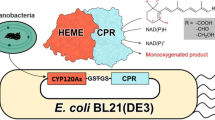
Abstract
Cytochrome CYP102A1 (P450 BM3) of Priestia megaterium (bas. Bacillus megaterium) has several unique functional features and thus provides an ideal object for directed evolution and other synthetic applications. Previously, the CYP102A1-LG23 mutant with 14 mutations in the heme part was obtained that hydroxylates several androstanes at C7β with the formation of products with the anti-inflammatory and neuroprotective activities. In this study, synthetic cyp102A1-LG23 gene encoding the P450 BM3 mutant was expressed as a component of either monocistronic operon or bicistronic operon containing the gdh (glucose dehydrogenase, GDH) or zwf2 (glucose 6-phosphate dehydrogenase, G6PD) gene in Mycolicibacterium smegmatis BD cells. The recombinant bacteria were able hydroxylate androst-4-ene-3,17-dione (AD) into 7β-OH-AD. Their biocatalytic activity was increased twice by increasing the solubility of CYP102A1-LG23 protein in the cells and supplementing the cells with the additional cofactor regeneration system by introducing GDH and G6PD. The maximum 7β-OH-AD yield (37.68 mol%) was achieved by co-expression of cyp102A1-LG23 and gdh genes in M. smegmatis. These results demonstrate the possibility of using synthetic genes to obtain recombinant enzymes and expand our understanding of the processes involved in steroid hydroxylation by bacterial cytochromes. The data obtained can be used to develop new approaches for microbiological production of 7β-hydroxylated steroids in genetically modified Mycolicibacterium species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Steroid compounds exhibit a broad range of biological activities that account for their wide application in medicine. Steroids are used to treat inflammation, endocrine diseases, neurodegenerative disorders, certain cancers, etc. [1].
The biological activity of a steroid depends on the degree of oxidation of its cycloalkane core and the presence of functional oxygen-containing groups in the molecule. Hydroxylated steroids are commonly known to be more active than higher activity compared to their nonhydroxylated analogs. Hydroxyl groups attached to the cycloalkane core increase the polarity and solubility of the molecule and affect its toxicity and sorption properties. Regiospecific and stereospecific positioning of hydroxyl groups is important for steroid binding with the respective cell receptors triggering metabolic reaction cascades in the body [2-5].
Chemical synthesis of hydroxylated steroids often includes multiple steps and is difficult (if possible at all) to perform. Hydroxylation of inactive C–H bonds with the use of microorganisms or enzymes may help to solve the problem [1, 6]. One of the promising approaches involves construction and use of enzymes with regiospecific and stereospecific activities toward steroid compounds [7, 8].
Cytochrome P450 monooxygenases (P450s or CYPs) are of particular interest for gene and protein engineering, as they play a crucial role in oxidative functionalization of inactive carbon atoms. Cytochrome P450 BM3 (CYP102A1) of Priestia megaterium (bas. Bacillus megaterium) has several unique functional features and thus provides an ideal object for directed evolution and other synthetic applications. In contrast to most P450s, P450 BM3 is a water-soluble protein. Another important feature is bifunctionality ensured by the two-component structure of this protein, i.e., the natural fusion between the P450 monooxygenase domain and reductase domain. P450 BM3 is the fastest catalyst among all currently known P450s. Its turnover number reaches 17,000 m–1 (with arachidonic acid as a substrate) [9], which is several orders of magnitude higher than the turnover numbers of the majority of mammalian P450s [10]. The reason for such a high catalytic activity might be the presence of redox partners in the same protein molecule, which ensures rapid electron transfer from the flavin to the heme [11].
Natural P450 BM3 is not involved in steroid metabolism [12]. Several mutant variants of this protein have been obtained by directed evolution, including P450 BM3-LG23, which has 14 mutations in the heme part and hydroxylates several androstanes as steroid substrates [13].
