Abstract
Apolipoprotein A-I (ApoA-I) is a key component of reverse cholesterol transport in humans. In the previous studies, we demonstrated expression of the apoA-I gene in human monocytes and macrophages; however, little is known on the regulation of the apoA-I expression in macrophages during the uptake of modified low-density lipoprotein (LDL), which is one of the key processes in the early stages of atherogenesis leading to formation of foam cells. Here, we demonstrate a complex nature of the apoA-I regulation in human macrophages during the uptake of oxidized LDL (oxLDL). Incubation of macrophages with oxLDL induced expression of the apoA-I gene within the first 24 hours, but suppressed it after 48 h. Both effects depended on the interaction of oxLDL with the TLR4 receptor, rather than on the oxLDL uptake by the macrophages. The oxLDL-mediated downregulation of the apoA-I gene depended on the ERK1/2 and JNK cascades, as well as on the NF-κB cascade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Atherosclerosis is a systemic chronic disease of elastic and muscular arteries resulting from disruption of the lipid and protein metabolism and accompanied by deposition of cholesterol and some lipoproteins in the blood vessel intima [1]. Apolipoprotein A-I (ApoA-I) is a key component of the human cholesterol reverse transport. As a major component of the high-density lipoprotein (HDL), ApoA-I promotes cholesterol release from the tissues and its transport to the liver for further oxidation and excretion from the body [2]. Beside participation in the cholesterol reverse transport, ApoA-I acts a cofactor of lecithin-cholesterol acyltransferase [3], displays antioxidant properties [4], and suppresses inflammatory response. In particular, ApoA-I blocks macrophage activation by T lymphocytes and inhibits production of tumor necrosis factor α (TNFα) and interleukin 1β (IL-1β) [5, 6]. ApoA-I also suppresses another proinflammatory factor – C-reactive protein [7]. On the other hand, ApoA-I is a negative marker of acute inflammatory response. Thus, inflammation development is accompanied by significant downregulation of the apoA-I gene expression in the liver and small intestine, while the ApoA-I protein in circulation is replaced in the HDL by the serum amyloid and then degraded by serum proteases [5]. It was established that HDL is the major transporter of cholesterol to the sites of steroid hormone synthesis in steroidogenic tissues and organs of mammals (including humans) and that the process is controlled by ApoA-I protein [8].
In humans, ApoA-I is synthesized predominately in liver and small intestine [9]. Earlier, we demonstrated the presence of both ApoA-I mRNA and protein in human macrophages and monocytes, although the functions of ApoA-I in these cells remain obscure [10-12]. Endogenous ApoA-I displays anti-inflammatory activity in macrophages. Suppression of the ApoA-I biosynthesis by RNA interference promoted synthesis and secretion of TNFα, upregulated expression of the lipopolysaccharide (LPS) receptor TLR4, and activated the inflammatory response of macrophages to LPS [10]. In contrast to hepatocytes, where ApoA-I secretion results in production of blood plasma HDL, ApoA-I secreted by macrophages remains bound to the outer surface of the macrophage plasma membrane (macrophage surface ApoA-I). This binding occurs mostly due to the ApoA-I interaction with the cassette transporter ABCA1. Moreover, the level of ABCA1 in the macrophages strongly correlates with the amount of the cell surface ApoA-I, since inhibition of the ApoA-I synthesis results in the decreased ABCA1 content [10]. Of particular interest are the data on the antiatherogenic role of the human apoA-I gene after its transfer to mouse macrophages with the use of viral vectors. It was demonstrated that delivery of the apoA-I gene to the macrophages of mice deficient in the apoA-I gene (apoA-I–/–) reduced formation of fatty streaks on the arterial walls and promoted cholesterol reverse transport from the macrophages to the liver [13]. Delivery of the apoE-deficient bone marrow cells that were infected with a retroviral vector expressing human apoA-I to the apoA-I–/– mice significantly reduced the atherosclerotic lesion areas in the aorta [14, 15]. Similar results were obtained by the delivery of the lentiviral vectors expressing human apoA-I gene to the mouse macrophages [16]. Therefore, available data indicate important antiatherogenic role of the apoA-I expression in the macrophages.
The apoA-I gene has three promoters. The classic promoter (positions –41 to +1 relative to the transcription start) has the TATA box at position –39 [17, 18]. The alternative proximal and distal promoters have the transcription starts at positions –153 and –353 relative to the transcription start of the classic apoA-I promoter [19]. Hepatic enhancer (positions –222 to –110) [17] controls the apoA-I expression in liver and includes sites A (–214 to –192), B (–169 to –146), and C (–134 to –119) [18]. In hepatocytes, sites A and C bind the apoA-I gene repressors LXRα, LXRβ [20], ARP1 [21], PPARγ [22] as well as the activators HNF4α [23], RXRα [24], PPARα [25], while the transcription factors HNF3β (FOXA2) [26], and FOXO1 [27, 28] bind to the site B.
Unlike the relatively the well-studied regulation of the apoA-I expression in hepatocytes and enterocytes, little is known on its control in macrophages, in which only the classic apoA-I promoter is active [10]. Earlier, we demonstrated that TNFα and hypoxia induce the apoA-I gene transcription and ApoA-I synthesis in the macrophages [10-12]. Interestingly, regulation of apoA-I in hepatocytes mirrors its regulation in macrophages. Thus, PPARα activates apoA-I expression in hepatocytes, but suppresses it in macrophages, while other nuclear receptors (LXRα and LXRβ) repress apoA-I transcription in hepatocytes and activate it in macrophages [11].
