Abstract
Insulin-like growth factors 1 and 2 (IGF-1 and IGF-2) play a key role in the maintenance of the nervous tissue viability. IGF-1 and IGF-2 exhibit the neuroprotective effects by stimulating migration and proliferation of nervous cells, activating cellular metabolism, inducing regeneration of damaged cells, and regulating various stages of prenatal and postnatal development of the nervous system. The availability of IGFs for the cells is controlled via their interaction with the IGF-binding proteins (IGFBPs) that inhibit their activity. On the contrary, the cleavage of IGFBPs by specific proteases leads to the IGF release and activation of its cellular effects. The viability of neurons in the nervous tissue is controlled by a complex system of trophic factors secreted by auxiliary glial cells. The main source of IGF for the neurons are astrocytes. IGFs can accumulate as an extracellular free ligand near the neuronal membranes as a result of proteolytic degradation of IGFBPs by proteases secreted by astrocytes. This mechanism promotes interaction of IGFs with their genuine receptors and triggers intracellular signaling cascades. Therefore, the release of IGF by proteolytic cleavage of IGFBPs is an important mechanism of neuronal protection. This review summarizes the published data on the role of IGFs and IGFBPs as the key players in the neuroprotective regulation with a special focus on the specific proteolysis of IGFBPs as a mechanism for the regulation of IGF bioavailability and viability of neurons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Insulin-like growth factor (IGF)-dependent signaling regulates cell growth, proliferation, viability and more. This signaling takes place in every organ, system, and tissue. IGF signaling controls cell motility, including cell migration and proliferation and triggers the mechanisms that stimulate cell growth and development, maintain cell survival, influence cell metabolism, and provide the neuroprotective and cardioprotective effects (Fig. 1) [1].
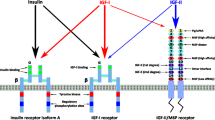
In eukaryotes, IGF signaling involves several ligand-receptor subsystems with partially cross talking pathways , including six IGF-binding proteins (IGFBPs) that can undergo protease-specific cleavage (Fig. 2, a and b). Among the ligands are insulin interacting with its genuine receptor (insulin receptor, IR), IGF-1 that displays a high-affinity to both IGF-1R (IFG-1 receptor) and IR/IGF-1R hybrid receptor; IGF-2 that binds to IFG-2R (IGF-2 receptor) as well as mannose 6-phosphate/IGF-2 receptor [2].
IGF signaling. a) Main ligands and receptors; high- and low-affinity ligand–receptor interactions are shown with solid and dashed lines, respectively. b) Mechanism of IGF release from the complex with IGFBP. IGFBP 1-6 interact with IGF-1 or IGF-2 resulting in the decreased IGF availability to the cells. Proteases specifically cleave IGFBPs in the complexes with IGF, leading to the IGF release and activation of IGF cellular effects.
Components of the IGF signaling are expressed virtually in all organs and tissues. In most tissues, expression of IGF signaling proteins in embryogenesis is significantly higher than in an adult organism [3].
At the whole-body level, IGF-1 and IGF-2 (also known as somatomedins C and A, respectively) mediate the tissue-related effects of the growth hormone (GH, somatotropin). GH induces hepatic expression of the IGF-1 gene, thus triggering its activity. In embryogenesis, IGF activates fetal growth, especially of the bone and muscle tissues, mammary glands, and prostate [4]. The concentration of IGF-1 in the mother’s serum increases along with the fetal growth and development; both IGF-1 mRNA and protein have been found at various stages of gestation, starting from preimplantation to the fully developed fetus. Postnatally, IGF mediates tissue growth and remodeling. For example, IGF signaling plays a crucial role in the development of reproductive system, in particular, oogenesis, sex determination, normal testicular function and spermatogenesis, ovulation, production of steroids, follicle viability, and placenta formation [5]. IGF signaling is also crucial for the development of skeletal muscle tissue. It was found that mutations in the nucleotide sequences of the IGF-1 and IGF-2 genes result in the retardation of the skeletal muscle growth and development [6]. The deficit of IGF negatively affects the nervous system development. Children with the IGF-1 insufficiency displayed various cranio-facial abnormalities due to the GH insensitivity, although their cognitive abilities were similar to the children of the same age [7, 8]. Children with reduced paracrine and autocrine activities of IGF-1 caused by mutations in the IGF-1 or IGF-1R genes suffered from microcephaly and demonstrated decreased cognitive abilities [9, 10].
At the cellular level, IGF-1 and IGF-2 activate cell migration, proliferation, differentiation, and angiogenesis. It was demonstrated that during the myoblast-to-myocyte cell differentiation, IGFs trigger both cell proliferation and differentiation simultaneously, whereas in osteoclasts, chondrocytes, and neural cells, IGFs induce only the process of cell differentiation [6-13]. IGF promotes cell survival and exerts the anti-apoptotic effect in various cell lines [14-17].
IGF signaling regulates multiple metabolic pathways. IGFs activate protein production and inhibit protein proteolytic degradation, resulting in the stimulation of cell growth [18]. In vivo and in vitro studies have shown that IGF-1 promotes the glucose uptake by the cells, thus contributing to the maintenance of a stable glucose level inside and outside the cell [19]. Moreover, IGFs activate metabolism of free fatty acids, downregulate secretion of insulin and GH, and enhance cell sensitivity to insulin. Low IGF-1 levels in the serum are associated with the development of insulin resistance [20].
IGF-1 and IGF-2 are involved in the development or suppression of various pathological conditions. Multiple studies have demonstrated that IGFs play a crucial role in carcinogenesis; for instance, both IGF-1 and IGF-2 promote proliferation of cancer cells [21]. High IGF-1 levels correlate with the development of pancreatic, breast, hepatic, and colorectal cancers [22-25]. The risk of developing gastrointestinal cancer in patients with acromegaly (disorder associated with excessive GH production) is two times higher than in healthy individuals [26]. In some cancer types, IGF-1 is expressed in malignant tissues during carcinogenesis and promotes the viability of cancer cells. Moreover, IGF-1 and IGF-2 can activate tumor metastasis [21].
On the contrary, IGFs prevent the development of some pathological conditions in case of cardiovascular and neurodegenerative diseases. As an agent increasing cell sensitivity to insulin, IGF-1 is used to treat the patients with severe insulin resistance in type 1 and type 2 diabetes, resulting in the improved glycemic control, lowered blood glucose, and increased insulin sensitivity [27]. Several studies have shown that IGF-1 and IGF-2 exert the cardioprotective and atheroprotective effects on the cardiac tissue [28-30]. Reduced IGF-1 serum levels correlate with the development of coronary heart disease and stroke [28]. Currently, IGF-1 is considered as a promising therapeutic agent for the treatment of cardiovascular diseases. IGF-1 and IGF-2 promote the survival of nervous tissue cells, in particular, neurons [31]; their neuroprotective effect was demonstrated in the Huntington’s [32] and Alzheimer’s [33] diseases.
Therefore, IGF signaling influences multiple processes at the cellular, tissue, and organ levels. In this review, we summarize the data on the role of IGFs and IGFBPs in the nervous system development and homeostasis. In addition, we present at a new angle the mechanism of IGF release via specific proteolysis of IGFBPs that promotes the activation of motility and increases viability of nervous tissue cells, in particular, neurons.
THE ROLE OF IGF SIGNALING IN THE NERVOUS TISSUE
IGF signaling in embryonic and adult nervous system. During embryogenesis, the genes for IGF-I and IGF-2 and their genuine receptors IGF-1 receptor (IGF-1R) and mannose 6-phosphate/IGF-2 receptor (M6P/IGF-2R), respectively, are expressed at different levels in various brain areas. IGF-1 expression is observed in all regions of the central nervous system, in both neurons and all types of glial cells. High brain levels of IGF correlate with proliferation of neural precursors. The highest IGF-1 expression occurs at the early postnatal stage followed by its decline to a lower level which is then maintained during the lifetime. Frequently, the maximal IGF-1 expression is observed in the regions with active proliferation, differentiation, and growth of nerve cells. IGF-1 and IGF-1R are typically detected in a close vicinity to each other, thereby confirming the idea that IGF-1 serves as a paracrine and autocrine regulator in brain development [34, 35].
The highest IGF-2 expression in the nervous tissue occurs during embryogenesis. In adult brain, IGF-2 is expressed in the meninges and choroid plexuses [34]. Expression of the IGF-2 gene in rat hypothalamus, hippocampus, and cerebellum can be activated by intravenous administration of GH or somatotropin-releasing hormone [36].
IGF-1R and insulin receptor (IR), which interacts with IGFs, have been found in multiple brain areas. Expression of IGF-1R and IR is downregulated in the postnatal period compared to embryonic period [37].
The expression patterns of IGFBPs in the nervous system are different. For instance, IGFBP-1 is not normally found in the brain, but under certain pathologies, e.g., glioblastoma, its expression can be elevated [38]. IGFBP-2 and IGFBP-5 are among most abundant brain proteins of the IGFBP family. The highest expression of IGFBP-2 was found in cortical astrocytes [39], presumably, due to the activation of NMDA receptors [40]. IGFBP-4 was detected in various brain areas during embryogenesis and postnatal period as a protein involved in the maintenance of cerebellar plasticity [41]. IGFBP-3 is not expressed in the nervous tissue, but can pass through the blood–brain barrier [42].
During rat embryogenesis, mRNA of IGF-1 is found in the peripheral nervous system and such areas of central nervous system as cervical-thoracic spinal cord, and epithelium of the ventral plate of the conus medullaris [43, 44]. IGF-1 and IGF-2 are expressed in the neural target areas, including groups of limb progenitor cells, but not in developing peripheral nerves [45]. IGF-1 and IGF-1R were found in neurons of developing trigeminal ganglia [43] and related axonal target areas in the face skin [46]. IGF-2 is predominantly expressed in the brain stem cells and neurons of the ventral plate [47]. In mice, IGF-2 is expressed in the neural crest and its derivatives [48], including cranial sensory, dorsal root, and sympathetic ganglia, as well as in the adrenal medulla [49]. In adulthood, IGF-1 is found in the ventral horn, sympathetic and dorsal root ganglia, and in the axons and Schwann cells of the sciatic nerve [50].
IGF signaling plays a crucial role in the development of nervous system, cell homeostasis maintenance, adaptation to stress factors, and neuroplasticity. Below, we describe various aspects related to the activity of IGF signaling components in the nervous system (see also Fig. 3) [34, 35, 42].
IGF signaling and neurogenesis. IGF-1 promotes neurogenesis starting from week three of gestation, when neural stem cells begin to proliferate, migrate from the subventricular zone, and undergo differentiation with the production of neurotransmitters and neurotrophic factors. IGF-1 activates these processes, as well as the events resulting in the formation of synaptic contacts (axons and dendrites). In the postnatal period, IGF-1 continues to exert its stimulatory effect on neurogenesis, as neurogenesis occurs during the lifetime in certain brain areas, e.g., dentate gyrus of the hippocampus (important for learning and memory), subventricular zone (experiments in mice showed that cells migrate to the olfactory bulb), and striatum (voluntary motor control) [51].