Because actinobacteria of the genus Mycolicibacterium are capable of utilizing sterols (cholesterol and phytosterols) commonly used to produce therapeutic steroids and their precursors [14, 15]. The nonpathogenic fast-growing Mycolicibacterium smegmatis (syn. Mycobacterium smegmatis) strain mc2 155 provides a convenient host to express heterologous steroidogenic genes because this strain contains an efficient system for the transport of exogenous steroid compounds and has a high transformation rate [16]. We have previously introduced deletions into kshB (3-ketosteroid Δ1-dehydrogenase) and kstD (reductase subunit of 3-ketosteroid 9α-hydroxylase) to prevent complete destruction of the cycloalkane core in steroids to obtain the mutant M. smegmatis BD strain that oxidized phytosterol and cholesterol into androst-4-ene-3,17-dione (AD) [15].
In this work, M. smegmatis BD was used to obtain recombinant strains expressing heterologous P450 BM3-LG23 and hydroxylating AD at C7β (Fig. 1). AD hydroxylation, a reaction that is required for oxidative functionalization of steroids, is one of the most difficult to perform artificially. Along with C7β hydroxylation, the hydroxyl group was introduced at C1β. Production of 1β-hydroxysteroids and 1β,7β-dihydroxysteroids with the use of mutant P450 BM3 variants has not been reported before.
Our approach can be used to construct other microbial catalysts based on CYP102A1-LG23; it opens a possibility of a one-step production of hydroxylated androstenes from phytosterol.
MATERIALS AND METHODS
Reagents. Acetamide (AcA) (Sigma-Aldrich, USA), AD (Steraloids, USA), agarose (Invitrogen, United Kingdom), methyl-β-cyclodextrin (MCD) (Wacker Chemie, Germany), ethidium bromide (Serva, Germany), kanamycin (BioKhim, Russia), hygromycin, ampicillin, glycerol, yeast extract, peptone, agar-agar (Panreac, Spain), tryptone (Dia-M, Russia), Tween-80 (Serva), DNA-modifying enzymes, DNA extraction kits (Thermo Fisher Scientific, USA), and silica gel (Fluka, USA) were used in the study. Other reagents were from Russian manufacturers and were of analytical grade.
Bacterial strains, plasmids, and cultivation conditions. Primers, bacterial strains, and plasmids used in this work are shown in Table 1.
Escherichia coli strain DH5α was used to clone the plasmid constructs. The bacteria were cultivated in LB medium at 37°C at 200 rpm [19]. Transformed cells were selected using kanamycin (50 µg/ml), hygromycin (50 µg/ml), or ampicillin (100 µg/ml).
Mycolicibacterium smegmatis BD cells were grown in M3 medium at 37°C at 200 rpm [15]. Transformed cells were selected using hygromycin (50-75 µg/ml).
Synthetic sequences and site-directed mutagenesis. Nucleotide sequences of the wild-type cyp102A1 were retrieved from KEGG (BG04_163; Bacillus megaterium NBRC 15308) and GenBank (ACCESSION J04832, VERSION J04832.1); the amino acid sequence of the heme fragment of CYP102A1-LG23 was retrieved from RCSB PDB (PDB ID: 6LY4). The original nucleotide sequence of gdh (glucose dehydrogenase, GDH) was from GenBank (ACCESSION AY930464, VERSION AY930464.1; Bacillus megaterium AS1.223).
Synthetic sequences of mutant cyp102A1-LG23 [13] and gdh genes were optimized for efficient heterologous expression in M. smegmatis cells using the OptimumGeneTM algorithm. The optimized sequences were synthesized by GenScript (USA) and cloned into the pBluescript II SK(+) vector to produce pSKLG23 and pSKGDH plasmids, respectively (Table 1). The optimized synthetic sequence of zwf2 gene coding for type 2 glucose 6-phosphate dehydrogenase (G6PD) of Mycobacterium tuberculosis H37Rv was obtained from pNS25 [18].
The pMyNTA vector was constructed using the pMyNT shuttle expression vector [17] by introducing the NdeI site at the position of the ATG start codon via site directed mutagenesis carried out according to the QuickChange™ Site-Directed Mutagenesis System (QCM) protocol (Stratagene, USA). The oligonucleotide primers NdeIlF and NdeIlR (Table 1) were used to amplify pMyNT. The resulting mixture was digested with the DpnI restriction endonuclease and cloned in E. coli DH5α cells.