An increased concentration of low-density lipoprotein (LDL) and LDL-bound cholesterol in the blood plasma is one of the major risk factors in the development of ischemic heart disease (clinical manifestation of atherosclerosis) [29]. After entering the intima of the arteries, LDL are retained there due to interactions with the negatively charged components of the extracellular matrix (heparin sulfates) and are oxidized by free radicals and enzymes (myeloperoxidases and lipoxygenases) generating oxidized forms (oxLDL) [30]. oxLDL activates endothelial cells [31], thus promoting migration of monocytes to the intima, where they differentiate into macrophages and actively capture oxLDL via scavenger receptors (SR-A, CD36, SR-B1, LOX1, etc.). Uncontrolled scavenging of oxLDL by the macrophages results in accumulation of the lipid droplets [expansion of endoplasmic reticulum (ER) containing cholesterol esters] and macrophage transformation into foam cells, the major cellular component of atherosclerotic plaques [32, 33]. Besides being captured by the macrophages, oxLDL also plays signaling role, although the data on the ability of oxLDL to trigger signaling cascades in monocytes and macrophages are contradictory. Some researches claim that oxLDL interacts with the CD36–TLR2–TLR4 [34], CD36–TLR6–TLR4 [35], and TLR4–DC–SIGN [36] receptor complexes, which results in initiation of the inflammatory response and activation of oxLDL uptake by macrophages. On the other hand, a number of studies have demonstrated that the proinflammatory activity of oxLDL preparations is due to their contamination with the endotoxin, while the endotoxin-free oxLDL, on the contrary, suppresses synthesis of the proinflammatory cytokines by the macrophages [37, 38].
Little is known on the apoA-I regulation during the uptake of oxLDL by macrophages, key process of the early stages of atherogenesis resulting in formation of foam cells. Here, we demonstrate intricate dynamics of the apoA-I expression in human macrophages upon oxLDL uptake. The first 24 h of incubation of THP-1 macrophages (human monocytic acute leukemia cells) with oxLDL were characterized by the induction of the apoA-I expression (at both mRNA and protein levels), while after 48 h of incubation, the apoA-I expression was suppressed. Both effects depended on the oxLDL interaction with the TLR4 receptor, but were not directly related to the oxLDL uptake by the macrophages. Suppression of the apoA-I expression after incubation with oxLDL for 48 h depended on activation of the ERK1/2 and JNK (but not p38) signaling cascades and the transcription factor NF-κB signaling pathway.
MATERIALS AND METHODS
Cell cultures and differentiation of macrophages from peripheral blood monocytes. Human acute monocytic leukemia THP-1 cells were obtained from the Vertebrate Cell Culture Collection of the Institute of Cytology, Russian Academy of Sciences. Cells were cultured in a RPMI-1640 medium (Biolot, Russia) supplemented with 10% fetal calf serum (FCS; HyClone Laboratories, USA) in 5% CO2 at 37°C. Differentiation of THP-1 monocytes into macrophages was induced by addition of 50 ng/ml (81 nM) phorbol 12-myristate 13-acetate (PMA) for 24 h. Next, PMA was washed off, the medium was replaced with fresh RPMI-1640 supplemented with 10% FCS, and the cells were differentiated for another 48 h. Total time of THP-1 cell differentiation was 3 days for the experiments on 24-h incubation with oxLDL or 4 days for the incubation with oxLDL for 48 h.
Preserved donor blood no longer suitable for transfusion was purchased from the Blood Transfusion Station (St. Petersburg, Russia); all donors have signed the informed consent for the use of their blood. To obtain primary macrophages, mononuclear cells were isolated from the blood by Ficoll density gradient centrifugation as described in [39]. For this, 15 ml of a Ficoll solution with density of 1.077 (Biolot) was placed in a 50-ml centrifuge tube; 35 ml of blood was layered onto Ficoll, and the tubes were centrifuged for 30 min at 2000 rpm at 18°C in a Z400K centrifuge (Hermle Labortechnik, Germany). Mononuclear cells were collected from the interphase and washed twice with Hanks’ solution. The pellet was resuspended in RPMI-1640 supplemented with 10% FCS, plated into 96-well plates (100,000 cells per well), and incubated at 37°C in 5% CO2 for 2 h for monocyte adhesion. The wells were washed with Hanks’ solution to remove unattached cells (lymphocytes), and fresh RPMI-1640 medium supplemented with 10% FCS was added to the wells. The cells were differentiated into macrophages at 37°C in 5% CO2 for five days (in experiments on the incubation with oxLDL for 24 h) or six days (in experiments on the incubation with oxLDL for 48 h).
Antibodies and inhibitors. ApoA-I on the cell surface was detected with mouse monoclonal antibodies against human ApoA-I (Bio-Rad, USA) and secondary Alexa 647-labeled rabbit F(ab′)2 antibodies against mouse IgG (Abcam, Great Britain). TLR4 was blocked with goat polyclonal antibodies against human TLR4 (R&D Systems, USA). The following inhibitors of MAP kinase-mediated and NF-κB signaling cascades were used: U0126 (MEK1/2 inhibitor), SB203580 (p38 kinase inhibitor), SP600125 (JNK inhibitor), QNZ (NF-κB inhibitor), and TO901317 (synthetic agonist of LXRα and LXRβ nuclear receptors) (Biomol, Germany).