IGF-1 activates proliferation, migration, and maturation of glial cell (oligodendrocytes, astrocytes, and microglia) through the juvenile period and maintains the viability of glial progenitor cells in the adult brain. IGF-1 also promotes differentiation of glial cells in response to tissue damage [52]. Local paracrine/autocrine IGF-1 sources are essential for the normal development of the entire nervous system. Children with reduced endocrine IGF-1 levels due to the GH insensitivity typically display normal cognitive abilities despite craniofacial abnormalities. At the same time children with the IGF-1 gene deletion or mutations in the IGF-1R gene and, therefore, reduced paracrine/autocrine activity of IGF-1, suffer from microcephaly and cognitive impairments [53]. The endocrine activity of IGF also plays an important role in the development of the nervous system. In preterm infants, the levels of circulating IGF-1 and IGFBP-3 correlate with the brain volume in the postnatal period [54].
Less is known about the IGF-2 role in neurodevelopment. It was found that hypermethylation of maternally imprinted IGF-2 gene is a potential risk factor for the development of the neural tube defects [55].
Overexpression/deficit of IGF signaling components in the neural cells. Overexpression of the IGF-1 in mice resulted in the increased number of brain cells and, therefore, brain enlargement [56], whereas the knockout of the IGF-1 gene caused an impaired somatic and dendritic growth of neurons [57]. Neither overexpression, nor the knockout of the IGF-2 gene caused any noticeable effect on the developing brain [58, 59]. The knockout of the IGF-1R gene led to the developmental disorders in multiple organs, including the brain, which was manifested in the underdevelopment of nerve fibers, as well as reduction in the volume and increase in the number of neurons [60]. The knockout of the IR gene had no profound effect on the nervous system functioning [61]. Overexpression of IGF-1 in mice revealed that IGF-1 increased the number of neurons by promoting proliferation and suppressing apoptosis of these cells and stimulated synaptogenesis during the development of the nervous system [62]. IGF-1 signaling is interrelated with the pathways triggered by other factors involved in the nervous system functioning. Among these factors are neurotransmitters, transcription factors regulating neurogenesis, and growth factors, e.g., fibroblast growth factor (FGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and brain-derived neurotrophic factors (BDNFs), which together support proliferation of neural stem cells [63].
In many cases, alterations in the expression of IGFBP genes do not lead to the impaired brain development; however, there are exceptions. Overexpression of the IGFBP-1 gene in the brain leads to the delayed brain development and decreased proliferation of glial cell in response to traumatic injury [64]. Overexpression of the IGFBP-5 gene inhibits brain growth [65]. Overexpression of the IGFBP-6 gene in the brain results in the delayed development of cerebellar structures [66].
Therefore, IGFs and their regulation by IGFBPs are essential factors in the organism development during embryogenesis, postnatal period, and adulthood. The action of IGF-1 and IGF-2 on the central and peripheral nervous systems development is related to various processes, such as cell migration, proliferation, and differentiation, as well as the maintenance of the viability of different types of nervous tissue cells.
EFFECT OF IGFs ON THE MOTILITY AND SURVIVAL OF NEURAL CELLS
Neurons. Axonal guidance and growth. IGF-1 specifically affects the axonal guidance in the olfactory neurons. Both IGF-1 and IGF-1R are expressed in the developing olfactory bulb during embryogenesis. IGF-1 was found to act as a chemoattractant in the primary cultures of olfactory and cerebellar granule neurons. In vitro experiments on the growth cone turning demonstrated that the growth cones of both olfactory and cerebellar granule neurons showed a marked positive motility along the IGF-1 gradient. Expression of IGF-1 and IGF-2 in the neural crest cells and neural target areas suggested the importance of these cytokines in the neuronal migration, axonal guidance, and related neurotrophic support [67]. Axonal growth, which is important in the neural development, especially for the peripheral motor and sensory neurons, involves IGF-1 signaling via IGF-1R. Apart from stimulating axonal growth, IGF-1 initiated cell motility in the two neuroblastoma cell lines: neuronal SH-SY5Y cells and SHEP Schwann cells [68].
Neurons of chick dorsal root ganglia exposed to IGF-1 and grown in the medium supplemented with 2% fetal calf serum have not only survived but also demonstrated accelerated axonal growth [69]. Moreover, axonal growth of the myenteric plexus neurons was activated by both IGF-1 and IGF-2 [70]. On the other hand, Kimpinski and Mearow [71] demonstrated that IGF-1 affected the axonal growth in a culture of adult rat cells of the dorsal root ganglia. The authors compared the action of IGF-1 with the action of EGF, FGF, and nerve growth factor (NGF). It was found that the effect of IGF-1 on the axonal growth was similar to that exerted by NGF. In addition, IGF-1 promoted axonal growth in the corticospinal motor and vestibulospinal neurons [71]. IGF-1 and IGF-2 stimulated the branching of dendrites and increased the density and stability of dendritic spines [35].
Repair of peripheral nerve damage and tissue regeneration. IGF expression correlates with regeneration after peripheral nerve injury. IGF-1 was detected in the axons and Schwann cells of the sciatic nerve; in the case of nerve damage, IGF-1 accumulated in the injured axons within 2 h after the trauma [72]. In rats, the level of IGF-1 at site of injury reached its maximum two weeks after sciatic nerve dissection. Moreover, IGF-1 contributed to the elongation of regenerating axons [73].
Astrocytes. Astrocytes constitute the majority of brain cells (in mammals, an average astrocyte/neuron ratio is 10/1). The body of a neuron, except its synaptic contacts, is fully covered with the membranes of astrocytes. Astrocytes produce a great diversity of neuroprotective molecules; the neuron–astrocyte dyad represents a system composed of the functional cell and its own neuroprotector. Astrocytes ensure physical and nutritive support for neurons, maintain the blood–brain barrier, and modulate synaptic transmission. It is not surprising that components of the IGF signaling are not only found in astrocytes, but also play an important role in maintaining the functional activity of the nervous tissue in general. IGF-1 activates astrocyte proliferation, affects expression of the cytoskeletal proteins (e.g., glial acidic protein and vimentin), promotes the activity of nuclear and mitochondrial enzymes, stimulates production of growth factors (erythropoietin), upregulates expression of gap junction proteins (connexin-43), regulates glutamate influx and intracellular signaling, and activates glucose metabolism [74].
Oligodendrocytes and microglial cells. Oligodendrocyte differentiation is related to the upregulation of myelin expression and production of trophic factors necessary for the neuronal survival and axonal integrity. IGF-1 promotes differentiation of oligodendrocyte precursors cell and, therefore, myelination of axons. There is a solid evidence showing that IGFs are involved in oligodendrocyte differentiation and survival, myelin production, and Schwann cell survival and motility. IGF-1 expression in the microglia after epileptic seizures is elevated and may play a role in the minimization of cell damage [75].
Therefore, IGFs are essential in the development and homeostasis of the nervous tissue. The effects of IGF-1 and IGF-2 on cells are mediated by their binding to the genuine receptors. The bioavailability of IGF-1 and IGF-2 for receptors is mainly regulated by two mechanisms: either by interaction with IGFBPs (which prevent IGF binding to the receptors) or by IGFBP proteolysis by specific proteases (that results in the IGF release and related biological effects). In the next section of the review, we describe the structural features of IGFs and IGFBPs, as well as the major mechanism providing IGF-1 and IGF-2 bioavailability via proteolytic degradation of IGFBPs in the nervous tissue in details.
IGFs AND IGFBPs: STRUCTURE AND FUNCTIONS
IGFs. IGF-1 and IGF-2 belong to the family of regulatory proteins that also includes insulin, relaxin, and some invertebrate hormones [76, 77]. Structurally and functionally, IGFs resemble insulin. IGFs and insulin activate similar signaling pathways, but their overall effects are different. Insulin mainly regulates cell metabolism, whereas IGFs induce cell growth, development, and differentiation [78].
IGF-1 is a 70-amino acid polypeptide (7649 Da) that contains three disulfide bonds [79]; IGF-2 molecule consists of 67 amino acids (7471 Da) [80]. The homology between IGF-1 and IGF-2 is 62%, whereas the homology between IGFs and insulin is only 50% [80].
The IGF molecule consists of the A, B, C, and D domains (Fig. 4). The 3D structure of IGFs is similar to that of insulin: like insulin, IGFs contains three α-helices (one in the B domain, and two in the A domain). Other amino acids in the primary sequences of IGF-1 and IGF-2 do not form secondary structure elements [81]. The A domain consists of the second and third α-helices, whereas the B-domain is made up of the first α-helix (counting from the N-terminus). The A and B domains of IGFs are homologous to the A and B chains of insulin, respectively, and contain a large number of highly conserved amino acid substitutions accounting for the similarity of their spatial structures. The IGF molecules also have the C and D domains absent in proinsulin [79, 80] (Fig. 4). Despite the high structural homology, IGF-1 and IGF-2 differ significantly in the C-terminal parts of their molecules, as well as in the region located near the start of the third α-helix, which might explain a higher affinity of IGF-2R to IGF-2 than to IGF-1 [81].
Alignment of IGF-1, IGF-2, and insulin sequences. Amino acids identical or similar between all three proteins are highlighted in light red, between IGFs – in blue, between IGF-1 and insulin – in green, between IGF-2 and insulin – in yellow; non-overlapping or lacking residues are highlighted in gray and depicted by dots, respectively. Residue numbering (A1-A21 and B1-B30) corresponds to the positions of amino acid residues in insulin A- and B-chains, respectively. Bottom panel, 3D- structures of IGF-1, IGF-2, and insulin (PDB structures 2C8R, 1GZR, 2L29) are shown. A, D, B, and C are depicted in red, yellow, cian blue, and green, respectively (adapted from [82]).
IGF binding proteins (IGFBPs). The key feature of the IGFBP family proteins is the ability for a specific high-affinity binding of IGFs, which mostly inhibits the biological effects of the latter [83]. However, in some cases, the binding to IGFBP may promote the action of IGF [84]. The inhibitory effect of IGFBPs may be based on the fact that the IGF binding to IGFBP creates steric difficulties for the IGF binding to the genuine receptor, whereas the mechanisms underlying the IGFBP-mediated activation of IGFs remain unknown [85]. Seven IGF-binding proteins (IGFBP1-7) with molecular weights ranging from 22.8 up to 31.3 kDa (216-289 amino acids) have been identified [86]. All IGFBPs have a common structure and consist of three domains of a similar size: highly conserved N- and C-terminal domains and the linker L-domain, whose structure differs in different IGFBPs.
All IGFBPs form high-affinity binary complexes with IGFs [83] through the N- and C-terminal domains. The L-domain is not involved in the direct interaction with IGFs and contains sites for glycosylation, phosphorylation, and protease-specific cleavage (Fig. 5). Compared to the full-sized proteins, the N- or C-terminal domains interact with IGFs with a much lower affinity. For instance, the affinity of the N-terminal fragment to IGFs is 10 to 1000-fold lower compared to IGFBPs [87]. The L-domain interacts with the cell surface, heparin, and IGFALS protein (insulin-like growth factor-binding protein complex acid labile subunit) [83].