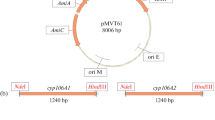
Monocistronic plasmid constructs for cyp102A1-LG23 expression in Mycolicibacterium cells. DNA cloning was performed according to a standard protocol [20] using pMyNT shuttle expression vector (Fig. 2a) and its derivative pMyNTA (Fig. 2b).
Genetic elements of monocistronic and bicistronic constructs. a) Map of the pMyNT plasmid expression vector. b) Map of the pMyNTA plasmid expression vector. c) Plasmid pNS38 with the cyp102A1-LG23 gene. d) Plasmid pVP1 with the cyp102A1-LG23 gene. e) Plasmid pVP2 with the cyp102A1-LG23 and gdh bicistronic expression cassette. f) Plasmid pVP3, with the cyp102A1-LG23 and zwf2 bicistronic expression cassette. AmiC, AmiA, AmiD, and AmiS, components of the acetamidase promoter; 6 × His, hexahistidine tag; HygR, hygromycin resistance gene; RBS, ribosome-binding site.
The pSKLG23 plasmid was digested with the NcoI and BamHI restriction enzymes. The cyp102A1-LG23 gene was ligated with pMyNT linearized with the same enzymes. The resulting recombinant plasmid carrying the cyp102A1-LG23 gene with the nucleotide sequence coding for the N-terminal hexahistidine tag (6 × His-tag) was designated pNS38 (Table 1, Fig. 2c).
The pNS38 plasmid was consecutively digested with NcoI, treated with the Klenow fragment of E. coli DNA polymerase I, and hydrolyzed with BamHI. The pMyNTA vector was treated consecutively with NdeI, Klenow fragment, and BamHI. The cyp102A1-LG23 gene was cloned in linearized pMyNTA vector to produce recombinant pVP1 plasmid (Table 1, Fig. 2d).
Bicistronic plasmid constructs for cyp102A1-LG23/gdh and cyp102A1-LG23/zwf2 coexpression in Mycolicibacterium cells. The pSKGDH plasmid was digested with BamHI and HindIII restriction endonucleases, and the gdh gene was ligated into pVP1 vector linearized with the same restriction enzymes; the resulting plasmid was designated pVP2 (Table 1, Fig. 2e).
The zwf2 gene was amplified by PCR with the ClaIzwf2F and ClaIzwf2R primers (Table 1) using the pNS25 plasmid as a template [18]. The amplified zwf2 gene was digested with ClaI, treated with the Klenow fragment, and ligated into the pVP1 vector treated consecutively with BamHI and Klenow fragment to produce the pVP3 plasmid (Table 1, Fig. 2f).
Gene expression in recombinant Mycolicibacterium strains. Electrocompetent cells of the mutant M. smegmatis BD strain were transformed with the pMyNT vector or pNS38, pVP1, pVP2, and pVP3 genetic constructs by electroporation [21] and plated onto M3 agar supplemented with 75 µg/ml hygromycin. Suspension cultures of M. smegmatis BD cells transformed with the plasmids were grown in 5 ml of liquid M3 medium containing 50 µg/ml hygromycin at 37°C at 200 rpm for 12-15 h and used for inoculation of 50 ml of the same medium in 750-ml flasks. The cells were grown with shaking in an incubator at 37°C at 200 rpm to OD600 0.8-1.0. Heterologous gene expression was induced by adding AcA to a final concentration of 2 g/liter. Induced cultures were grown at 25°C at 200 rpm for 48 h and centrifuged at 5020g at 4°C for 15 min. The cells were resuspended in 20 ml of buffer (50 mM KH2PO4, 50 mM NaCl, pH 7.4) and disintegrated by sonication with a Q500 ultrasonic homogenizer (USA) according to the manufacturer’s protocol. Cell debris was removed by centrifugation at 27,300g at 4°C for 2.5 h. Proteins were analyzed by polyacrylamide gel electrophoresis in the presence of SDS (SDS-PAGE) in 10% gel [22].