Purification, oxidation, and fluorescent labeling of LDL. LDL was isolated from the human blood plasma obtained from the preserved donor blood. To prevent cell contamination with the exogenous human ApoA-I, we also used LDL purified from the bovine serum (Biolot). LDL was isolated by sequential preparative ultracentrifugation as described in [40] using a Beckman Coulter Optima LE80K ultracentrifuge (Beckman Coulter, USA). For this, NaBr was added to the human plasma or bovine serum to the density of 1.019, and the solution was centrifuged for 18 h at 12°C at 40,000 rpm in a 50.3 Ti rotor. The upper phase containing very low-density lipoprotein was discarded. Density of the remaining solution was increased to 1.064 with NaBr, and the samples were centrifuged under the same conditions for 22 h. The upper LDL-containing phase was collected and dialyzed against phosphate buffered saline (PBS, pH 7.6) for 24 h at 4°C. Protein concentration was determined by the Lowry method [40]. LDL was oxidized as described by Jialal and Chait [41] by adding CuSO4 to the final concentration of 5 µM and incubation at 37°C for 18 h with the access of atmospheric oxygen. oxLDL was dialyzed against PBS twice for 24 h at 4°C. During the second dialysis procedure, 0.5 mM EDTA was added to the solution to remove residual copper ions. The extent of LDL oxidation was determined by measuring concentration of the thiobarbituric acid reactive substances (TBARS) [42]. oxLDL samples (100 µl; protein concentration 2-4 mg/ml) were incubated with c 0.5 ml of 20% acetic acid (pH 3.5) and 0.5 ml of 0.78% aqueous solution of thiobarbituric acid at 95°C for 45 min. Next, the samples were centrifuged at 4000 rpm for 5 min (Eppendorf, USA) and absorbance of the red pigment in the supernatant was measured at 532 nm. Calibration curve was constructed using malondialdehyde bis(dimethyl acetal) as a source of malonic aldehyde as described by Esterbauer and Cheeseman [43]. TBARS concentration in the oxLDL preparations used in the study was 30-45 nmol/mg of protein and 7-15 nmol/mg of protein for the native LDL. LDL was fluorescently labeled before oxidation using tetrafluorophenyl ester of the photostable hydrophilic dye Alexa 488 (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions.
To eliminate the effects of possible bacterial contamination in the experiments with oxLDL, polymyxin B (Sigma, USA) was added to the cell preparations to bind and inactivate bacterial LPS.
Monitoring of foam cell formation. THP-1 macrophages were washed three times with PBS, fixed in 4% formaldehyde in PBS for 10 min, washed with 60% isopropanol, and stained for 10 min with 3% Oil Red O (Sigma) in 60% isopropanol. After washing with 60% isopropanol, the cells were additionally stained with hematoxylin for 2 min and washed three times with PBS. The number of lipid droplets in the cells was determined under a Zeiss Axiovert 40CFL microscope (Zeiss, Germany).
RNA isolation and RT-qPCR. Total RNA was isolated from the cultured cells using an RNA STAT-60 kit (Tel-Test, USA) according to the manufacturer’s instructions. Residual genomic DNA was removed by treatment with RNase-free DNase I (Roche Applied Science, Switzerland) for 30 min at 37°C. The reaction was stopped by adding EDTA to the final concentration of 2 mM, and DNase I was inactivated by heating at 70°C for 15 min. RNA concentration and purity were evaluated using a Synergy 2 plate reader (BioTek, USA). The 260/280 nm absorbance ratio was over 2, and the 260/230 nm absorbance ratio was over 1.7. Absence of RNA degradation was confirmed by electrophoresis in 1% agarose gel based on the integrity of ribosomal RNAs. Reverse transcription (RT) was performed using the same amount of total RNA (1 µg) for all samples, oligo-dT primer, 3′-primers specific for the apoA-I, ABCA1, and GRP78 genes, and reagents from Promega, USA.
Quantitative PCR (qPCR) was performed using the Taqman or SYBR Green protocols in a CFX-96 cycler (Bio-Rad). All reagents were from Sintol, Russia. Primers and fluorescent probes for the apoA-I, ABCA1, and reference genes cyclophilin A, β-actin, and rplp0 were described previously in [10, 22, 28, 44]. The following primers and probes designed with the Primer3 software (https://primer3.org/) were used for detection of the GRP78 mRNA: forward primer 5′-GTTCTTGCCGTTCAAGGTG-3′; reverse primer 5′-TTTCCCAAATAAGCCTCAGC-3′; and probe 5′-Cy5.5-TGCTCCTGAAGAAATTTCTGCCATGG-RTQ2-3′. Expression of the apoA-I mRNA was estimated relative to expression of the reference genes in the multiplex PCR. The results were normalized using geometrical mean for the three reference genes as described in [45]. The number of cycles for each gene was determined with a CFX-96 RealTime PCR System (Bio-Rad), so that the fluorescence level was ten times higher than the standard deviation of the background fluorescence. Relative expression of the apoA-I mRNA (% of control) was determined by the formula:
2(Ct(control) – Ct(experiment))*100.
Western assay. Cells were lysed in RIPA-50 buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 0.1% SDS and 0.01% NaN3, 1 mM PMSF, pH 7.4). Proteins were separated by 12% SDS-PAGE, transferred to nitrocellulose membrane (Amersham, Hybond-C, 0.45 µm pore size). The membrane was blocked in a 5% dried skim milk solution in PBS with 0.02% Tween 20 for 1 h at room temperature, immunoblotted with mouse monoclonal antibody against human ApoA-I for 12 h at 4°C, triple-rinsed in PBS with 0.02% Tween 20 and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive bands were detected using Enhanced Chemiluminescence system of detection (ECL) by Chemidoc XRS+ system (Bio-Rad). The quantification of western blotting results was performed by the densitometry with Image J software (https://imagej.nih.gov/ij/index.html).