Posttranslational modifications and adhesion to the extracellular matrix (ECM) components may affect the affinity of IGFBP binding to IGFs, thus impacting the availability of IGF to the cells. A large portion of circulating IGFs exists in the IGFBP-bound state, which affects the stability of the serum IGF. The most stable are the ternary complexes composed of IGF, IGFBP, and IGFALS [88, 89]. The N-terminal domains in all IGFBPs except IGFBP-6, contain 6 disulfide bonds (IGFBP-6 has 5 disulfide bonds) and conserved GCGCC motif (IGFBP-6 lacks two adjacent cysteine residues in this motif) [85, 90]. The N-terminal domain also contains a high-affinity IGF-binding site consisting of three-stranded β-sheet stabilized by two disulfide bonds [91-93]. As exemplified by IGFBP-6, the structure of the C-terminal domain is similar to that of thyroglobulin type-1 fold and comprises an α-helix, three-stranded β-sheet, and three loops. The first loop is between the α-helix and β-sheet; the second loop separates the strands in the β-sheet, and the third loop is located after the β-sheet. It is assumed that the C-terminal domain accounts for multiple IGF-independent effects of IGFBPs. For instance, the C-terminal domains of IGFBP-1 and IGFBP-2 contain the integrin-binding motif [94]; the C-terminal domains of IGFBP-3 and IGFBP-5 contain the motifs enriched in basic amino acids that bind heparin, IGFALS [95, 96], plasminogen activator-1 inhibitor [97], and transferrin, participate in the association with cell surface and ECM [98], and are responsible for the IGFBP nuclear import [99].
IGFBP-1 (molecular weight, 28 kDa [100]) interacts with IGF-1 and IGF-2 with similar affinity [101]. IGFBP-1 phosphorylation at serine residues in the linker (Ser101, Ser119) and C-terminal domain (Ser169) increases its affinity to IGF-1 [102]. Ser101 is the major phosphorylation site in IGFBP-1 [102]. IGFBP-1 phosphorylation promotes protein interaction with IGF-1, whereas dephosphorylation, on the contrary, activates IGF-1 release from the complex [103]. The observed relationship between the casein kinase 2 activation and activity of the mTOR pathway in the case of nutrient deficit results in the increased level of IGFBP-1 phosphorylation, lower IGF bioavailability, and intrauterine growth retardation [104]. High IGFBP-1 expression levels and high serum concentration of this protein were observed in embryogenesis and early childhood, whereas in the adulthood, both parameters decline [105].
IGFBP-2. According to the amino acid sequence, the molecular weight of IGFBP-2 is 31.3 kDa [94]. IGFBP-2 interacts with IGF-2 with a 10 to 20-fold higher affinity than with IGF-1 [101]. Similar to IGFBP-1, the C-terminal domain of IGFBP-2 contains the Arg-Glu-Asp motif responsible for the binding to the cell surface integrins [94]. The linker domain of IGFBP-2 contains the heparin-binding motif PKKLRP [106] presumably involved in IGFBP-2 binding to the cell surface glycosaminoglycans [107]. Interactions between IGFBP-2 and ECM components ensure cell proliferation and migration induced by the IGFBP-2 nuclear translocation [108]. It was shown in vitro that IGFBP-2 interaction with glycosaminoglycans decreases its affinity to IGF-1 approximately three times, which facilitates the binding of IGF-1 to its genuine receptor [107]. The heparin-binding motif mediates the IGFBP-2 interaction with the receptor tyrosine phosphatase-β (RPTPβ) [109]. Moreover, the C-terminal domain of IGFBP-2 also contains another heparin-binding motif [110]. The linker domain of IGFBP-2 includes the nuclear localization signal (NLS) 179PKKLRPP185 that ensures interaction between IGFBP-2 and α-importin, followed by the nuclear import of the former. After entering the nucleus, IGFBP-2 upregulates VEGF expression, thereby stimulating angiogenesis [111]. IGFBP-2 contains no glycosylation sites [112], and no IGFBP-2 phosphorylation was observed despite the presence of potential phosphorylation site in its sequence [113]. In the nervous system, IGFBP-2 plays an important role in the regulation of the IGF activity (see “IGFBP proteolysis in the nervous system” below) [114].
IGFBP-3. The majority of circulating IGF-1 and IGF-2 molecules are found in the high-molecular-weight complexes (apparent molecular weight, ~150 kDa) also including IGFBP-3 and IGFALS (85 kDa). The stability of IGF within these complexes is substantially higher than that of the free IGF or in the binary complexes with IGFBPs [88]. IGFBP-3 is the only IGFBP isoform capable of transporting IGFs in the content of the ternary complexes [83]. An apparent molecular weight for IGFBP-3 is ~50 kDa. In the plasma, IGFBP-3 circulates as a glycoprotein [115].
IGFBP-3 binds IGF-1 and IGF-2 with approximately equal affinities [115]. The N-terminal domain of IGFBP-3 contains phosphorylation [116] and glycosylation [112] sites. IGFBP-3 may either inhibit or facilitate the IGF activity [117]. Due to the presence of NLS in the C-terminal domain, IGFBP-3 is translocated to the nucleus via the importin-β-dependent pathway [99]. In the nucleus, IGFBP-3 interacts with receptors (RXR-α, PPAR-γ, vitamin D receptor, Nur77), thus affecting cell apoptosis, proliferation, and differentiation [118]. IGFBP-3 phosphorylation by the dsDNA-dependent kinase (DNA-PK) promotes its nuclear accumulation and interaction with nuclear components. At the same time, IGFBP-3 phosphorylation decreases its affinity to IGF-1, which can result in the IGF-1 release in the nucleus [119]. IGFBP-3 interaction with DNA-PK and nuclear EGF receptor activates cell response to the DNA-damaging agents [120]. The C-terminal domains of IGFBP-3 and IGFBP-5 contain the heparin-binding motif accounting for the binding of these proteins to the cell surface [98]. It is possible that IGFBP-3 and IGFBP-5 also bind to fibrinogen and fibrin via the heparin-binding motif, which may provide IGF-1 accumulation at the sites of mechanical tissue damage [121]. The heparin-binding motif participates in the IGFBP-3 binding to plasminogen [122]. At the same time, IGFBP-3 is cleaved by plasmin [123]. IGFBP-3 also binds to fibronectin [124]. When IGFBP-3 attaches to the cell surface, its affinity to IGF-1 decreases tenfold, which may account for the IGFBP-3-mediated stimulation of IGF-1 activity [125]. In vitro, IGFBP-3 inhibits the IGF-dependent cell proliferation and maintenance of cell viability, i.e., the processes leading to carcinogenesis [126]. IGFBP-3 can either suppress [127] or activate angiogenesis [128].
IGFBP-4. IGFBP-4 is the smallest protein among IGFBPs (molecular weight, 25 kDa; 237 amino acid residues). Both non-glycosylated and glycosylated IGFBP-4 was found in the human serum (molecular weight of the glycosylated protein is slightly above 30 kDa) [129]. IGFBP-4 can be N-glycosylated at Asn104 in the C-terminal domain [130, 131], although this modification does not affect its binding to IGF [132]. Compared to other IGFBPs that contain 18 cysteine residues, IGFBP-4 has two additional cysteines in the linker domain [129, 132]. The affinity of IGFBP-4 to IGF-2 (dissociation constant KD, 0.2 nM) exceeds by more than an order of magnitude its affinity to IGF-1 (KD, 4.5 nM) [133]. IGFBP-4 has a single binding site for IGF-1 and IGF-2 [134]. IGFBP-4 is cleaved by PAPP-A between Met135 and Lys136 in the linker domain, resulting in the increased IGF bioavailability and manifestation of its effects [135]. PAPP-A-specific IGFBP-4 proteolysis is IGF-dependent. It was suggested that the binding of IGF (especially, IGF-2) makes IGFBP-4 a preferable target for PAPP-A [136]. Apart from PAPP-A, IGFBP-4 can be cleaved by matrix metalloproteinases, e.g., MMP-2, MMP-7, and MMP-9 [137]. IGFBP-4 mainly inhibits the IGF-1/IGF-2 activity, but can also facilitate it.
IGFBP-5. IGFBP-5 has a molecular weight of 28.5 kDa [138]. About half of the circulating IGFBP-5 is found in the ternary complexes with IGFALS and IGF-1/IGF-2. The remaining protein exists either as a binary complex with IGF-1 or IGF-2 or in a free form [83]. The major IGFALS-binding site in IGFBP-5 is located in the C-terminal domain and contains basic amino acid residues [139]. The central domain of IGFBP-5 contains another IGFALS-binding site [140]. The IGFBP-5 affinity to IGF-2 compared to IGF-1 is 3 to 10-fold higher [129]. The adhesion of IGFBP-5 to the extracellular matrix decreases 7-fold its affinity to IGF-1. The extracellular matrix components inhibit the IGFBP-5 proteolysis. Thus, IGFBP-5 binding to the ECM makes the accumulation of IGF-1 on the cell surface possible and promotes its interaction with the cell receptors [141, 142]. IGFBP-5 binds with the ECM components, such as glycosaminoglycans in some proteoglycans, type III and IV collagens, laminin, fibronectin, plasminogen activator inhibitor I, thrombospondin, and osteopontin [97, 141, 143]. The linker and the C-terminal domain of IGFBP-5 contain conserved motifs composed of basic amino acid residues that ensure the binding between IGFBP-5 and glycosaminoglycans, in particular, heparin. The binding of IGFBP-5 to heparin lowers 17-fold its affinity to IGF-1 [144]. Phosphorylation and O-glycosylation of residues in the IGFBP-5 linker domain reduces the efficiency of IGFBP-5 binding to heparin, but does not affect its interaction with IGF and IGFALS [145]. The C-terminal domain of IGFBP-5 contains the NLS sequence that accounts for the IGFBP-5 nuclear import via the importin-β-dependent pathway [99, 146]. In the nucleus, IGFBP-5 affects gene expression via the N-terminal region [147]. IGFBP-5 interacts with the nuclear protein FHL2 involved in the activation of transcription of certain genes, as well as with the nucleolar protein nucleolin. Regardless of the presence of IGF, metalloproteases PAPP-A and PAPP-A2 cleave IGFBP-5 at a single specific site in the linker domain [148].
IGFBP-6. IGFBP-6 is a glycoprotein with a molecular weight of 34 kDa [149]; the “protein part” of IGFBP-6 corresponds to 22.847 kDa. The affinity of IGFBP-6 for IGF-2 exceeds its affinity for IGF-1 from 10 to 100 times [150]. As mentioned earlier, the structure of the N-terminal domain of IGFBP-6 differs from that found in other IGFBPs. IGFBP-6 suppresses the IGF-2-induced cell proliferation, vital activity, and metabolism in some cell lines [151, 152], and acts as a relatively specific inhibitor of IGF-2 effects [153]. The linker of IGFBP-6 can be O-glycosylated, resulting in the inhibition of IGFBP-6 binding to glycosaminoglycans and cell membranes, as well as IGFBP-6 proteolysis, thus sustaining the inhibitory effect of IGFBP-6 on IGF-2. However, glycosylation per se does not affect the IGF-2 binding to IGFBP-6 [154]. IGFBP-6 undergoes proteolysis by the acid-activated cathepsin D-like protease that cleaves both IGFBP-6 and IGFBP-3 [155]. All IGFBPs are hydrolyzed by MMP-7 to ensure IGF availability to IGF-1R [156]. IGFBP-6 is also cleaved by MMP-9 and MMP-12, which might result in the increased IGF-1 bioavailability to IGF-1R and abolish the inhibitory effect of IGFBP-6 on oligodendrocyte maturation [157]. Moreover, IGFBP-6 is hydrolyzed by MMP-2, presumably resulting in the activation of angiogenesis [158].