AD bioconversion by recombinant Mycolicibacterium strains. Suspension cultures recombinant Mycolicibacterium strains were grown and induced as described above. AD (aqueous suspension with MCD, 1 : 1.5, mol/mol) was added simultaneously with AcA to a final concentration of 200 mg/liter. Glucose solution was added to the fermentation medium (to 10 g/liter) together with the inducer and then daily. The cultures were incubated at 25°C at 200 rpm for 4 days. The samples of culture liquid (0.5 ml) were collected every 24 h. Steroids were analyzed by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC).
Biomass production assessment. The growth of bacterial cultures was assessed by measuring OD600 in an Eppendorf BioSpectrometer Basic spectrophotometer (Germany). The strains were grown in liquid M3 medium (supplemented with 50 µg/ml hygromycin in the case of transformants) at 37°C at 200 rpm for 48 h.
Thin-layer chromatography. Steroids were extracted with a double volume of ethyl acetate. The extracts were applied onto ALUGRAM SIL G/UV254 chromatographic plates (Macherey-Nagel, Germany) and fractionated using benzene : acetone (2 : 1, v/v) as a mobile phase. Steroids were detected in UV light (254 nm) in a CN-15. LC UV Darkroom viewing chamber (Vilber Lourmat, France).
High-performance liquid chromatography. An aliquot of the culture liquid was combined with an equal volume of acetonitrile; the mixture was incubated for 12 h and centrifuged at 2100g for 8 min. The supernatant was analyzed using an Agilent Infinity 1260 chromatography system (Agilent Technologies, USA) with a Symmetry RP-18 column (5 µm, 4.6 × 250 mm) and a Symmetry RP-18 precolumn (5 µm, 3.9 × 20 mm) (Waters, USA). Mobile phase: solution A, acetonitrile : THF (tetrahydrofuran) : water (10 : 10 : 80, v/v); solution B, 100% acetonitrile. Flow rate, 1 ml/min; gradient elution (1st stage: eluent A 100%; 2nd stage: eluent A 40% – eluent B 60%); column temperature, 50°C. Steroids were detected at 254 nm.
Isolation and structural analysis of hydroxylated AD derivatives. The culture liquid (~200 ml) collected after 48-h incubation was centrifuged at 27,300g at 4°C for 1 h. The supernatant was extracted with ethyl acetate (70 ml) three times, and pooled ethyl acetate extract was evaporated under reduced pressure. Steroids were separated by column chromatography on silica gel 90 (0.2-0.5 mm), and AD hydroxylation products were isolated by a stepwise elution with hexane : ethyl acetate : ethanol:
1st stage: hexane 70% ‒ ethyl acetate 30%;
2nd stage: hexane 50% ‒ ethyl acetate 50%;
3rd stage: hexane 30% ‒ ethyl acetate 70%;
4th stage: ethyl acetate 100%;
5th stage: ethyl acetate 70% ‒ ethanol 30%;
6th stage: ethyl acetate 50% ‒ ethanol 50%;
7th stage: ethyl acetate 30% ‒ ethanol 70%;
8th stage: ethanol 100%.
The resulting fractions were evaporated until dry, dissolved in 500 µl of ethyl acetate, and purified by TCL on ALUGRAM SIL G/UV254 plates (Macherey-Nagel, Germany) in benzene : acetone (3 : 1, v/v). Steroid compounds were detected in UV light in a CN-15. LC UV Darkroom viewing chamber (Vilber Lourmat) at 254 nm.
The structure of steroid components was verified by 1H-NMR spectroscopy. The spectra were recorded with a Bruker Avance 400 NMR spectrometer (Bruker, Germany) at 400 and 100.6 MHz. Chemical shifts were measured relative to tetramethylsilane (1H-NMR).
Statistical data processing. Experimental data were obtained in three biological replicates. Results are presented as mean +/– standard deviation.
RESULTS
Recombinant bacterial producers were obtained using the mutant M. smegmatis BD strain (Table 1) [15]. A set of plasmids was constructed to express synthetic cyp102A1-LG23 as a single gene and in the content of bicistronic operons with the gdh or zwf2 genes (Table 1) under control of the inducible acetamidase (Ami) promoter.