Flow cytometry. The cells were fixed in 4% formaldehyde for 10 min at 22°C, washed three times with PBS containing 0.1 M glycine, and incubated for 40 min at 22°C in the blocking buffer [PBS containing 1% BSA, 3% FCS, nonspecific human IgG (1 µg/ml), and 0.02% (v/v) Tween 20]. The cells were incubated with mouse monoclonal antibodies against human ApoA-I (dilution, 1/250) in PBS containing 1% BSA and 0.02% (v/v) Tween 20 for 2 h at 22°C, washed three times with PBS, and incubated with the secondary Alexa 647-labeled rabbit F(ab′)2 anti-mouse IgG antibodies (dilution, 1/1000) in PBS containing 1% BSA and 0.02% (v/v) Tween 20 for 1 h at 22°C. The cells were washed 3 times with PBS, fixed with 1% formaldehyde in PBS, and used in flow cytometry experiments. The cells incubated with the secondary antibodies but not with the primary anti-ApoA-I antibodies were used as a control of the immune staining specificity (isotype control). Flow cytometry and cell sorting were performed using an Epics Altra flow cytometer (Beckman Coulter) and FCSalyzer software (https://sourceforge.net/projects/fcsalyzer/).
Statistical analysis. The results are presented as a mean ± standard error of the mean (SE). Normality of distribution was verified with the Shapiro–Wilk test. Significance of differences between the groups was estimated using the unpaired Student’s t-test. Multiple comparisons were performed using the Dunnett criterion. Difference between the groups was considered significant at p < 0.05. Statistical analysis was performed using Microsoft Excel program.
RESULTS
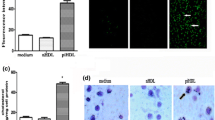
Incubation of macrophages with oxLDL stimulated apoA-I expression after 24 h and suppressed it after 48 and 72 h. The key process in formation of atherosclerotic plaques is activation of the uptake of modified LDL by macrophages accompanied by formation of foam cells [1, 32, 33]. It seemed interesting to investigate dynamics of the apoA-I expression during the oxLDL uptake by the THP-1 macrophages and formation of foam cells, which was monitored by staining the cells with lipophilic Oil Red O dye and hematoxylin (Fig. 1). Incubation of the macrophages with oxLDL (50 µg/ml) for 24 and 48 h resulted in a gradual accumulation of lipid droplets typical for the foam cells in the cell cytoplasm. At the same time, no complete filling of the macrophage cytoplasm with lipid droplets and necrotic cell death characteristic of the late stages of the foam cell formation were observed at the used oxLDL concentrations and incubation times (Fig. 1).
oxLDL is the major source of oxidized forms of cholesterol, which are natural ligands of the LXR nuclear receptors. Earlier, we demonstrated that activation of LXR receptors in the monocytes and macrophages is accompanied by activation of the apoA-I expression. Hence, it can be expected that the uptake of the oxLDL by macrophages should upregulate the apoA-I expression due to accumulation of the LXR ligands in the cells. To verify this hypothesis, THP-1 macrophages (Fig. 2a) or primary macrophages differentiated from the human peripheral blood monocytes (Fig. 2b), were incubated with oxLDL (50 µg/ml) for 24, 48, and 96 h. For comparison, control cells were incubated for the same periods of time with the synthetic LXR agonist. Analysis of gene expression by RT-qPCR showed that the 24 h incubation with oxLDL upregulated expression of the apoA-I gene to the level comparable to the activation induced by the LXR agonist. However, already after 48 h of incubation, the synthesis of the ApoA-I mRNA was suppressed, with even more pronounced suppression observed after 96 h. At the same time, the stimulating effect of the LXR agonist progressively increased over the entire incubation period.
Dependence of the apoA-I expression in THP-1 macrophages (a, c-e) and primary macrophages differentiated from human peripheral monocytes (b) on the time of incubation with oxLDL (50 µg/ml): a and c-e) THP-1 cells were differentiated in the presence of PMA (50 ng/ml) for 2 days and then incubated with human plasma oxLDL (oxLDL-h, 50 µg/ml), bovine plasma oxLDL (oxLDL-b, 50 µg/ml), or LXR agonist TO901317 (5 µM) for the indicated periods of time; b) monocytes isolated from the peripheral blood of healthy donors were differentiated for 4 days and then incubated with human plasma oxLDL (oxLDL-h, 50 µg/ml), bovine plasma oxLDL (oxLDL-b, 50 µg/ml), or LXR agonist TO901317 (5 µM) for the indicated periods of time; c) dependence of the ApoA-I mRNA level on the oxLDL concentration after incubation for 24 and 48 h. a-c) Total RNA was isolated and expression of the ApoA-I mRNA was determined by RT-qPCR. All data were normalized to the expression of three reference genes (cyclophilin A, β-actin, and rplp0). The data are shown as mean ± SE (error bars). Difference between the experimental and control groups was evaluated using the Student’s t-test; # p < 0.05. d) ApoA-I protein levels in THP-1 macrophages after incubation with oxLDL (50 µg/ml) for 24 and 48 h (according to Western blot analysis and densitometry from three independent experiments); y-axis, relative content of ApoA-I protein in arbitrary units, AU (the content of ApoA-I in untreated cells was taken as one AU). The amount of ApoA-I protein was normalized to the β-actin content. e) Flow cytometry analysis of THP-1 macrophages incubated with human oxLDL (50 µg/ml) for 24 and 48 h. The diagram shows mean values of the fluorescence intensity ± SE (error bars). Difference between the experimental and control groups was evaluated with the Student’s t-test; * p < 0.05.