IGFBP PROTEOLYSIS AS A MECHANISM FOR THE REGULATION OF IGF BIOAVAILABILITY IN THE NERVOUS TISSUE
As mentioned above, proteolysis of IGFBP–IGF complexes ensures IGF bioavailability to the cell surface receptors and, therefore, is one of the key mechanisms that positively regulates cell migration and proliferation, thus contributing to the cell homeostasis. Since neurons are unable to maintain their own viability and rely on astrocytes secreting neurotrophic factors and IGFs, proteolytic degradation of IGFBPs by specific proteases contributes to the free IGF accumulation on the cell surface, that is especially important in nervous tissue.
Proteases cleaving IGFBPs. The cleavage of IGFBPs is protease-specific. For example, non-phosphorylated IGFBP-1 (but not its phosphorylated form) is cleaved by the protease secreted by decidual membrane cells [159]. IGFBP-3 is cleaved by plasminogen [123], PSA (prostate-specific antigen; serine protease of the kallikrein family isolated from the seminal fluid) [160], nerve growth factor displaying high structural similarity with PSA [161], and cathepsin-D-like protease [155]. PAPP-A (pregnancy-associated plasma protein A) specifically hydrolyzes IGFBP-4 [135]. Apart from PAPP-A, IGFBP-4 is cleaved by the matrix metalloproteinases MMP-2, MMP-7, and MMP-9 [137, 156]. PAPP-A and PAPP-A2 hydrolyze IGFBP-5 at a single site in the linker domain regardless of the presence of bound IGF [148]. PAPP-A also cleaves IGFBP-2 [162]. Similar to IGFBP-3, IGFBP-6 is cleaved by the acid-activated cathepsin D-like protease [155]. IGFBP-6 is also cleaved by MMP-2, which results in the IGF release and activation of angiogenesis [158]. All IGFBPs are hydrolyzed by MMP-7, thus ensuring IGF availability for IGF-1R [156]. By activating cell growth and viability, IGFBP proteolysis plays a crucial role in the tumor cell viability and tumor progression. Recent findings suggest that IGFBP-2 and IGFBP-4 proteolysis are particularly involved in carcinogenesis.
IGFBP proteolysis in the nervous system has been investigated over last years. We believe that it represents an important mechanism of promoting IGF release near the cell surface and its interaction with genuine receptor. Proteolysis of IGFBPs ensures IGF bioavailability, thus supporting the functioning of neural cells. It is especially important for the viability of neurons, because neurons require additional trophic factors to maintain their viability. The data on the proteolysis of IGFBPs in the nervous system suggest that (i) IGFs released due to the IGFBP proteolysis exert the neuroprotective activity, (ii) proteolysis occurs mainly on the astrocyte surface and involves specific proteases secreted by the astrocytes. Some examples of specific IGFBP proteolysis in the nervous system are described below. For instance, IGFBP-2 undergoes proteolysis in proliferating (but not in differentiating) astrocytes [114]. IGFBP-5, which is one of the most abundant IGFBPs in the brain, is hydrolyzed by the tissue kallikrein; brain regions expressing IGFBP-5 co-localize with the areas of kallikrein expression. Therefore, it is possible that kallikrein is involved in the local regulation of IGF bioavailability [163]. Fibroblast growth factor-2 (FGF2) activates IGFBP proteolysis by a serine protease in the extracellular fluid in the near-membrane pool of IGFBP-2, resulting in the increased bioavailability of IGFs for their genuine receptors [164]. Glial cells derived from mesenchymal stem cells secrete IGFBP-4 that increases the survival of neurons in the case of oxygen deficit and glucose starvation by regulating the extracellular levels of IGF-1 and IGF-2. These cells were also found to express PAPP-A [165], which cleaves IGFBPs at specific sites and increases the IGF bioavailability [135]. In the nervous tissue, IGFBP-6 is hydrolyzed by MMP-9 and MMP-12, which results in the elevated IGF-1 bioavailability for IGF-1R and abolishes the inhibitory effect of IGFBP-6 on the maturation of oligodendrocytes [157]. Moreover, in some cases, neurons intrinsically initiate IGFBP proteolysis. For instance, the electrical activity of neurons in some regions of the nervous system activates MMP-9, which cleaves IGFBP-3 with the release of IGF-1 [166].
Hence, the release of IGF resulting from the IGFBP proteolysis by astrocyte-secreted specific proteases and accumulation of free IGF near the neuronal membrane may play an important role in the neuronal homeostasis. In some cases, neurons themselves can initiate this process (Fig. 6).
The role of IGFBP proteolysis in the nervous system. Extracellular IGFs mainly exist as complexes with IGFBPs. Astrocytes secrete various proteases that cleave IGFBPs in the complexes with IGFs, thus increasing the IGF level near the membranes of neurons and astrocytes and promoting the IGF interaction with the cell receptors, which results in the triggering of the IGF signaling and corresponding cellular effects.
CONCLUSION
One of the physiological features of the nervous tissue is that the functioning of neurons critically depends on the cell environment, in particular, astrocytes that support the extracellular homeostasis via secretion and internalization of various trophic factors. Free extracellular IGFs in the nervous tissue can appear via (i) secretion by the nervous system cells and (ii) specific proteolysis of IGFBPs resulting in the IGF release. What is intriguing is that compared to the IGF secretion by neurons and astrocytes, proteolysis-mediated release of IGF mainly depends on astrocytes secreting specific proteases. Free IGFs interact with their genuine receptors and induce intracellular signaling cascades leading to the activation of multiple cell processes. How does such interplay between different types of cells occur? How do astrocytes “sense” that the level of IGF should be increased or decreased? These topics still remain the terra incognita. Taking into consideration the key role of insulin-like factors in the stimulation of neuronal cell growth and proliferation, as well as their neuroprotective effects, we are certain that further investigations of the IGF signaling in the nervous system will shed a light on the fundamental adaptive mechanisms of the nervous tissue and nervous system as a whole.
Abbreviations
- IGF:
-
insulin-like growth factor
- IGFALS:
-
insulin-like growth factor-binding protein complex acid labile subunit
- IGF-1R:
-
insulin-like growth factor 1 receptor
- IGFBP:
-
insulin-like growth factor binding protein
- IR:
-
insulin receptor
- MMP:
-
matrix metalloproteinase
- PAPP-A:
-
pregnancy-associated plasma protein A
References
Annunziata, M., Granata, R., and Ghigo, E. (2011) The IGF system, Acta Diabetol., 48, 1-9, https://doi.org/10.1007/s00592-010-0227-z.
Bach, L. A. (2004) The insulin-like growth factor system: towards clinical applications, Clin. Biochem. Rev., 25, 155-164.
Kiepe, D., Ciarmatori, S., Haarmann, A., and Tönshoff, B. (2006) Differential expression of IGF system components in proliferating vs. differentiating growth plate chondrocytes: the functional role of IGFBP-5, Am. J. Physiol. Endocrinol. Metab., 290, E363-371, https://doi.org/10.1152/ajpendo.00363.2005.
Le Roith, D. L. (2003) The insulin-like growth factor system, Exp. Diab. Res., 4, 205-212, https://doi.org/10.1155/EDR.2003.205.
Neirijnck, Y., Papaioannou, M. D., and Nef, S. (2019) The insulin/IGF system in mammalian sexual development and reproduction, Int. J. Mol. Sci., 20, E4440, https://doi.org/10.3390/ijms20184440.
Clemmons, D. R. (2009) Role of IGF-I in skeletal muscle mass maintenance, Trends Endocrinol. Metab., 20, 349-356, https://doi.org/10.1016/j.tem.2009.04.002.
Kornreich, L., Horev, G., Schwarz, M., Karmazyn, B., and Laron, Z. (2002) Craniofacial and brain abnormalities in Laron syndrome (primary growth hormone insensitivity), Eur. J. Endocrinol., 146, 499-503, https://doi.org/10.1530/eje.0.1460499.
Kranzler, J. H., Rosenbloom, A. L., Martinez, V., and Guevara-Aguirre, J. (1998) Normal intelligence with severe insulin-like growth factor I deficiency due to growth hormone receptor deficiency: a controlled study in a genetically homogeneous population, J. Clin. Endocrinol. Metab., 83, 1953-1958, https://doi.org/10.1210/jcem.83.6.4863.
Abuzzahab, M. J., Schneider, A., Goddard, A., Grigorescu, F., Lautier, C., Keller, E., Kiess, W., Klammt, J., Kratzsch, J., Osgood, D., Pfäffle, R., Raile, K., Seidel, B., Smith, R. J., Chernausek, S. D., and Intrauterine Growth Retardation (IUGR) Study Group (2003) IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation, N. Engl. J. Med., 349, 2211-2222, https://doi.org/10.1056/NEJMoa010107.
Woods, K. A., Camacho-Hübner, C., Savage, M. O., and Clark, A. J. (1996) Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene, N. Engl. J. Med., 335, 1363-1367, https://doi.org/10.1056/NEJM199610313351805.
Feng, J., and Meng, Z. (2021) Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway, Exp. Ther. Med., 22, 891, https://doi.org/10.3892/etm.2021.10323.
Zhang, X., Zhang, L., Cheng, X., Guo, Y., Sun, X., Chen, G., Li, H., Li, P., Lu, X., Tian, M., Qin, J., Zhou, H., and Jin, G. (2014) IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3K/Akt pathway, PLoS One, 9, e113801, https://doi.org/10.1371/journal.pone.0113801.
Zhou, Q., Li, B., Zhao, J., Pan, W., Xu, J., and Chen, S. (2016) IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo, In vitro Cell Dev. Biol. Anim., 52, 356-364, https://doi.org/10.1007/s11626-015-9969-9.
Beneit, N., Martín-Ventura, J. L., Rubio-Longás, C., Escribano, Ó., García-Gómez, G., Fernández, S., Sesti, G., Hribal, M. L., Egido, J., Gómez-Hernández, A., and Benito, M. (2018) Potential role of insulin receptor isoforms and IGF receptors in plaque instability of human and experimental atherosclerosis, Cardiovasc. Diabetol., 17, 31, https://doi.org/10.1186/s12933-018-0675-2.
Dupont, J., Fernandez, A. M., Glackin, C. A., Helman, L., and LeRoith, D. (2001) Insulin-like growth factor 1 (IGF-1)-induced twist expression is involved in the anti-apoptotic effects of the IGF-1 receptor, J. Biol. Chem., 276, 26699-26707, https://doi.org/10.1074/jbc.M102664200.
Gehmert, S., Sadat, S., Song, Y.-H., Yan, Y., and Alt, E. (2008) The anti-apoptotic effect of IGF-1 on tissue resident stem cells is mediated via PI3-kinase dependent secreted frizzled related protein 2 (Sfrp2) release, Biochem. Biophys. Res. Commun., 371, 752-755, https://doi.org/10.1016/j.bbrc.2008.04.151.
Han, Y., Wang, S., Wang, Y., and Zeng, S. (2019) IGF-1 inhibits apoptosis of porcine primary granulosa cell by targeting degradation of BimEL, Int. J. Mol. Sci., 20, E5356, https://doi.org/10.3390/ijms20215356.
LeRoith, D., and Yakar, S. (2007) Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1, Nat. Clin. Pract. Endocrinol. Metab., 3, 302-310, https://doi.org/10.1038/ncpendmet0427.
