44].
The effects of cell stimulation with extracellular Hsp90 on the phosphorylation of Akt, p38, and JNK. Cells were treated with eHsp90 (50 μg/mL) and lysed. Cell lysates were analyzed by Western blotting with antibodies to Akt, p38, and JNK, and their phosphoryaltes forms, as well as with antibodies to beta-actin (control sample loading).
Mitogen-activated protein kinases (MAPK) represent a family of conserved serine/threonine-specific protein kinases involved in various fundamental cellular processes, including proliferation, motility, differentiation, the stress response, apoptosis, and other processes. MAPK include kinases regulated by extracellular signals (ERK1/2), c-Jun-NH2-terminal protein kinases 1, 2, and 3 (JNK), p38 (p38α, β, γ, and δ isoforms) and some other kinases. ERK-mediated signaling is a key cascade of the MAPK-dependent signal pathway [45] and is the most important component in cell motility [46]. The activation of the ERK1/2-mediated signaling pathway in response to cell stimulation by eHsp90 has been shown for cell cultures of colorectal cancer, primary dermal fibroblasts, prostate cancer, and glioblastoma cells [8, 10, 11, 13]. We also found that the inhibition of ERK1/2 effectively blocked eHsp90-dependent activation of A-172 and HT1080 cells but had little effect on basal migration and cell invasion (Tables 2 and 3). This demonstrates the key role of the ERK-mediated signaling pathway in eHsp90-dependent stimulation of migration and invasion of various cell types.
The JNK signaling pathway is another path of MAPK-dependent signaling associated with the cell stress response to external influences and stimuli [47]. The JNK1/2/3 inhibitor moderately reduced both basal and Hsp90-induced migration and cell invasion (Tables 1 and 2). We did not observe an increase in the phosphorylated form of JNK after 2-h activation of cells by eHsp90 (Fig. 2). Summarizing these data, one could suggest a definite, but not essential, participation of a JNK-mediated signaling pathway in the eHsp90-dependent activation of cells of both cell cultures. In a few studies performed on the colorectal cancer cell lines it was also demonstrated that JNK signaling probably did not play an important role in Hsp90-dependent cell stimulation [48].
p38-Mediated signaling is another MAPK-dependent signaling cascade, activated by cell treatment with cytokines and in response to many stress stimuli (ultraviolet light, heat and osmotic shock, etc.) [49]. We found that two p38α/β MAPK inhibitors, SB203580 and SB202190, did not affect the basal and Hsp90-induced cell migration and invasion (Tables 2 and 3). The quantity of the p38 phosphorylated form did not increase in the cells during their treatment with eHsp90 as well (Fig. 2). This indicates that in A-172 and HT1080 cells p38-dependent signaling did not participate in Hsp90-induced stimulation of cell motility, which is consistent with data obtained on colorectal cancer cell lines [48].
The NF-κB signal pathway is associated with the activation of the NF-κB transcription factor by cytokines, T- and B-cell mitogens, TLR ligands (for example, lipopolysaccharides), and stress effects. IKK (IκB kinase) integrates numerous pathways activated by the NF-κB pathway [50]. When cells are activated, IKK phosphorylates the inhibitory protein IκB and forms a complex with NF-κB in the cytoplasm. IκB phosphorylation causes its degradation and the release of NF-κB, which is translocated into the nucleus and activates the transcription of many genes. The block of IKK activation leads to the inhibition of the NF-κB signaling pathway. It was previously shown that eHSP90 causes the association of LRP1 with IKKα and IKKβ and activates the LRP1/IKK/NF-κB-signaling cascade in some colorectal cancer cell lines [48]. We also demonstrated that inhibition of IKK did not actually affect basal migration and invasion of A-172 and HT1080 cells; however, it dramatically decreased the eHsp90-dependent activation of cells (Tables 2 and 3). This suggests an important role for the NF-κB-based signaling pathway in eHsp90-dependent stimulation of cell migration and invasion.