oxLDL preparations isolated from the human blood can contain impurities of exogenous ApoA-I. Since the level of ApoA-I expression in the macrophages is very low, exogenous ApoA-I can significantly distort the results of the ApoA-I content estimation upon macrophage incubation with oxLDL. To prevent the possibility of such contamination, oxLDL preparations isolated from the bovine serum can be used [10]. On the other hand, it is impossible to rule out that the action of oxLDL on the macrophages is species-specific. To verify this suggestion, we conducted a series of experiments on incubation of THP-1 macrophages and macrophages differentiated from the human peripheral blood monocytes with human and bovine oxLDL (Fig. 2, a and b). No significant difference between the effects of human and bovine oxLDL on the ApoA-I mRNA levels were observed at all incubation times. All following experiments were performed with bovine oxLDL. Figure 2c shows dependence of the ApoA-I mRNA level on the oxLDL dose. oxLDL stimulated ApoA-I mRNA expression starting from 15 µg/ml, but suppressed it at the concentrations of 25 µg/ml and above; both stimulation and suppression being dose-dependent. Upregulation of the apoA-I expression by THP-1 macrophages after 24 h incubation with oxLDL was accompanied by the increase in the ApoA-I protein content in the cells (Fig. 2d). Interestingly, content of the ApoA-I protein after 48 h of incubation was at the level typical for the control (unstimulated) cells, unlike the level of ApoA-I mRNA, which was lower than in the control (Fig. 2d). Previous studies of the ApoA-I functions in the macrophages have revealed that the amount of the ApoA-I protein bound to the outer surface of the plasma membrane is more important than the total cellular content of ApoA-I [10]. In this regard, it seemed interesting to estimate the effect of oxLDL on the surface ApoA-I (Fig. 2e). It was found that incubation of the THP-1 macrophages with oxLDL for 24 h led to the increase in the surface ApoA-I; however, after 48 h, the content of the surface ApoA-I decreased below the level typical for unstimulated cells. These results indicate that even if oxLDL stimulated the apoA-I expression at the early stages of foam cell formation (presumably via accumulation of the LXR ligands), this effect was reversed already on the second day of the experiment.
Suppression of the apoA-I expression upon prolonged incubation with oxLDL is not related to the ER stress or lipid accumulation in the cells. Suppression of the apoA-I expression can occur via the following three mechanisms. Firstly, formation of foam cells can be associated with the ER stress development [46, 47] and lead to downregulation of the genes coding for the secretory proteins with signaling peptides that are translated into the ER lumen. Development of ER stress in the macrophages incubated with oxLDL for 48 h was verified from the expression of the GRP78 gene encoding for one of the ER chaperones, since expression of this gene is known to be activated under ER stress. THP-1 macrophages stimulated with tunicamycin, which blocks protein N-glycosylation in the ER and induces ER stress, were used as a positive control (Fig. 3a). However, we observed no ER stress in our experiments, as the incubation of macrophages with oxLDL did not stimulate the GRP78 expression.
Effect of the oxLDL uptake by the macrophages on the apoA-I expression: a) incubation of THP-1 macrophages with oxLDL does not induce the ER stress. THP-1 cells were differentiated in the presence of PMA (50 ng/ml) for 2 day and then incubated with oxLDL (50 µg/ml) for 24 and 48 h or with tunicamycin (1 µg/ml) for 24 h. Control samples contained 50 µg/ml BSA. Total RNA was isolated from the cells and the level of the GRP78 mRNA was determined by RT-qPCR. c-d) Dependence of the apoA-I and ABCA1 gene expression on the capture of oxLDL by THP-1 macrophages. THP-1 cells were differentiated in the presence of PMA (50 ng/ml) for 2 days and then incubated with fluorescently labeled (Alexa 488) oxLDL (50 µg/ml) for 48 h. b) Flow cytometry analysis and cell sorting of the THP-1 macrophages incubated with labeled oxLDL. Dotted line with gray filling under the curve, cells incubated with BSA (negative control); solid black line, cells incubated with Alexa 488-oxLDL; oxLDL– and oxLDL+, gates used for the cell sorting (low and high oxLDL content, respectively). Total RNA was isolated from the cells and used for analysis of the apoA-I (c) and ABCA1 (d) gene expression by RT-qPCR. The results were normalized to the expression of three reference genes (cyclophilin A, β-actin, and rplp0) and are shown as mean intensity ± SE (error bars) from 3 independent experiments. The significance of differences between the untreated cells (control) and cells incubated with oxLDL was evaluated using the Student’s t-test; * p < 0.05; n.d., no statistically significant differences between the macrophages with the high (oxLDL+) and low (oxLDL–) content of captured oxLDL.
Secondly, apoA-I expression could be affected by accumulation of the oxLDL metabolism products, some of which can act as ligands of nuclear receptors (PPARα, PPARγ, LXRα, LXRβ) that are capable of binding to the 5′-regulatory region of the apoA-I gene and modulating its expression. Although the hypothesis of LXR involvement in the oxLDL-mediated suppression of the ApoA-I biosynthesis was refuted by the data presented in Fig. 2, the question on the role of other nuclear receptors in this process remains open.
Thirdly, according to the published data, oxLDL interacts with the TLR4 receptor, leading to activation of the proinflammatory signaling in the cells [48]. Moreover, oxLDL internalization by the scavenger receptors, such as CD36 and LOX1, is accompanied by initiation of the signaling pathways in the macrophages [49, 50]. To verify the role of the second suggested mechanism in the suppression of ApoA-I synthesis during prolonged incubation with oxLDL, we performed flow cytometry sorting of the THP-1 macrophages after 48-h incubation of these cells with fluorescently labeled oxLDL. Under the used experimental conditions, only ~65% macrophages acquired the foam cell phenotype (Fig. 3b). Total RNA was isolated from the LDL+ and LDL– macrophages and used for estimation of the apoA-I mRNA levels vs. control cells incubated in the absence of oxLDL (Fig. 3c). The efficiency of sorting was controlled by evaluation of the ABCA1 gene expression (Fig. 3d), which is known to correlate positively with the amount of captured oxLDL [51]. We found that the content of ABCA1 mRNA in the LDL– cells was the same as in the control cells. At the same time, expression of the ABCA1 mRNA in the LDL+ cells was significantly upregulated, which was in agreement with the earlier published data. These results confirm validity of the chosen methodological approach. Under the same experimental conditions, the content of the ApoA-I mRNA was the same in the LDL+ and LDL– cells and comprised ~50% of the its level in the control cells. These results demonstrate that suppression of the apoA-I expression upon prolonged incubation with oxLDL was not related to accumulation of the lipid compounds in the macrophages and support the hypothesis on the role of signaling mechanisms in this process.