Saukkonen, T., Shojaee-Moradie, F., Williams, R. M., Amin, R., Yuen, K. C., Watts, A., Acerini, C. L., Umpleby, A. M., and Dunger, D. B. (2006) Effects of recombinant human IGF-I/IGF-binding protein-3 complex on glucose and glycerol metabolism in type 1 diabetes, Diabetes, 55, 2365-2370, https://doi.org/10.2337/db05-1646.
Ohlsson, C., Mohan, S., Sjögren, K., Tivesten, A., Isgaard, J., Isaksson, O., Jansson, J.-O., and Svensson, J. (2009) The role of liver-derived insulin-Like growth factor-I, Endocr. Rev., 30, 494-535, https://doi.org/10.1210/er.2009-0010.
Pollak, M. (2008) Insulin and insulin-like growth factor signalling in neoplasia, Nat. Rev. Cancer, 8, 915-928, https://doi.org/10.1038/nrc2536.
Christopoulos, P. F., Msaouel, P., and Koutsilieris, M. (2015) The role of the insulin-like growth factor-1 system in breast cancer, Mol. Cancer, 14, 43, https://doi.org/10.1186/s12943-015-0291-7.
Stuver, S. O., Kuper, H., Tzonou, A., Lagiou, P., Spanos, E., Hsieh, C. C., Mantzoros, C., and Trichopoulos, D. (2000) Insulin-like growth factor 1 in hepatocellular carcinoma and metastatic liver cancer in men, Int. J. Cancer, 87, 118-121, https://doi.org/10.1002/1097-0215(20000701)87:1<118::aid-ijc17>3.0.co;2-w.
Trajkovic-Arsic, M., Kalideris, E., and Siveke, J. T. (2013) The role of insulin and IGF system in pancreatic cancer, J. Mol. Endocrinol., 50, R67-74, https://doi.org/10.1530/JME-12-0259.
Vigneri, P. G., Tirrò, E., Pennisi, M. S., Massimino, M., Stella, S., Romano, C., and Manzella, L. (2015) The insulin/IGF system in colorectal cancer development and resistance to therapy, Front. Oncol., 5, https://doi.org/10.3389/fonc.2015.00230.
Jenkins, P. J. (2006) Cancers associated with acromegaly, Neuroendocrinology, 83, 218-223, https://doi.org/10.1159/000095531.
Ezzat, V. A., Duncan, E. R., Wheatcroft, S. B., and Kearney, M. T. (2008) The role of IGF-I and its binding proteins in the development of type 2 diabetes and cardiovascular disease, Diabetes Obes. Metab., 10, 198-211, https://doi.org/10.1111/j.1463-1326.2007.00709.x.
Colao, A. (2008) The GH-IGF-I axis and the cardiovascular system: clinical implications, Clin. Endocrinol. (Oxf), 69, 347-358, https://doi.org/10.1111/j.1365-2265.2008.03292.x.
Higashi, Y., Sukhanov, S., Anwar, A., Shai, S.-Y., and Delafontaine, P. (2010) IGF-1, oxidative stress and atheroprotection, Trends Endocrinol. Metab., 21, 245-254, https://doi.org/10.1016/j.tem.2009.12.005.
Suleiman, M.-S., Singh, R. J. R., and Stewart, C. E. H. (2007) Apoptosis and the cardiac action of insulin-like growth factor I, Pharmacol. Ther., 114, 278-294, https://doi.org/10.1016/j.pharmthera.2007.03.001.
Torres-Aleman, I. (2010) Toward a comprehensive neurobiology of IGF-I, Dev. Neurobiol., 70, 384-396, https://doi.org/10.1002/dneu.20778.
Humbert, S., Bryson, E. A., Cordelières, F. P., Connors, N. C., Datta, S. R., Finkbeiner, S., Greenberg, M. E., and Saudou, F. (2002) The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves Huntingtin phosphorylation by Akt, Dev. Cell, 2, 831-837, https://doi.org/10.1016/s1534-5807(02)00188-0.
Carro, E., Trejo, J. L., Gomez-Isla, T., LeRoith, D., and Torres-Aleman, I. (2002) Serum insulin-like growth factor I regulates brain amyloid-beta levels, Nat. Med., 8, 1390-1397, https://doi.org/10.1038/nm1202-793.
Chaker, Z., Aïd, S., Berry, H., and Holzenberger, M. (2015) Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging, Aging Cell, 14, 847-856, https://doi.org/10.1111/acel.12365.
O’Kusky, J., and Ye, P. (2012) Neurodevelopmental effects of insulin-like growth factor signaling, Front. Neuroendocrinol., 33, 230-251, https://doi.org/10.1016/j.yfrne.2012.06.002.
Frago, L. M., Pañeda, C., Dickson, S. L., Hewson, A. K., Argente, J., and Chowen, J. A. (2002) Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection, Endocrinology, 143, 4113-4122, https://doi.org/10.1210/en.2002-220261.
Baron-Van Evercooren, A., Olichon-Berthe, C., Kowalski, A., Visciano, G., and Van Obberghen, E. (1991) Expression of IGF-I and insulin receptor genes in the rat central nervous system: a developmental, regional, and cellular analysis, J. Neurosci. Res., 28, 244-253, https://doi.org/10.1002/jnr.490280212.
Nijaguna, M. B., Patil, V., Urbach, S., Shwetha, S. D., Sravani, K., Hegde, A. S., Chandramouli, B. A., Arivazhagan, A., Marin, P., Santosh, V., and Somasundaram, K. (2015) Glioblastoma-derived macrophage colony-stimulating factor (MCSF) induces microglial release of insulin-like growth factor-binding protein 1 (IGFBP1) to promote angiogenesis, J. Biol. Chem., 290, 23401-23415, https://doi.org/10.1074/jbc.M115.664037.
Bonham, L. W., Geier, E. G., Steele, N. Z. R., Holland, D., Miller, B. L., Dale, A. M., Desikan, R. S., Yokoyama, J. S., and Alzheimer’s Disease Neuroimaging Initiative (2018) Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer’s disease pathology and shows differential expression in transgenic mice, Front. Neurosci., 12, 476, https://doi.org/10.3389/fnins.2018.00476.
Holmin, S., Mathiesen, T., Langmoen, I. A., and Sandberg Nordqvist, A. C. (2001) Depolarization induces insulin-like growth factor binding protein-2 expression in vivo via NMDA receptor stimulation, Growth Horm. IGF Res., 11, 399-406, https://doi.org/10.1054/ghir.2001.0252.
Jiang, X., Zhao, J., Ju, L., Liu, Y., Wang, B., Zou, X., and Xu, C. (2013) Temporal expression patterns of insulin-like growth factor binding protein-4 in the embryonic and postnatal rat brain, BMC Neurosci., 14, 132, https://doi.org/10.1186/1471-2202-14-132.
Lewitt, M. S., and Boyd, G. W. (2019) The role of insulin-like growth factors and insulin-like growth factor-binding proteins in the nervous system, Biochem. Insights., 12, 117862641984217, https://doi.org/10.1177/1178626419842176.
Rotwein, P., Burgess, S. K., Milbrandt, J. D., and Krause, J. E. (1988) Differential expression of insulin-like growth factor genes in rat central nervous system, Proc. Natl. Acad. Sci. USA, 85, 265-269, https://doi.org/10.1073/pnas.85.1.265.
Santos, A., Yusta, B., Fernández-Moreno, M. D., and Blázquez, E. (1994) Expression of insulin-like growth factor-I (IGF-I) receptor gene in rat brain and liver during development and in regenerating adult rat liver, Mol. Cell. Endocrinol., 101, 85-93, https://doi.org/10.1016/0303-7207(94)90222-4.
Streck, R. D., Wood, T. L., Hsu, M. S., and Pintar, J. E. (1992) Insulin-like growth factor I and II and insulin-like growth factor binding protein-2 RNAs are expressed in adjacent tissues within rat embryonic and fetal limbs, Dev. Biol., 151, 586-596, https://doi.org/10.1016/0012-1606(92)90196-n.
Ayer-le Lievre, C., Ståhlbom, P. A., and Sara, V. R. (1991) Expression of IGF-I and -II mRNA in the brain and craniofacial region of the rat fetus, Development, 111, 105-115, https://doi.org/10.1242/dev.111.1.105.
Wood, T. L., Brown, A. L., Rechler, M. M., and Pintar, J. E. (1990) The expression pattern of an insulin-like growth factor (IGF)-binding protein gene is distinct from IGF-II in the midgestational rat embryo, Mol. Endocrinol., 4, 1257-1263, https://doi.org/10.1210/mend-4-8-1257.
Lee, J. E., Pintar, J., and Efstratiadis, A. (1990) Pattern of the insulin-like growth factor II gene expression during early mouse embryogenesis, Development, 110, 151-159, https://doi.org/10.1242/dev.110.1.151.
Stylianopoulou, F., Efstratiadis, A., Herbert, J., and Pintar, J. (1988) Pattern of the insulin-like growth factor II gene expression during rat embryogenesis, Development, 103, 497-506, https://doi.org/10.1242/dev.103.3.497.
Hansson, H. A., Rozell, B., and Skottner, A. (1987) Rapid axoplasmic transport of insulin-like growth factor I in the sciatic nerve of adult rats, Cell Tissue Res., 247, 241-247, https://doi.org/10.1007/BF00218305.
Ernst, A., and Frisén, J. (2015) Adult neurogenesis in humans – common and unique traits in mammals, PLoS Biol., 13, e1002045, https://doi.org/10.1371/journal.pbio.1002045.
Jessen, K. R. (2004) Glial cells, Int. J. Biochem. Cell Biol., 36, 1861-1867, https://doi.org/10.1016/j.biocel.2004.02.023.
Witsch, J., Szafranski, P., Chen, C.-A., Immken, L., Simpson Patel, G., Hixson, P., Cheung, S. W., Stankiewicz, P., and Schaaf, C. P. (2013) Intragenic deletions of the IGF1 receptor gene in five individuals with psychiatric phenotypes and developmental delay, Eur. J. Hum. Genet., 21, 1304-1307, https://doi.org/10.1038/ejhg.2013.42.
Puche, J. E., and Castilla-Cortázar, I. (2012) Human conditions of insulin-like growth factor-I (IGF-I) deficiency, J. Transl. Med., 10, 224, https://doi.org/10.1186/1479-5876-10-224.
Wu, L., Wang, L., Shangguan, S., Chang, S., Wang, Z., Lu, X., Zhang, Q., Wang, J., Zhao, H., Wang, F., Guo, J., Niu, B., Guo, J., and Zhang, T. (2013) Altered methylation of IGF2 DMR0 is associated with neural tube defects, Mol. Cell Biochem., 380, 33-42, https://doi.org/10.1007/s11010-013-1655-1.
Mathews, L. S., Hammer, R. E., Behringer, R. R., D’Ercole, A. J., Bell, G. I., Brinster, R. L., and Palmiter, R. D. (1988) Growth enhancement of transgenic mice expressing human insulin-like growth factor I, Endocrinology, 123, 2827-2833, https://doi.org/10.1210/endo-123-6-2827.