The obtained recombinant strains did not differ in the cultivation and morphological properties when grown on M3 agar but showed a slightly lower growth rate in the liquid medium compared with the parental M. smegmatis BD strain (Fig. 3).
The highest level of CYP102A1-LG23 expression was observed in Mycolicibacterium cells carrying the pNS38 plasmid (Fig. 4a, lane 2). Fractionation of cell homogenate showed that the recombinant protein accumulated almost fully in inclusion bodies (Fig. 4b, lane 2d). When the hexahistidine tag was removed from the N-terminus of the mutant cytochrome, up to 40-50% of the protein occurred in a soluble form (Fig. 4b; lanes 3d, 3s). GDH was detected in the soluble cell fraction (Fig. 4b, lane 4s), while G6PD was found in the membrane fraction (Fig. 4b, lane 5d). The recombinant proteins synthesized upon induction with AcA showed expected molecular weights: CYP102A1-LG23, 118 kDa; G6PD, 57 kDa; and GDH, 28 kDa. In all recombinant strains, production of the second protein (GDH or G6PD) encoded in the bicistronic construct was much lower than that of the first protein (Fig. 4a; lanes 4, 5).
SDS-PAGE analysis of (a) heterologous gene expression and (b) solubility of recombinant proteins in the AcA-induced cultures of recombinant M. smegmatis BD strains. M, molecular weight markers (Bio-Rad, USA); s, supernatant; d, cell debris. Plasmids: 1) pMyNT (control); 2) pNS38; 3) pVP1; 4) pVP2; 5) pVP3. Arrows indicate CYP102A1-LG23 (118 kDa), GDH (28 kDa), and G6PD (57 kDa).
In vivo production of 7β-hydroxyandrost-4-ene-3,17-dione (7β-OH-AD) by the strain carrying the pNS38 plasmid (Fig. 5) was low (15.56 mol%) indicating low enzymatic activity of the insoluble protein. Decreasing the cyp102A1-LG23 expression using a lower concentration of the inducer failed to noticeably improve the activity and solubility of the enzyme (data no shown), while removal of the 6 × His-tag (plasmid pVP1) improved the solubility of P450 BM3-LG23, resulting in the two-fold (to 32.75 mol%) increase in the efficiency of substrate bioconversion (Fig. 5).
However, even in the case of improved protein solubility, the conversion of AD to 7β-OH-AD was incomplete. To further increase the efficiency of substrate bioconversion, we investigated whether the activity of CYP102A1-LG23 could be elevated by providing a necessary level of reduced cofactors via coexpression of gdh (pVP2) or zwf2 (pVP3).
In all the cases, 7β-OH-AD was accumulated as a major metabolite, while the content of 7α-OH-AD did not exceed 1.5 mol%. 7β-Hydroxylation of AD by the strains carrying the bicistronic constructs pVP2 and pVP3 was 9-15% more efficient (37.68 and 35.63 mol%, respectively) than by the strain with the monocistronic pVP1 construct (Fig. 5). The highest yield of 7β-OH-AD was achieved within 48 h, after which the concentration of the target product decreased together with a decrease in the AD content (Fig. 5).
We also observed introduction of the OH group at the C1β position (in addition to C7β hydroxylation) in the recombinant strains (Fig. 6, Table 2). Production of 1β-OH- and 1β,7β-diOH-steroids by P450-BM3 mutant variants has not been described before.
DISCUSSION
Introduction of functional oxygen-containing groups into the cycloalkane core is one of the most important and difficult tasks in the synthesis of therapeutic steroids. New prospects in this area are associated with the creation and use of recombinant bacterial P450 cytochromes that have a higher solubility and activity than most eukaryotic cytochromes [5]. The most promising direction is the use of bacillary P450 BM3 cytochrome that possesses unique biocatalytic features [9, 11]. One of the outstanding achievements was the creation of its mutant variant CYP102A1-LG23 capable of hydroxylating steroids at C7β [13]. In this work, we optimized the nucleotide sequence of this mutant enzyme for expression in Mycolicibacterium cells. The optimized gene sequence was synthesized and used for the construction of plasmids for heterologous expression of cyp102A1-LG23 as a part of either monocistronic operon or bicistronic operons with the gdh or zwf2 genes coding for GDH and G6PD, respectively, in M. smegmatis BD cells.