The role of TLR4 in the apoA-I expression regulation in the macrophages incubated with oxLDL. The most probable candidate for the oxLDL receptor is TLR4, which is a natural receptor of LPS. Interaction of oxLDL with TLR4 also causes activation of the proinflammatory cascades. To verify the possible role of TLR4 in the regulation of the apoA-I expression upon the action of oxLDL, TLR4 was blocked with the corresponding antibodies (Fig. 4). In the case of 24 h incubation, blocking of TLR4 resulted in the reversion of the stimulating effect of oxLDL (Fig. 4a), while in the case of 48 h incubation, preliminary treatment of the macrophages with the anti-TLR4 antibodies completely abolished the effect of oxLDL, but not the effect of the LXR ligand (Fig. 4b). Therefore, both the stimulatory (24 h incubation) and inhibitory (48 h incubation) effects of oxLDL on the ApoA-I mRNA level in the macrophages was determined by the interaction of oxLDL with TLR4 and activation of the related signaling pathways.
Role of TLR4 in the regulation of the apoA-I expression in the macrophages incubated with oxLDL for 24 h (a) and 48 h (b). THP-1 cells were differentiated in the presence of PMA (50 ng/ml) for 2 days and then treated with antibodies against human TLR4 (anti-TLR4 AB; 5 µg/ml) for 15 min. Control samples were treated with human nonspecific IgG (5 µg/ml). Next, the cells were incubated with oxLDL (50 µg/ml) or LXR agonist TO901317 (5 µM) for 24 or 48 h. Control samples contained BSA (50 µg/ml). Total RNA was isolated from the cells and used for estimation of the ApoA-I mRNA level by RT-qPCR. The results were normalized to the expression of three reference genes (cyclophilin A, β-actin, and rplp0) and are shown as mean ± SE (error bars) from 3 independent experiments. The significance of differences between the untreated cells (control) and cells incubated with oxLDL or TO901317 was evaluated using the Dunnett criterion; # p < 0.05.
The role of MAP kinase and NF-κB signaling cascades in the TLR4-mediated regulation of the apoA-I expression. It was demonstrated that the TLR4 activation in macrophages initiates a number of signaling pathways, such as MAP kinase (p38, ERK1/2, JNK1/2/3) cascades and cascade resulting in induction of the proinflammatory transcription factor NF-κB [52]. Here, we studied the role of proinflammatory cascades in the THP-1 macrophages incubated with oxLDL for 48 h to exclude possible interference with the TLR4-independent signaling pathways. To elucidate the role of MAP kinase and NF-κB cascades in the suppression of the apoA-I gene upon macrophage incubation with oxLDL for 48 h, we used the corresponding inhibitors (Fig. 5). Inhibition of the NF-κB, JNK, and ERK1/2 pathways resulted in suppression of the apoA-I expression in THP-1 macrophages, thus indicating involvement of these signaling pathways in the regulation of the apoA-I gene. At the same time, incubation of macrophages with oxLDL in the presence of the signaling inhibitors did not cause further decrease in the ApoA-I mRNA level. Therefore, suppression of the ApoA-I synthesis by oxLDL involves ERK1/2 and JNK (but not p38) cascades and NF-κB signaling pathway (if the effect of oxLDL is independent on these signaling cascades, the effect of their inhibitors and oxLDL on the ApoA-I mRNA level would have been cumulative). Therefore, we can conclude that suppression of the apoA-I expression upon 48 h incubation with oxLDL is determined by the TLR4-mediated activation of the NF-κB, JNK, and ERK1/2 (but not p38).
Role of TLR4-mediated signaling cascades in suppression of the apoA-I expression in the macrophages incubated with oxLDL for 48 h. THP-1 cells were differentiated in the presence of PMA (50 ng/ml) for 2 days and then treated with the p38 MAPK inhibitor SB203580 (25 µM), JNK1/2/3 inhibitor SP600125 (10 µM), MEK1/2 inhibitor U0126 (10 µM), or NF-κB inhibitor QNZ (10 nM) for 1 h. Next, the cells were incubated with oxLDL (50 µg/ml) or BSA (50 µg/ml, control) for 48 h. Expression of the apoA-I gene was evaluated by RT-qPCR. The results were normalized to the expression of three reference genes (cyclophilin A, β-actin, and rplp0) and are shown as mean ± SE (error bars) from 3 independent experiments. The significance of differences between the cells incubated with oxLDL and BSA (control) was evaluated using the Dunnett criterion; # p < 0.05.