Cheng, C. M., Mervis, R. F., Niu, S.-L., Salem, N., Witters, L. A., Tseng, V., Reinhardt, R., and Bondy, C. A. (2003) Insulin-like growth factor 1 is essential for normal dendritic growth, J. Neurosci. Res., 73, 1-9, https://doi.org/10.1002/jnr.10634.
van Buul-Offers, S. C., de Haan, K., Reijnen-Gresnigt, M. G., Meinsma, D., Jansen, M., Oei, S. L., Bonte, E. J., Sussenbach, J. S., and Van den Brande, J. L. (1995) Overexpression of human insulin-like growth factor-II in transgenic mice causes increased growth of the thymus, J. Endocrinol., 144, 491-502, https://doi.org/10.1677/joe.0.1440491.
Dikkes, P., Hawkes, C., Kar, S., and Lopez, M. F. (2007) Effect of kainic acid treatment on insulin-like growth factor-2 receptors in the IGF2-deficient adult mouse brain, Brain Res., 1131, 77-87, https://doi.org/10.1016/j.brainres.2006.11.022.
Liu, J. P., Baker, J., Perkins, A. S., Robertson, E. J., and Efstratiadis, A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r), Cell, 75, 59-72, https://doi.org/10.1016/S0092-8674(05)80084-4.
Schubert, M., Gautam, D., Surjo, D., Ueki, K., Baudler, S., Schubert, D., Kondo, T., Alber, J., Galldiks, N., Küstermann, E., Arndt, S., Jacobs, A. H., Krone, W., Kahn, C. R., and Brüning, J. C. (2004) Role for neuronal insulin resistance in neurodegenerative diseases, Proc. Natl. Acad. Sci. USA, 101, 3100-3105, https://doi.org/10.1073/pnas.0308724101.
Carson, M. J., Behringer, R. R., Brinster, R. L., and McMorris, F. A. (1993) Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice, Neuron, 10, 729-740, https://doi.org/10.1016/0896-6273(93)90173-O.
Zielinski, R., Przytycki, P. F., Zheng, J., Zhang, D., Przytycka, T. M., and Capala, J. (2009) The crosstalk between EGF, IGF, and Insulin cell signaling pathways – computational and experimental analysis, BMC Syst. Biol., 3, 88, https://doi.org/10.1186/1752-0509-3-88.
Murphy, L. J. (2000) Overexpression of insulin-like growth factor binding protein-1 in transgenic mice, Pediatr. Nephrol., 14, 567-571, https://doi.org/10.1007/s004670000347.
D’Ercole, A. J., Ye, P., and O’Kusky, J. R. (2002) Mutant mouse models of insulin-like growth factor actions in the central nervous system, Neuropeptides, 36, 209-220, https://doi.org/10.1054/npep.2002.0893.
Bienvenu, G., Seurin, D., Grellier, P., Froment, P., Baudrimont, M., Monget, P., Le Bouc, Y., and Babajko, S. (2004) Insulin-like growth factor binding protein-6 transgenic mice: postnatal growth, brain development, and reproduction abnormalities, Endocrinology, 145, 2412-2420, https://doi.org/10.1210/en.2003-1196.
Leventhal, P. S., Shelden, E. A., Kim, B., and Feldman, E. L. (1997) Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance, J. Biol. Chem., 272, 5214-5218, https://doi.org/10.1074/jbc.272.8.5214.
Meyer, G. E., Shelden, E., Kim, B., and Feldman, E. L. (2001) Insulin-like growth factor I stimulates motility in human neuroblastoma cells, Oncogene, 20, 7542-7550, https://doi.org/10.1038/sj.onc.1204927.
Bothwell, M. (1982) Insulin and somatemedin MSA promote nerve growth factor-independent neurite formation by cultured chick dorsal root ganglionic sensory neurons, J. Neurosci. Res., 8, 225-231, https://doi.org/10.1002/jnr.490080212.
Mulholland, M. W., Romanchuk, G., Simeone, D. M., and Flowe, K. (1992) Stimulation of myenteric plexus neurite outgrowth by insulin and insulin-like growth factors I and II, Life Sci., 51, 1789-1796, https://doi.org/10.1016/0024-3205(92)90049-u.
Kimpinski, K., and Mearow, K. (2001) Neurite growth promotion by nerve growth factor and insulin-like growth factor-1 in cultured adult sensory neurons: role of phosphoinositide 3-kinase and mitogen activated protein kinase, J. Neurosci. Res., 63, 486-499, https://doi.org/10.1002/jnr.1043.
Zochodne, D. W., and Cheng, C. (2000) Neurotrophins and other growth factors in the regenerative milieu of proximal nerve stump tips, J. Anat., 196 (Pt 2), 279-283, https://doi.org/10.1046/j.1469-7580.2000.19620279.x.
Nachemson, A. K., Lundborg, G., and Hansson, H. A. (1990) Insulin-like growth factor I promotes nerve regeneration: an experimental study on rat sciatic nerve, Growth Factors, 3, 309-314, https://doi.org/10.3109/08977199009003673.
Noriega-Prieto, J. A., Maglio, L. E., Zegarra-Valdivia, J. A., Pignatelli, J., Fernandez, A. M., Martinez-Rachadell, L., Fernandes, J., Núñez, Á., Araque, A., Alemán, I. T., and de Sevilla, D. F. (2020) IGF-I governs cortical inhibitory synaptic plasticity by astrocyte activation, BioRxiv, https://doi.org/10.1101/2020.02.11.942532.
Long, K. L. P., Breton, J. M., Barraza, M. K., Perloff, O. S., and Kaufer, D. (2021) Hormonal regulation of oligodendrogenesis I: effects across the lifespan, Biomolecules, 11, 283, https://doi.org/10.3390/biom11020283.
Kawakami, A., Kataoka, H., Oka, T., Mizoguchi, A., Kimura-Kawakami, M., Adachi, T., Iwami, M., Nagasawa, H., Suzuki, A., and Ishizaki, H. (1990) Molecular cloning of the Bombyx mori prothoracicotropic hormone, Science, 247, 1333-1335, https://doi.org/10.1126/science.2315701.
Lagueux, M., Lwoff, L., Meister, M., Goltzené, F., and Hoffmann, J. A. (1990) cDNAs from neurosecretory cells of brains of Locusta migratoria (Insecta, Orthoptera) encoding a novel member of the superfamily of insulins, Eur. J. Biochem., 187, 249-254, https://doi.org/10.1111/j.1432-1033.1990.tb15302.x.
Hakuno, F., and Takahashi, S.-I. (2018) IGF1 receptor signaling pathways, J. Mol. Endocrinol., 61, T69-T86, https://doi.org/10.1530/JME-17-0311.
Rinderknecht, E., and Humbel, R. E. (1978) The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin, J. Biol. Chem., 253, 2769-2776, https://doi.org/10.1016/S0021-9258(17)40889-1.
Rinderknecht, E., and Humbel, R. E. (1978) Primary structure of human insulin-like growth factor II, FEBS Lett., 89, 283-286, https://doi.org/10.1016/0014-5793(78)80237-3.
Torres, A. M., Forbes, B. E., Aplin, S. E., Wallace, J. C., Francis, G. L., and Norton, R. S. (1995) Solution structure of human insulin-like growth factor II. Relationship to receptor and binding protein interactions, J. Mol. Biol., 248, 385-401, https://doi.org/10.1016/s0022-2836(95)80058-1.
Papaioannou, A., Kuyucak, S., and Kuncic, Z. (2016) Elucidating the activation mechanism of the insulin-family proteins with molecular dynamics simulations, PLoS One, 11, e0161459, https://doi.org/10.1371/journal.pone.0161459.
Firth, S. M., and Baxter, R. C. (2002) Cellular actions of the insulin-like growth factor binding proteins, Endocr. Rev., 23, 824-854, https://doi.org/10.1210/er.2001-0033.
Rechler, M. M. (1993) Insulin-like growth factor binding proteins, Vitam. Horm., 47, 1-114, https://doi.org/10.1016/s0083-6729(08)60444-6.
Sitar, T., Popowicz, G. M., Siwanowicz, I., Huber, R., and Holak, T. A. (2006) Structural basis for the inhibition of insulin-like growth factors by insulin-like growth factor-binding proteins, Proc. Natl. Acad. Sci. USA, 103, 13028-13033, https://doi.org/10.1073/pnas.0605652103.
Rajaram, S., Baylink, D. J., and Mohan, S. (1997) Insulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functions, Endocr. Rev., 18, 801-831, https://doi.org/10.1210/edrv.18.6.0321.
Bach, L. A., Headey, S. J., and Norton, R. S. (2005) IGF-binding proteins – the pieces are falling into place, Trends Endocrinol. Metab., 16, 228-234, https://doi.org/10.1016/j.tem.2005.05.005.
Frystyk, J., Hussain, M., Skjaerbaek, C., Pørksen, N., Froesch, E. R., and Orskov, H. (1999) The pharmacokinetics of free insulin-like growth factor-I in healthy subjects, Growth Horm. IGF Res., 9, 150-156, https://doi.org/10.1054/ghir.1999.0100.
Guler, H. P., Zapf, J., Schmid, C., and Froesch, E. R. (1989) Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates, Acta Endocrinol. (Copenh), 121, 753-758, https://doi.org/10.1530/acta.0.1210753.
Chandrashekaran, I. R., Yao, S., Wang, C. C., Bansal, P. S., Alewood, P. F., Forbes, B. E., Wallace, J. C., Bach, L. A., and Norton, R. S. (2007) The N-terminal subdomain of insulin-like growth factor (IGF) binding protein 6. Structure and interaction with IGFs, Biochemistry, 46, 3065-3074, https://doi.org/10.1021/bi0619876.
Kalus, W., Zweckstetter, M., Renner, C., Sanchez, Y., Georgescu, J., Grol, M., Demuth, D., Schumacher, R., Dony, C., Lang, K., and Holak, T. A. (1998) Structure of the IGF-binding domain of the insulin-like growth factor-binding protein-5 (IGFBP-5): implications for IGF and IGF-I receptor interactions, EMBO J., 17, 6558-6572, https://doi.org/10.1093/emboj/17.22.6558.
Zesławski, W., Beisel, H. G., Kamionka, M., Kalus, W., Engh, R. A., Huber, R., Lang, K., and Holak, T. A. (2001) The interaction of insulin-like growth factor-I with the N-terminal domain of IGFBP-5, EMBO J., 20, 3638-3644, https://doi.org/10.1093/emboj/20.14.3638.
Headey, S. J., Keizer, D. W., Yao, S., Brasier, G., Kantharidis, P., Bach, L. A., and Norton, R. S. (2004) C-terminal domain of insulin-like growth factor (IGF) binding protein-6: structure and interaction with IGF-II, Mol. Endocrinol., 18, 2740-2750, https://doi.org/10.1210/me.2004-0248.
Binkert, C., Landwehr, J., Mary, J. L., Schwander, J., and Heinrich, G. (1989) Cloning, sequence analysis and expression of a cDNA encoding a novel insulin-like growth factor binding protein (IGFBP-2), EMBO J., 8, 2497-2502, https://doi.org/10.1002/j.1460-2075.1989.tb08386.x.
Firth, S. M., Ganeshprasad, U., and Baxter, R. C. (1998) Structural determinants of ligand and cell surface binding of insulin-like growth factor-binding protein-3, J. Biol. Chem., 273, 2631-2638, https://doi.org/10.1074/jbc.273.5.2631.
Twigg, S. M., Kiefer, M. C., Zapf, J., and Baxter, R. C. (1998) Insulin-like growth factor-binding protein 5 complexes with the acid-labile subunit. Role of the carboxyl-terminal domain, J. Biol. Chem., 273, 28791-28798, https://doi.org/10.1074/jbc.273.44.28791.