M. smegmatis BD was used as a host due to the presence of an efficient system for steroid transport [15] and possibility of one-step production of 7β-hydroxylated androstenes from phytosterols [23]. Previously, this strain has been successfully tested for the expression of genes of bacillary CYP106A1 and CYP106A2 cytochromes. The recombinant strain expressing the cyp106A2 gene selectively hydroxylated AD at C15β [15].
Overexpression of CYP102A1-LG23 led to the generation of insoluble inclusion bodies and, consequently, low efficiency of C7β-hydroxylation in vivo. Presumably, protein aggregation was caused by the presence of the N-terminal polyhistidine tag that had a negative effect on the solubility, structure, and/or folding of P450 BM-LG23. It is known that addition of the His-tag can affect protein function and stability. Recently, there has been an increasing number of publications reporting a negative effect of His-tags on the structure, oligomeric state, kinetics, and catalytic activity of proteins [24]. Deletion of the hexahistidine tag from the N-terminus allowed up to increase the content of the soluble functionally active protein up to 50% without affecting its total yield, which increased the content of produced 7β-OH-AD two-fold.
Cofactor engineering is an efficient approach to improving the performance in production of valuable metabolites. For example, overexpression of NADPH-regenerating G6PD noticeably increased production of ɛ-caprolactone in E. coli [25]. The balance between NADP+ and NADPH has been found to play a key role in the regulation of phytosterol conversion to 7β-OH-AD in Mycolicibacterium neoaurum [23]. To maintain the pool of reduced NADPH, GDH, or G6PD were coexpressed with CYP102A1-LG23 in M. smegmatis BD. Glucose was added to the fermentation medium as a cosubstrate to achieve more efficient NADPH regeneration [23, 26]. Introduction of enzymes for additional cofactor regeneration allowed to increase the activity of the recombinant system by 15%. In all the cases, 7β-OH-AD was accumulated as a major metabolite and 7α-OH-AD was detected in trace amounts, which was in agreement with the published data [13, 23]. Two new compounds (1β-OH-AD and 1β,7β-diOH-AD) were obtained for the first time as the products of hydroxylation reaction catalyzed by the mutant variants of P450 BM3 cytochrome. Microbiological C1-hydroxylation of steroids (in particular, at the C1β position) is a relatively rare phenomenon [27] that should be investigated further.
CONCLUSIONS
In this study, we achieved heterologous expression of the unique mutant cytochrome CYP102A1-LG23 in M. smegmatis BD cells for the purpose of 7β-OH-AD synthesis from AD. The enzymatic activity of the recombinant Mycolicibacterium bacteria was increased by increasing the content of protein active form and using a cofactor regeneration system, as both factors limited AD conversion to 7β-OH-AD in M. smegmatis. The maximum product yield (37.68 mol%) was achieved with the M. smegmatis BD (pVP2) strain expressing cyp102A1-LG23 and gdh. These results contribute to the understanding of the catalytic features of recombinant P450 enzymes and can be used as a starting point to develop a system for producing valuable C7β-hydroxylated steroids from inexpensive precursors (phytosterols and cholesterol) via microbial transformation by genetically modified Mycolicibacterium bacteria.
Abbreviations
- 7β-OH-AD:
-
7β-hydroxyandrost-4-ene-3,17-dione
- AD:
-
androst-4-ene-3,17-dione
- G6PD:
-
glucose 6-phosphate dehydrogenase
- GDH:
-
glucose dehydrogenase
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
References
Donova, M. V. (2017) Steroid bioconversions, Methods Mol. Biol., 1645, 1-13, https://doi.org/10.1007/978-1-4939-7183-1_1.