Focal adhesion kinase (FAK) is a cytosolic tyrosine kinase. It is one of the components of cell adhesive contacts with the extracellular matrix (focal contacts) [51]. In the area of the focal contacts FAK is associated with the Src tyrosine kinase. They function in conjunction, launching several FAK/Src-controlled signal pathways involved, among other processes, in cell motility [52]. FAK inhibitor reduced the basal migration and invasion of HT1080 cells and to a slightly higher degree, of A-172 cells, confirming the role of FAK in cell motility. FAK inhibitor significantly reduced Hsp90-induced migration and invasion of HT1080 cells and almost completely blocked Hsp90-induced migration of A-172 cells (Tables 1 and 2). The results show the important role of the FAK-dependent signaling in eHsp90-dependent stimulation of A-172 and HT1080 cells, which agrees with the data of others [11], where the critical, in which the crucial role of FAK in the motogenic eHsp90 activity in prostate cancer cells was established.
Many signal transduction pathways are initiated and modulated by non-receptor tyrosine kinases of the Src family. We showed that PP2, a Src inhibitor, slightly reduced basal cell migration and invasion, but almost completely blocked the stimulation of cell migration and invasion induced by eHsp90. This indicates the important role of the tyrosine kinases of the Src family in eHsp90-induced stimulation of A-172 and HT1080 cells (Tables 2 and 3). It was also shown earlier for multiform glioblastoma cells that eHsp90-LRP1-mediated activation of Src is necessary for the subsequent phosphorylation and activation of Akt [10]. The important role of Src in the phosphorylation and activation of Akt and, therefore, in the activation of the Akt-dependent signaling pathway was demonstrated when the cells are activated by other stimuli [44]. The involvement of the Src family of tyrosine kinases in the eHsp90-dependent activation of cell migration and invasion may also be associated with their role in FAK/Src-mediated signaling necessary for cell motility [11, 52]. In addition, Src can participate in Src-Rho-signaling. Rho is a Src-signaling effector [53]. In turn, the Rho effectors stimulate the assembly of myosin filaments and the stabilization of actin–myosin interactions [54]. In particular, the Rho-associated serine-threonine kinase ROCK1 is a small GTPase effector. It also regulates the actomyosin cytokeleton of cells, thereby participating in cell motility and metastasis [55]. It was shown earlier that the treatment of cells with eHSP90 led to the activation of Src–Rho signaling in human glioma cells [56]. We also showed that inhibition of ROCK1 had little effect on basal cell migration and invasion, while eHsp90-induced cell invasion was significantly inhibited, especially in HT1080 cells (Tables 2 and 3). This proves the essential role of ROCK1 in eHsp90-induced stimulation of cell motility.
Matrix metalloproteinases are regulatory molecules that participate in ECM remodeling and in processing cytokine and growth factors, which, in turn, modulate the activity of ligands [57]. We found that inhibition of a wide range of matrix metalloproteinases (MMP1/2/3/7/9) with blatimastat had little effect on basal cell migration and invasion, as well as Hsp90-induced cell migration. At the same time Hsp90-induced cell invasion was substantially reduced, which was particularly obvious for A-172 cells (Tables 2 and 3). This is consistent with the data obtained from earlier studies indicating the essential role of matrix metalloproteinases in cell invasion stimulated by eHsp90 [18–25].
Our data indicate that the stimulation of cells by eHsp90 is a complex coordinated process that involves many components. eHsp90, acting as a motogenic ligand, interacts with various surface cellular receptors (LRP1 and HER2) and activates several pathways of intracellular signaling, which is accompanied by the activation of signaling pathways that provide cellular motility (FAK–Src, Src–Rho–ROCK1). eHsp90 also activates cell invasion by interacting with extracellular proteolytic enzymes. There are significant differences in the pathways and mechanisms of activation of individual components associated with eHsp90-dependent stimulation of migration and invasion of cells in vitro for different cell types.