Nam, T. J., Busby, W., and Clemmons, D. R. (1997) Insulin-like growth factor binding protein-5 binds to plasminogen activator inhibitor-I, Endocrinology, 138, 2972-2978, https://doi.org/10.1210/endo.138.7.5230.
Booth, B. A., Boes, M., Andress, D. L., Dake, B. L., Kiefer, M. C., Maack, C., Linhardt, R. J., Bar, K., Caldwell, E. E., and Weiler, J. (1995) IGFBP-3 and IGFBP-5 association with endothelial cells: role of C-terminal heparin binding domain, Growth Regul., 5, 1-17.
Schedlich, L. J., Le Page, S. L., Firth, S. M., Briggs, L. J., Jans, D. A., and Baxter, R. C. (2000) Nuclear import of insulin-like growth factor-binding protein-3 and -5 is mediated by the importin beta subunit, J. Biol. Chem., 275, 23462-23470, https://doi.org/10.1074/jbc.M002208200.
Baxter, R. C., Martin, J. L., and Wood, M. H. (1987) Two immunoreactive binding proteins for insulin-like growth factors in human amniotic fluid: relationship to fetal maturity, J. Clin. Endocrinol. Metab., 65, 423-431, https://doi.org/10.1210/jcem-65-3-423.
Oh, Y., Müller, H. L., Lee, D. Y., Fielder, P. J., and Rosenfeld, R. G. (1993) Characterization of the affinities of insulin-like growth factor (IGF)-binding proteins 1-4 for IGF-I, IGF-II, IGF-I/insulin hybrid, and IGF-I analogs, Endocrinology, 132, 1337-1344, https://doi.org/10.1210/endo.132.3.7679979.
Jones, J. I., Busby, W. H., Wright, G., Smith, C. E., Kimack, N. M., and Clemmons, D. R. (1993) Identification of the sites of phosphorylation in insulin-like growth factor binding protein-1. Regulation of its affinity by phosphorylation of serine 101, J. Biol. Chem., 268, 1125-1131, https://doi.org/10.1016/S0021-9258(18)54050-3.
Yu, J., Iwashita, M., Kudo, Y., and Takeda, Y. (1998) Phosphorylated insulin-like growth factor (IGF)-binding protein-1 (IGFBP-1) inhibits while non-phosphorylated IGFBP-1 stimulates IGF-I-induced amino acid uptake by cultured trophoblast cells, Growth Horm. IGF Res., 8, 65-70, https://doi.org/10.1016/s1096-6374(98)80323-7.
Abu Shehab, M., Damerill, I., Shen, T., Rosario, F. J., Nijland, M., Nathanielsz, P. W., Kamat, A., Jansson, T., and Gupta, M. B. (2014) Liver mTOR controls IGF-I bioavailability by regulation of protein kinase CK2 and IGFBP-1 phosphorylation in fetal growth restriction, Endocrinology, 155, 1327-1339, https://doi.org/10.1210/en.2013-1759.
Liu, F., Powell, D. R., Styne, D. M., and Hintz, R. L. (1991) Insulin-like growth factors (IGFs) and IGF-binding proteins in the developing rhesus monkey, J. Clin. Endocrinol. Metab., 72, 905-911, https://doi.org/10.1210/jcem-72-4-905.
Cardin, A. D., and Weintraub, H. J. (1989) Molecular modeling of protein-glycosaminoglycan interactions, Arteriosclerosis, 9, 21-32, https://doi.org/10.1161/01.atv.9.1.21.
Russo, V. C., Bach, L. A., Fosang, A. J., Baker, N. L., and Werther, G. A. (1997) Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb, Endocrinology, 138, 4858-4867, https://doi.org/10.1210/endo.138.11.5472.
Russo, V. C., Schütt, B. S., Andaloro, E., Ymer, S. I., Hoeflich, A., Ranke, M. B., Bach, L. A., and Werther, G. A. (2005) Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion, Endocrinology, 146, 4445-4455, https://doi.org/10.1210/en.2005-0467.
Xi, G., Shen, X., Rosen, C. J., and Clemmons, D. R. (2019) IRS-1 functions as a molecular scaffold to coordinate IGF-I/IGFBP-2 signaling during osteoblast differentiation, J. Bone Miner Res., 34, 2331, https://doi.org/10.1002/jbmr.3800.
Kuang, Z., Yao, S., Keizer, D. W., Wang, C. C., Bach, L. A., Forbes, B. E., Wallace, J. C., and Norton, R. S. (2006) Structure, dynamics and heparin binding of the C-terminal domain of insulin-like growth factor-binding protein-2 (IGFBP-2), J. Mol. Biol., 364, 690-704, https://doi.org/10.1016/j.jmb.2006.09.006.
Azar, W. J., Zivkovic, S., Werther, G. A., and Russo, V. C. (2014) IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells, Oncogene, 33, 578-588, https://doi.org/10.1038/onc.2012.630.
Firth, S. M., and Baxter, R. C. (1999) Characterisation of recombinant glycosylation variants of insulin-like growth factor binding protein-3, J. Endocrinol., 160, 379-387, https://doi.org/10.1677/joe.0.1600379.
Coverley, J. A., and Baxter, R. C. (1997) Phosphorylation of insulin-like growth factor binding proteins, Mol. Cell. Endocrinol., 128, 1-5, https://doi.org/10.1016/s0303-7207(97)04032-x.
Chesik, D., De Keyser, J., and Wilczak, N. (2007) Insulin-like growth factor binding protein-2 as a regulator of IGF actions in CNS: implications in multiple sclerosis, Cytokine Growth Factor Rev., 18, 267-278, https://doi.org/10.1016/j.cytogfr.2007.04.001.
Martin, J. L., and Baxter, R. C. (1986) Insulin-like growth factor-binding protein from human plasma. Purification and characterization, J. Biol. Chem., 261, 8754-8760, https://doi.org/10.1016/S0021-9258(19)84446-0.
Hoeck, W. G., and Mukku, V. R. (1994) Identification of the major sites of phosphorylation in IGF binding protein-3, J. Cell Biochem., 56, 262-273, https://doi.org/10.1002/jcb.240560220.
Martin, J. L., Lin, M. Z., McGowan, E. M., and Baxter, R. C. (2009) Potentiation of growth factor signaling by insulin-like growth factor-binding protein-3 in breast epithelial cells requires sphingosine kinase activity, J. Biol. Chem., 284, 25542-25552, https://doi.org/10.1074/jbc.M109.007120.
Baxter, R. C. (2015) Nuclear actions of insulin-like growth factor binding protein-3, Gene, 569, 7-13, https://doi.org/10.1016/j.gene.2015.06.028.
Schedlich, L. J., Nilsen, T., John, A. P., Jans, D. A., and Baxter, R. C. (2003) Phosphorylation of insulin-like growth factor binding protein-3 by deoxyribonucleic acid-dependent protein kinase reduces ligand binding and enhances nuclear accumulation, Endocrinology, 144, 1984-1993, https://doi.org/10.1210/en.2002-220798.
Lin, M. Z., Marzec, K. A., Martin, J. L., and Baxter, R. C. (2014) The role of insulin-like growth factor binding protein-3 in the breast cancer cell response to DNA-damaging agents, Oncogene, 33, 85-96, https://doi.org/10.1038/onc.2012.538.
Campbell, P. G., Durham, S. K., Hayes, J. D., Suwanichkul, A., and Powell, D. R. (1999) Insulin-like growth factor-binding protein-3 binds fibrinogen and fibrin, J. Biol. Chem., 274, 30215-30221, https://doi.org/10.1074/jbc.274.42.30215.
Campbell, P. G., Durham, S. K., Suwanichkul, A., Hayes, J. D., and Powell, D. R. (1998) Plasminogen binds the heparin-binding domain of insulin-like growth factor-binding protein-3, Am. J. Physiol., 275, E321-331, https://doi.org/10.1152/ajpendo.1998.275.2.E321.
Angelloz-Nicoud, P., and Binoux, M. (1995) Autocrine regulation of cell proliferation by the insulin-like growth factor (IGF) and IGF binding protein-3 protease system in a human prostate carcinoma cell line (PC-3), Endocrinology, 136, 5485-5492, https://doi.org/10.1210/endo.136.12.7588299.
Gui, Y., and Murphy, L. J. (2001) Insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) binds to fibronectin (FN): demonstration of IGF-I/IGFBP-3/fn ternary complexes in human plasma, J. Clin. Endocrinol. Metab., 86, 2104-2110, https://doi.org/10.1210/jcem.86.5.7472.
Conover, C. A. (1991) Glycosylation of insulin-like growth factor binding protein-3 (IGFBP-3) is not required for potentiation of IGF-I action: evidence for processing of cell-bound IGFBP-3, Endocrinology, 129, 3259-3268, https://doi.org/10.1210/endo-129-6-3259.
Baxter, R. C. (2014) IGF binding proteins in cancer: mechanistic and clinical insights, Nat. Rev. Cancer., 14, 329-341, https://doi.org/10.1038/nrc3720.
Liu, B., Lee, K.-W., Anzo, M., Zhang, B., Zi, X., Tao, Y., Shiry, L., Pollak, M., Lin, S., and Cohen, P. (2007) Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis, Oncogene, 26, 1811-1819, https://doi.org/10.1038/sj.onc.1209977.
Granata, R., Trovato, L., Lupia, E., Sala, G., Settanni, F., Camussi, G., Ghidoni, R., and Ghigo, E. (2007) Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms, J. Thromb. Haemost., 5, 835-845, https://doi.org/10.1111/j.1538-7836.2007.02431.x.
Kiefer, M. C., Masiarz, F. R., Bauer, D. M., and Zapf, J. (1991) Identification and molecular cloning of two new 30-kDa insulin-like growth factor binding proteins isolated from adult human serum, J. Biol. Chem., 266, 9043-9049, https://doi.org/10.1016/S0021-9258(18)31549-7.
Konev, A. A., Smolyanova, T. I., Kharitonov, A. V., Serebryanaya, D. V., Kozlovsky, S. V., Kara, A. N., Feygina, E. E., Katrukha, A. G., and Postnikov, A. B. (2015) Characterization of endogenously circulating IGFBP-4 fragments – novel biomarkers for cardiac risk assessment, Clin. Biochem., 48, 774-780, https://doi.org/10.1016/j.clinbiochem.2015.05.010.
Konev, A. A., Serebryanaya, D. V., Koshkina, E. V., Rozov, F. N., Filatov, V. L., Kozlovsky, S. V., Kara, A. N., Katrukha, A. G., and Postnikov, A. B. (2018) Glycosylated and non-glycosylated NT-IGFBP-4 in circulation of acute coronary syndrome patients, Clin. Biochem., 55, 56-62, https://doi.org/10.1016/j.clinbiochem.2018.03.004.
Chelius, D., Baldwin, M., Lu, X., and Spencer, E. (2001) Expression, purification and characterization of the structure and disulfide linkages of insulin-like growth factor binding protein-4, J. Endocrinol., 168, 283-296, https://doi.org/10.1677/joe.0.1680283.
Laursen, L. S., Kjaer-Sorensen, K., Andersen, M. H., and Oxvig, C. (2007) Regulation of insulin-like growth factor (IGF) Bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5, Mol. Endocrinol., 21, 1246-1257, https://doi.org/10.1210/me.2006-0522.