Wojtal, K., Trojnar, M. K., and Czuczwar, S. J. (2006) Endogenous neuroprotective factors: neurosteroids, Pharmacol. Rep., 58, 335-340.
Fegan, K. S., Rae, M. T., Critchley, H. O. D., and Hillier, S. G. (2008) Anti-inflammatory steroid signalling in the human peritoneum, J. Endocrinol., 196, 369-376, https://doi.org/10.1677/joe-07-0419.
Ali Shah, S. A., Sultan, S., and Adnan, H. S. (2013) A whole-cell biocatalysis application of steroidal drugs, Orient. J. Chem., 29, 389-403, https://doi.org/10.13005/ojc/290201.
Szaleniec, M., Wojtkiewicz, A. M., Borowski, T., Bernhardt, R., and Donova, M. (2018) Bacterial steroid hydroxylases: enzyme classes, their functions and comparison of their catalytic mechanisms, Appl. Microbiol. Biotechnol., 102, 8153-8171, https://doi.org/10.1007/s00253-018-9239-3.
Julsing, M. K., Cornelissen, S., Bühler, B., and Schmid, A. (2008) Heme-iron oxygenases: powerful industrial biocatalysts? Curr. Opin. Chem. Biol., 12, 177-186, https://doi.org/10.1016/j.cbpa.2008.01.029.
Bureik, M., and Bernhardt, R. (2007) in Modern Biooxidation. Enzymes, Reactions and Applications (Schmid, R. D., and Urlacher, V. B., eds) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp. 155-176.
Fernández-Cabezón, L., Galán, B., and García, J. L. (2018) New insights on steroid biotechnology, Front. Microbiol., 9, 958, https://doi.org/10.3389/fmicb.2018.00958.
Munro, A. W., Leys, D. G., McLean, K. J., Marshall, K. R., Ost, T. W., et al. (2002) P450 BM3: the very model of a modern flavocytochrome, Trends Biochem. Sci., 27, 250-257, https://doi.org/10.1016/s0968-0004(02)02086-8.
Guengerich, F. P. (1991) Reactions and significance of cytochrome P-450 enzymes, J. Biol. Chem., 266, 10019-10022, https://doi.org/10.1016/S0021-9258(18)99177-5.
Munro, A. W., Daff, S., Coggins, J. R., Lindsay, J. G., and Chapman, S. K. (1996) Probing electron transfer in flavocytochrome P-450 BM3 and its component domains, Eur. J. Biochem., 239, 403-409, https://doi.org/10.1111/j.1432-1033.1996.0403u.x.
Finnigan, J. D., Young, C., Cook, D. J., Charnock, S. J., and Black, G. W. (2020) Cytochromes P450 (P450s): A review of the class system with a focus on prokaryotic P450s, Adv. Protein Chem. Struct. Biol., 122, 289-320, https://doi.org/10.1016/bs.apcsb.2020.06.005.
Li, A., Acevedo-Rocha, C. G., D’Amore, L., Chen, J., Peng, Y., et al. (2020) Regio- and stereoselective steroid hydroxylation at C7 by cytochrome P450 monooxygenase mutants, Angew. Chem. Int. Ed., 59, 12499-12505, https://doi.org/10.1002/ange.202003139.
Tang, R., Ren, X., Xia, M., Shen, Y., Tu, L., et al. (2021) Efficient one-step biocatalytic multienzyme cascade strategy for direct conversion of phytosterol to C-17-hydroxylated steroids, Appl. Environ. Microbiol., 87, e00321-00321, https://doi.org/10.1128/AEM.00321-21.
Karpov, M. V., Nikolaeva, V. M., Fokina, V. V., Shutov, A. A., Kazantsev, A. V., et al. (2022) Creation and functional analysis of Mycolicibacterium smegmatis recombinant strains carrying the bacillary cytochromes CYP106A1 and CYP106A2 genes, Appl. Biochem. Microbiol., 58, 947-957, https://doi.org/10.1134/S0003683822090058.
Snapper, S. B., Melton, R. E., Mustafa, S., Kieser, T., and Jacobs, W. R. Jr. (1990) Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis, Mol. Microbiol., 4, 1911-1919, https://doi.org/10.1111/j.1365-2958.1990.tb02040.x.