CONCLUSIONS
HER2 is involved in eHsp90-induced stimulation of cell migration and invasion of human glioblastoma A-172 and fibrosarcoma HT1080 cell lines in vitro. eHsp90-induced migration and invasion of A-172 and HT1080 cells is accompanied by the activation of ERK1/2-, IKK/NF-κB-, FAK-, ROCK1- and Src-dependent signal pathways, and weak activation of JNK, while the p38-dependent signal cascade is not activated. The eHsp90 activates the PI3K-Akt signaling pathway in A-172 cells, while in HT1080 cells Akt is activated independently of PI3K. Matrix metalloproteinases are also involved in eHsp90-induced invasion of A-172 and HT1080 cells in vitro.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
REFERENCES
J. Li and J. Buchner, Biomed. J. 36, 106 (2013).
A. S. Sreedhar, E. Kalmar, P. Csermely, and Y.F. Shen, FEBS Lett. 562, 11 (2004).
D. Picard, Cell. Mol. Life Sci. 59 (10), 1640 (2002).
W. Li, Y. Li, S. Guan, et al., EMBO J. 26, 1221 (2007).
S. Tsutsumi, K. Beebe, and L. Neckers, Future Oncol. 5, 679 (2009).
X. Wang, X. Song, W. Zhuo, et al., Proc. Natl. Acad. Sci. U. S. A. 106, 21 288 (2009).
S. Basu, R. J. Binder, T. Ramalingam, and P. K. Srivastava, Immunity 14, 303 (2001).
J. S. Chen, Y. M. Hsu, C. C. Chen, et al., J. Biol. Chem. 285, 25 458 (2010).
C. F. Cheng, J. Fan, M. Fedesco, et al., Mol. Cell. Biol. 28, 3344 (2008).
U. Gopal, J. E. Bohonowych, C. Lema-Tome, et al., PLoS One 6, 17 649 (2011).
M. W. Hance, K. Dole, U. Gopal, et al., J. Biol. Chem. 287 (45), 37 732 (2012).
P. Jayaprakash, H. Dong, M. Zou, et al., J. Cell Sci. 128, 1475 (2015).
F. Tsen, A. Bhatia, K. O’Brien, et al., Mol. Cell Biol. 33, 4947 (2013).
S. Tsutsumi, B. Scroggins, F. Koga, et al., Oncogene 27, 2478 (2008).
D. Thuringer, A. Hammann, N. Benikhlef, et al., J. Biol. Chem. 286, 3418 (2011).
A. P. Lillis, I. Mikhailenko, and D. K. Strickland, J. Thromb Haemost. 3, 1884 (2005).
K. Sidera, M. Gaitanou, D. Stellas, et al., J. Biol. Chem. 283, 2031 (2008).
D. Stellas, A. El Hamidieh, and E. Patsavoudi, BMC Cell Biol. 11, 51 (2010).
B. K. Eustace, T. Sakurai, J. K. Stewart, et al., Nat. Cell Biol. 6, 507 (2004).
Y. Yang, R. Rao, J. Shen, et al., Cancer Res. 68, 4833 (2008).
X. Wang, X. Song, W. Zhuo, et al., Proc. Natl. Acad. Sci. U. S. A. 106, 21 288 (2009).
J. D. Sims, J. McCready, and D. G. Jay, PLoS One 6, e18848 (2011).
X. Song, X. Wang, W. Zhuo, et al., J. Biol. Chem. 285, 40 039 (2010).
F. Lagarrigue, S. Dupuis-Coronas, D. Ramel, et al., Cancer Res. 70, 6978 (2010).
A. L. Correia, H. Mori, E. I. Chen, et al., Genes Dev. 27, 805 (2013).
M. C. Hunter, K. L. O’Hagan, A. Kenyon, et al., PLoS One 9, e86842 (2014).
J. Bohonowych, M. Hance, K. Nolan, et al., Prostate 74, 395 (2014).
M. Schafer and S. Werner, Nat. Rev. Mol. Cell Biol. 9, 628 (2008).
Yu. Skarga, V. Vrublevskaya, Y. Evdokimovskaya, and O. Morenkov, Biomed. Chromatogr. 23 (11), 1208 (2009).