Qin, X., Strong, D. D., Baylink, D. J., and Mohan, S. (1998) Structure-Function analysis of the human insulin-like growth factor binding protein-4, J. Biol. Chem., 273, 23509-23516, https://doi.org/10.1074/jbc.273.36.23509.
Boldt, H. B., Bale, L. K., Resch, Z. T., Oxvig, C., Overgaard, M. T., and Conover, C. A. (2013) Effects of mutated pregnancy-associated plasma protein-A on atherosclerotic lesion development in mice, Endocrinology, 154, 246-252, https://doi.org/10.1210/en.2012-1552.
Laursen, L. S., Overgaard, M. T., Søe, R., Boldt, H. B., Sottrup-Jensen, L., Giudice, L. C., Conover, C. A., and Oxvig, C. (2001) Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A, FEBS Lett., 504, 36-40, https://doi.org/10.1016/S0014-5793(01)02760-0.
Prudova, A., auf dem Keller, U., Butler, G. S., and Overall, C. M. (2010) Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics, Mol. Cell. Proteomics, 9, 894-911, https://doi.org/10.1074/mcp.M000050-MCP201.
Shimasaki, S., Shimonaka, M., Zhang, H. P., and Ling, N. (1991) Identification of five different insulin-like growth factor binding proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human, J. Biol. Chem., 266, 10646-10653.
Firth, S. M., Clemmons, D. R., and Baxter, R. C. (2001) Mutagenesis of basic amino acids in the carboxyl-terminal region of insulin-like growth factor binding protein-5 affects acid-labile subunit binding, Endocrinology, 142, 2147, https://doi.org/10.1210/endo.142.5.8284.
Twigg, S. M., Kiefer, M. C., Zapf, J., and Baxter, R. C. (2000) A central domain binding site in insulin-like growth factor binding protein-5 for the acid-labile subunit, Endocrinology, 141, 454-457, https://doi.org/10.1210/endo.141.1.7375.
Jones, J. I., Gockerman, A., Busby, W. H., Camacho-Hubner, C., and Clemmons, D. R. (1993) Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I, J. Cell Biol., 121, 679-687, https://doi.org/10.1083/jcb.121.3.679.
Nam, T. J., Busby, W. H., and Clemmons, D. R. (1994) Human fibroblasts secrete a serine protease that cleaves insulin-like growth factor-binding protein-5, Endocrinology, 135, 1385-1391, https://doi.org/10.1210/endo.135.4.7523096.
Nam, T.-J., Busby, W. H., Rees, C., and Clemmons, D. R. (2000) Thrombospondin and osteopontin bind to insulin-like growth factor (IGF)-binding protein-5 leading to an alteration in IGF-I-stimulated cell growth, Endocrinology, 141, 1100-1106, https://doi.org/10.1210/endo.141.3.7386.
Hodgkinson, S. C., Napier, J. R., Spencer, G. S. G., and Bass, J. J. (1994) Glycosaminoglycan binding characteristics of the insulin-like growth factor-binding proteins, J. Mol. Endocrinol., 13, 105-112, https://doi.org/10.1677/jme.0.0130105.
Graham, M. E., Kilby, D. M., Firth, S. M., Robinson, P. J., and Baxter, R. C. (2007) The in vivo phosphorylation and glycosylation of human insulin-like growth factor-binding protein-5, Mol. Cell. Proteomics, 6, 1392-1405, https://doi.org/10.1074/mcp.M700027-MCP200.
Schedlich, L. J., Young, T. F., Firth, S. M., and Baxter, R. C. (1998) Insulin-like growth factor-binding protein (IGFBP)-3 and IGFBP-5 share a common nuclear transport pathway in T47D human breast carcinoma cells, J. Biol. Chem., 273, 18347-18352, https://doi.org/10.1074/jbc.273.29.18347.
Zhao, Y., Yin, P., Bach, L. A., and Duan, C. (2006) Several acidic amino acids in the N-domain of insulin-like growth factor-binding protein-5 are important for its transactivation activity, J. Biol. Chem., 281, 14184-14191, https://doi.org/10.1074/jbc.M506941200.
Overgaard, M. T., Boldt, H. B., Laursen, L. S., Sottrup-Jensen, L., Conover, C. A., and Oxvig, C. (2001) Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase, J. Biol. Chem., 276, 21849-21853, https://doi.org/10.1074/jbc.M102191200.
Martin, J. L., Willetts, K. E., and Baxter, R. C. (1990) Purification and properties of a novel insulin-like growth factor-II binding protein from transformed human fibroblasts, J. Biol. Chem., 265, 4124-4130, https://doi.org/10.1016/S0021-9258(19)39711-X.
Bach, L. A., Hsieh, S., Sakano, K., Fujiwara, H., Perdue, J. F., and Rechler, M. M. (1993) Binding of mutants of human insulin-like growth factor II to insulin-like growth factor binding proteins 1-6, J. Biol. Chem., 268, 9246-9254, https://doi.org/10.1016/S0021-9258(18)98342-0.
Leng, S. L., Leeding, K. S., Whitehead, R. H., and Bach, L. A. (2001) Insulin-like growth factor (IGF)-binding protein-6 inhibits IGF-II-induced but not basal proliferation and adhesion of LIM 1215 colon cancer cells, Mol. Cell. Endocrinol., 174, 121-127, https://doi.org/10.1016/S0303-7207(00)00444-5.
Schmid, C., Schlapfer, I., Keller, A., Waldvogel, M., Froesch, E. R., and Zapf, J. (1995) Effects of insulin-like growth factor (IGF) binding proteins (BPs)-3 and -6 on DNA synthesis of rat osteoblasts: further evidence for a role of auto-/paracrine IGF I but not IGF II in stimulating osteoblast growth, Biochem. Biophys. Res. Commun., 212, 242-248, https://doi.org/10.1006/bbrc.1995.1962.
Bach, L. (1999) Insulin-like growth factor binding protein-6: the “Forgotten” binding protein? Horm Metab Res., 31, 226-234, https://doi.org/10.1055/s-2007-978723.
Shalamanova, L., Kübler, B., Storch, S., Scharf, J.-G., and Braulke, T. (2008) Multiple post-translational modifications of mouse insulin-like growth factor binding protein-6 expressed in epithelial Madin-Darby canine kidney cells, Mol. Cell. Endocrinol., 295, 18-23, https://doi.org/10.1016/j.mce.2008.08.034.
Marinaro, J. A., Hendrich, E. C., Leeding, K. S., and Bach, L. A. (1999) HaCaT human keratinocytes express IGF-II, IGFBP-6, and an acid-activated protease with activity against IGFBP-6, Am. J. Physiol. Endocrinol. Metab., 276, E536-E542, https://doi.org/10.1152/ajpendo.1999.276.3.E536.
Nakamura, M., Miyamoto, S., Maeda, H., Ishii, G., Hasebe, T., Chiba, T., Asaka, M., and Ochiai, A. (2005) Matrix metalloproteinase-7 degrades all insulin-like growth factor binding proteins and facilitates insulin-like growth factor bioavailability, Biochem. Biophys. Res. Commun., 333, 1011-1016, https://doi.org/10.1016/j.bbrc.2005.06.010.
Larsen, P. H. (2006) Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice, J. Neurosci., 26, 2207-2214, https://doi.org/10.1523/JNEUROSCI.1880-05.2006.
Dean, R. A., Butler, G. S., Hamma-Kourbali, Y., Delbé, J., Brigstock, D. R., Courty, J., and Overall, C. M. (2007) Identification of candidate angiogenic inhibitors processed by matrix metalloproteinase 2 (MMP-2) in cell-based proteomic screens: disruption of vascular endothelial growth factor (VEGF)/heparin affin regulatory peptide (pleiotrophin) and VEGF/connective tissue growth factor angiogenic inhibitory complexes by MMP-2 proteolysis, MCB, 27, 8454-8465, https://doi.org/10.1128/MCB.00821-07.
Gibson, J. M., Aplin, J. D., White, A., and Westwood, M. (2001) Regulation of IGF bioavailability in pregnancy, Mol. Hum. Reprod., 7, 79-87, https://doi.org/10.1093/molehr/7.1.79.
Cohen, P., Graves, H. C., Peehl, D. M., Kamarei, M., Giudice, L. C., and Rosenfeld, R. G. (1992) Prostate-specific antigen (PSA) is an insulin-like growth factor binding protein-3 protease found in seminal plasma, J. Clin. Endocrinol. Metab., 75, 1046-1053, https://doi.org/10.1210/jcem.75.4.1383255.
Rajah, R., Bhala, A., Nunn, S. E., Peehl, D. M., and Cohen, P. (1996) 7S nerve growth factor is an insulin-like growth factor-binding protein protease, Endocrinology, 137, 2676-2682, https://doi.org/10.1210/endo.137.7.8770886.
Monget, P. (2002) Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation, Biol. Reprod., 68, 77-86, https://doi.org/10.1095/biolreprod.102.007609.
Iwadate, H., Sugisaki, T., Kudo, M., and Kizuki, K. (2003) Actions of insulin-like growth factor binding protein-5 (IGFBP-5) are potentially regulated by tissue kallikrein in rat brains, Life Sci., 73, 3149-3158, https://doi.org/10.1016/j.lfs.2003.06.010.
Russo, V. C., Rekaris, G., Baker, N. L., Bach, L. A., and Werther, G. A. (1999) Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells, Endocrinology, 140, 3082-3090, https://doi.org/10.1210/endo.140.7.6771.
Son, J.-W., Park, J., Kim, Y. E., Ha, J., Park, D. W., Chang, M.-S., and Koh, S.-H. (2019) Glia-like cells from late-passage human MSCs protect against ischemic stroke through IGFBP-4, Mol. Neurobiol., 56, 7617-7630, https://doi.org/10.1007/s12035-019-1629-8.
Nishijima, T., Piriz, J., Duflot, S., Fernandez, A. M., Gaitan, G., Gomez-Pinedo, U., Verdugo, J. M. G., Leroy, F., Soya, H., Nuñez, A., and Torres-Aleman, I. (2010) Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS, Neuron, 67, 834-846, https://doi.org/10.1016/j.neuron.2010.08.007.
Acknowledgments
The authors express sincere gratitude to V. E. Adashev for the help in creating the figures in the review. O. I. Klychnikov is thankful to the MSU School of Science and Education “Molecular Technologies of Living Systems and Synthetic Biology” for financial support.
Author information
Authors and Affiliations
Contributions
G.A.D. and D.V.S. conceived the theme, analyzed the literature and wrote the manuscript. O.I.K. edited the manuscript and contributed to the idea and the structure of the review. D.A.A. prepared all the figures. E.A.V. and A.G.K. gave valuable comments and suggestions. All authors read and approved the manuscript before publishing it.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest. This article does not contain description of studies with the involvement of humans or animal subjects performed by any of the authors.
Additional information
Translated from Uspekhi Biologicheskoi Khimii, 2023, Vol. 63, pp. 207-244.
Rights and permissions
About this article
Cite this article
Dya, G.A., Klychnikov, O.I., Adasheva, D.A. et al. IGF-Binding Proteins and Their Proteolysis as a Mechanism of Regulated IGF Release in the Nervous Tissue. Biochemistry Moscow 88 (Suppl 1), S105–S122 (2023). https://doi.org/10.1134/S0006297923140079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923140079