DISCUSSION
Functions of the endogenous ApoA-I in the macrophages, details of regulation of the apoA-I gene, and possible involvement of the endogenous ApoA-I in atherogenesis still remain poorly understood. The anti-inflammatory activity of ApoA-I in the macrophages demonstrated by us earlier and its ability to stabilize the ABCA1 cassette transporter [10], as well as the data on the effect of exogenous human apoA-I gene expression in the mouse macrophages on the development of atherosclerotic lesions [13-16] allow to state with confidence that ApoA-I has antiatherogenic effect in the macrophages. Here, we studied for the first time dynamics of the apoA-I expression in the macrophages in the process of oxLDL uptake, which leads to formation of the foam cells. Accumulation of lipids, in particular, cholesterol, in the macrophages results in activation of the genes coding for proteins involved in the reverse cholesterol transport, e.g., cassette transporters ABCA1 and ABCG1. The key role in this process belongs to the nuclear receptors PPARγ and LXRs, whose agonists are accumulated in the cells upon the oxLDL uptake [51]. The ABCA1 and ABCG1 genes are direct targets of the LXR transcription factors (regulatory regions of ABCA1 and ABCG1 contain LXR-binding sites), whereas the LXRβ gene is activated by PPARγ [51]. The regulatory region of human apoA-I gene also contains binding site for LXRs (site C in the hepatic enhancer) [20]. Moreover, LXR transcription factors in hepatocytes interact with the site C and suppress transcription of the apoA-I gene [20, 32]. Earlier, we demonstrated that LXRs retain its ability to interact with the site C in the macrophages; however, in these cells, LXRs activate expression of the apoA-I gene [11]. Therefore, it might have been expected that the uptake of oxLDL by macrophages would be accompanied not only by induction of the ABCA1 and ABCG1 expression, but also by activation of the apoA-I gene via the same LXR-dependent mechanism. However, experimental verification of this hypothesis revealed more complex mechanisms involved in the modulation of apoA-I gene activity upon the oxLDL uptake by the macrophages. Although incubation with oxLDL for 24 h upregulated expression of the apoA-I gene both at the mRNA and protein levels, this process was independent on accumulation of the LXR ligands, as follows from the results shown in Fig. 4a. In particular, blocking of TLR4 reversed the effect of oxLDL on the apoA-I activity. In macrophages, LXRs activate the apoA-I gene (see the effect of LXR agonist LXR TO901317, Fig. 2, a and b); hence, if the apoA-I activation after 24 h incubation was caused by accumulation of the LXR ligands, blocking TLR4 would have had no influence on the action of oxLDL or would have weakened the stimulating effect of these compounds. In reality, blocking TLR4 reversed the effect of oxLDL on the ApoA-I mRNA level, i.e., suppressed expression of the apoA-I gene already after 24 h of incubation. These results can be explained by the bidirectional effect of oxLDL on the apoA-I activity, such as stimulation of this gene via the TLR4 could be accompanied by initiation of other signaling cascades leading to the apoA-I downregulation. The stimulatory effect of TLR4 is strong, so the negative apoA-I regulation can be observed only upon the complete blocking of TLR4. Signaling pathways involved in this negative regulation remain unknown and have to be elucidated in future studies. The published data on the ability of oxLDL to initiate signaling cascades by interacting with TLR4 are contradictory (see “Introduction” section). Thus, some authors state that the observed activation of signaling cascades is caused by the binding of endotoxin, which contaminates the oxLDL preparations, to the TLR4 [37, 38]. To rule out this possibility, we added polymyxin B (compound that binds to and neutralizes endotoxin) to the preparations of oxLDL used in our experiments. Therefore, the obtained data indicate that activation of the ApoA-I synthesis in the macrophages incubated with oxLDL for 24 h was related to activation of the signaling cascades initiated by the oxLDL interaction with TLR4.
Investigation of the long-term effects of oxLDL on the apoA-I activity also produced unexpected results. Unlike the LXR ligand, stimulating effect of which increased with time, oxLDL progressively suppressed the apoA-I expression after 48 h of incubation. One of the possible explanations for this reversion of the effect could be the ER stress observed earlier upon the oxLDL uptake by the macrophages [46, 47]. However, we detected no ER stress in our experiments. The reasons for these discrepancies could be insufficiently long incubation times or low oxLDL concentrations used in the experiments. An indirect evidence in favor of this explanation is low level of apoptosis (<10%) in the macrophages incubated with oxLDL, which indicates that the studied cells were at the early stages of the foam cell formation, when the ER stress has not yet developed (data not shown).
Suppression of the apoA-I gene upon prolonged incubation with oxLDL cannot be explained by accumulation of the lipids and their derivatives in the cells. Cell sorting of the macrophages after incubation with the fluorescently labeled oxLDL showed that, unlike the expression of the ABCA1 gene induced exclusively in the cells with high amounts of captured oxLDL, expression of the apoA-I gene was suppressed to the same extent in the macrophages with a low and high lipid content. These results unambiguously indicate signaling mechanism of the oxLDL action. Blocking of the TLR4 receptor confirmed this hypothesis, as the inhibitory effect of oxLDL was abolished in this case. Therefore, suppression of the apoA-I activity upon prolonged incubation of the macrophages with oxLDL was associated with the TLR4-mediated signaling cascades. Interestingly, stimulation of the same receptor by the same ligand can cause the opposite effect on the target gene depending on the incubation time, as it has already been described for the effect of TLR4 activation of the gene expression in macrophages. Thus, expression of the Ctnnb1, Maff, Zfp36l1, Dnaja4, MOUSE UPF04, and Slbp genes was activated by the LPS binding to TLR4 for the first 2-4 h, but then was suppressed for the next 8 h [53]. Inhibitor analysis revealed important role of the ERK1/2, JNK, and NF-κB cascades in the suppression of the apoA-I gene upon the prolonged incubation of the macrophages with oxLDL. It was shown earlier that these MAP kinase cascades are involved in activation of the apoA-I gene in human macrophages by TNFα [11] or hypoxia [12], and that LXR transcription factors play an important role in this process. It is unlikely that LXR is involved in the transcription suppression by oxLDL; most probably, this role belongs to the transcription factors (e.g., FOXO1-3) interacting with the site B of the apoA-I hepatic enhancer [27, 28]. It should be noted that the action of the inhibitors of MAP kinases and NF-κB on the apoA-I activity in human macrophages likely depends on the degree of macrophage differentiation. Blocking any of the three main MAP kinase cascades or the NF-κB transcription factor in the monocytes results in the increase of the ApoA-I mRNA level [11]. As the cells undergo differentiation, the stimulatory effect of the inhibitors becomes less pronounced and then reverses to inhibition [11, 12]. Differences in the rate of monocyte differentiation into macrophages in different experiments, increase in the ApoA-I mRNA level in the macrophages during differentiation [10], as well as divergence of macrophages into the ApoA-I-enriched and ApoA-I-poor pools [10, 11] during differentiation, make it difficult to compare the influence of inhibitors on the basal levels of ApoA-I mRNA from different experiments. Nevertheless, this obstacle should not prevent the use of inhibitor analysis for elucidating the role of signaling cascades in the action of external stimuli on the apoA-I activity in human macrophages. Another potential difficulty in interpretation of the results of inhibitor analysis may be nonspecific activity of the inhibitors. For example, it was reported that high concentrations of SB203580 (p38 kinase inhibitor) suppress JNK kinases [54]. Although we did not observe such cross-inhibition (cell treatment with SB203580 did not produce the same effect as the specific JNK inhibitor SP600125), we cannot rule out that the used inhibitors affected other signaling pathways in the cells. Hence, our data on the role of MAP kinase cascades in the oxLDL-mediated suppression of the apoA-I expression in the macrophages should be viewed as preliminary and have to be verified in future studies (e.g., by using RNA interference).