Poulsen, C., Holton, S., Geerlof, A., Wilmanns, M., and Song, Y.-H. (2010) Stoichiometric protein complex formation and over expression using the prokaryotic native operon structure, FEBS Lett., 584, 669-674, https://doi.org/10.1016/j.febslet.2009.12.057.
Strizhov, N., Karpov, M., Sukhodolskaya, G., Nikolayeva, V., Fokina, V., et al. (2016) Development of mycobacterial strains producing testosterone, Proc. Nat. Acad. Sci. Belarus, Chem. Series, 3, 57-58.
Bertani, G. (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli, J. Bacteriol., 62, 293-300, https://doi.org/10.1128/jb.62.3.293-300.1951.
Sambrook, J., and Russell, D. W. (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, N.Y.
Daugelat, S., Kowall, J., Mattow, J., Bumann, D., Winter, R., et al. (2003) The RD1 proteins of Mycobacterium tuberculosis: expression in Mycobacterium smegmatis and biochemical characterization, Microbes Infect., 5, 1082-1095, https://doi.org/10.1016/s1286-4579(03)00205-3.
Laemmli, U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature, 227, 680-685, https://doi.org/10.1038/227680a0.
Zhao, Y.-Q., Liu, Y.-J., Ji, W.-T., Liu, K., Gao, B., et al. (2022) One-pot biosynthesis of 7β-hydroxyandrost-4-ene-3,17-dione from phytosterols by cofactor regeneration system in engineered Mycolicibacterium neoaurum, Microb. Cell Fact., 21, 59, https://doi.org/10.1186/s12934-022-01786-5.
Booth, W. T., Schlachter, C. R., Pote, S., Ussin, N., Mank, N. J., et al. (2018) Impact of an N-terminal polyhistidine tag on protein thermal stability, ACS Omega, 3, 760-768, https://doi.org/10.1021/acsomega.7b01598.
Lee, W.-H., Park, J.-B., Park, K., Kim, M.-D., and Seo, J.-H. (2007) Enhanced production of ɛ-caprolactone by overexpression of NADPH-regenerating glucose 6-phosphate dehydrogenase in recombinant Escherichia coli harboring cyclohexanone onooxygenase gene, Appl. Microbiol. Biotechnol., 76, 329-338, https://doi.org/10.1007/s00253-007-1016-7.
Wu, Y., Li, H., Zhang, X. M., Gong, J. S., Li, H., et al. (2015) Improvement of NADPH-dependent P450-mediated biotransformation of 7α, 15α-diOH-DHEA from DHEA by a dual cosubstrate-coupled system, Steroids, 101, 15-20, https://doi.org/10.1016/j.steroids.2015.05.005.
Dodson, R. M., Kraychy, S., Nicholson, R. T., and Mizuba, S. (1962) Microbiological transformations. IX. The 1β-hydroxylation of androstenedione, J. Org. Chem., 27, 3159-3164, https://doi.org/10.1021/jo01056a043.
Funding
This work was supported by the Russian Science Foundation (project no. 21-64-00024).
Author information
Authors and Affiliations
Contributions
V.Yu.P. planned and performed experiments, discussed the results, and prepared the manuscript; N.I.S. developed the study concepted and designed genetic constructs; M.V.K. planned the experiments, discussed the results, and prepared the manuscript; V.M.N. purified steroids; A.V.K. performed NMR analysis; O.I.S. performed the experiments; A.A.Sh. conducted HPLC analysis; M.V.D. supervised the study and edited the manuscript.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain experimental studies involving animals or human subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Poshekhontseva, V.Y., Strizhov, N.I., Karpov, M.V. et al. Expression of Synthetic cyp102A1-LG23 Gene and Functional Analysis of Recombinant Cytochrome P450 BM3-LG23 in the Actinobacterium Mycolicibacterium smegmatis. Biochemistry Moscow 88, 1347–1355 (2023). https://doi.org/10.1134/S0006297923090146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923090146