U. K. Laemmli, Nature 227 (5259), 680 (1970).
A. Citri, K. B. Skaria, and Y. Yarden, Exp. Cell Res. 284, 54 (2003).
C. J. Barnes and R. Kumar, Cancer Treat Res. 119, 1 (2004).
L. Maletínská, E. A. Blakely, K. A. Bjornstad, et al., Cancer Res. 60 (8), 2300 (2000).
R. C. Munch, M. D. Muhlebach, T. Schaser, et al., Mol. Ther. 19 (4), 686 (2011).
G. Perrot, B. Langlois, J. Devy, et al., Mol. Cell. Biol. 32 (16), 3293 (2012).
A. P. Lillis, I. Mikhailenko, and D. K. Strickland, J. Thromb. Haemost. 3, 1884 (2005).
B. Langlois, G. Perrot, C. Schneider, et al., PLoS One 5 (7), e11584 (2010).
N. Etique, L. Verzeaux, S. Dedieu, and H. Emonard, BioMed. Res. Int. Article ID 152163 (2013).
A. W. Orr, C. E. Pedraza, M. A. Pallero, et al., J. Cell Biol. 161, 1179 (2003).
B. Thapa, B. H. Koo, Y. H Kim., et al., Biochem. Biophys. Res. Commun. 450, 1696 (2014).
Y. Shi, E. Mantuano, G. Inoue, et al., Sci. Signal. 2 (68), 18 (2009).
A. Adamczyk, A. Grela-Wojewoda, M. Domagaia-Haduch, et al., J. Cancer 8 (1), 131 (2017).
K. Mahajan and N. P. Mahajan, J. Cell. Physiol. 227, 3178 (2012).
T. Jiang and Y. Qiu, J. Biol. Chem. 278 (18), 15 789 (2003).
G. Pagès, S. Guérin, D. Grall, et al., Science 286, 1374 (1999).
G. A. Smolen, J. Zhang, M. J. Zubrowski, et al., Genes Dev. 24, 2654 (2010).
Y. Keshet and R. Seger, Methods Mol. Biol. 661, 3 (2010).
W. S. Chen, C. C. Chen, L. L. Chen, et al., J. Biol. Chem. 288 (13), 9001 (2013).
A. Cuadrado and A. R. Nebreda, Biochem. J. 429 (3), 403 (2010).
F. Mercurio, H. Zhu, B. W. Murray, et al., Science 278 (5339), 860 (1997).
X. Zhao and J. L. Guan, Adv. Drug Deliv. Rev. 63 (8), 610 (2011).
L. A. Cary, J. F. Chang, and J. L. Guan, J. Cell Sci. 109 (Pt 7), 1787 (1996).
M. D. Haskell, A. L. Nickles, J. M. Agati, et al., J. Cell Sci. 114, 699 (2001).
K. Kimura, M. Ito, M. Amano, et al., Science 273, 245 (1996).
N. Rath and M. F. Olson, EMBO Rep. 13 (10), 900 (2012).
A. Daoud, U. Gopal, J. Kaur, and J. S. Isaacs, Oncotarget 8 (63), 106 807 (2017).
S. Löffek, O. Schilling, and C. W. Franzke, Eur. Respir. J. 38 (1), 191 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. N. Shipounova
Abbreviations: Hsp90, heat shock protein 90; eHsp90, extracellular Hsp90; LRP1, low density lipoprotein receptor-related protein 1; HER2, human epidermal growth factor receptor 2; PET, polyethylene terephthalate, PBS, phosphate-buffered saline; EGFR, Epidermal growth factor receptor; FBS, fetal bovine serum; BSA, bovine serum albumin; OD, optical density; PI3K, phosphoinositide 3-kinase, MAPK, mitogen-activated protein kinases; ERK, extracellular signal-regulated kinases; JNK, c-Jun N-terminal kinases; IKK, IκB kinase; FAK, Focal adhesion kinase.
Rights and permissions
About this article
Cite this article
Snigireva, A.V., Vrublevskaya, V.V., Zhmurina, M.A. et al. The Mechanisms of Stimulation of Migration and Invasion of Tumor Cells by Extracellular Heat Shock Protein 90 (eHsp90) in vitro. BIOPHYSICS 63, 931–939 (2018). https://doi.org/10.1134/S0006350918060258
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350918060258