Regulatory regions of the human apoA-I gene do not contain binding sites for NF-κB; nevertheless, this transcription factor is involved in the LPS– and TNFα-mediated suppression of the apoA-I expression in hepatocytes [43, 55], as well as in the induction of the apoA-I expression by TNFα [11] or hypoxia [12] in macrophages. The effect of NF-κB can be explained by its interaction with the nuclear receptor PPARα, which is a positive regulator of the apoA-I expression in the liver. Such interaction results in the mutual inhibition of both transcription factors (trans-repression) [55]. Interestingly, PPARα also regulates the apoA-I expression in the macrophages; however, in these cells, PPARα acts as a transcription repressor [11]. It is possible that the mechanisms of the NF-κB influence on the apoA-I expression in hepatocytes and macrophages are similar, but this issue requires further investigation.
In conclusion, we were the first to demonstrate changes in the expression of the apoA-I gene in human macrophages at the early stages of foam cell formation. These changes have a very complex and bidirectional dynamics. In particular, initial induction of the ApoA-I synthesis for the first 24 h is followed by the suppression of the apoA-I expression within 48 h. Both stimulation and suppression of the apoA-I expression are related to the interaction of oxLDL with TLR4 receptor rather than to the lipid accumulation in macrophages.
Abbreviations
- ApoA-I:
-
apolipoprotein A-I
- BSA:
-
bovine serum albumin
- ER:
-
endoplasmic reticulum
- FCS:
-
fetal calf serum
- LDL:
-
low-density lipoprotein
- oxLDL:
-
oxidized low-density lipoprotein
- PBS:
-
phosphate buffered saline
- PMA:
-
phorbol 12-myristate 13-acetate (activator of cell differentiation and/or apoptosis in cancer models)
- THP-1:
-
human monocytic cell line derived from an acute monocytic leukemia patient
- TNFα:
-
tumor necrosis factor α
References
Wolf, D., and Ley, K. (2019) Immunity and Inflammation in Atherosclerosis, Circ. Res., 124, 315-327, https://doi.org/10.1161/CIRCRESAHA.118.313591.
Zannis, V. I., Chroni, A., and Krieger, M (2006) Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL, J. Mol. Med., 84, 276-294, https://doi.org/10.1007/s00109-005-0030-4.
Nikiforova, A. A., Kheĭfets, G. M., Alksnis, E. G., Parfenova, N. S., and Klimov, A. N. (1988) HDL2b lipoproteins as an acceptor of cholesterol from erythrocyte membrane and the role of lecithin-cholesterol-acyltransferase during this process, Biochemistry (Moscow), 53, 1334-1338.
Shah, P. K., Kaul, S., Nilsson, J., and Cercek, B. (2001) Exploiting the vascular protective effects of high-density lipoprotein and its apolipoproteins: an idea whose time for testing is coming, Circulation, 104, 2376-2383, https://doi.org/10.1161/hc4401.098467.
Hyka, N., Dayer, J. M., Modoux, C., Kohno, T., Edwards, C. K. 3rd, et al. (2001) Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes, Blood, 97, 2381-2389, https://doi.org/10.1182/blood.v97.8.2381.
Burger, D., and Dayer, J. M. (2002) High-density lipoprotein-associated apolipoprotein A-I: the missing link between infection and chronic inflammation? Autoimmun Rev., 1, 111-117, https://doi.org/10.1016/s1568-9972(01)00018-0.
Wadham, C., Albanese, N., Roberts, J., Wang, L., Bagley, C. J., et al. (2004) High-density lipoproteins neutralize C-reactive protein proinflammatory activity, Circulation, 109, 2116-2122, https://doi.org/10.1161/01.CIR.0000127419.45975.26.
Connelly, M. A., and Williams, D. L. (2004) SR-BI and HDL cholesteryl ester metabolism, Endocr. Res., 30, 697-703, https://doi.org/10.1081/erc-200043979.
Walsh, A., Ito, Y., and Breslow, J. L. (1989) High levels of human apolipoprotein A-I in transgenic mice result in increased plasma levels of small high density lipoprotein (HDL) particles comparable to human HDL3, J. Biol. Chem., 264, 6488-6494.
Mogilenko, D. A., Orlov, S. V., Trulioff, A. S., Ivanov, A. V., Nagumanov, V. K., et al. (2012) Endogenous apolipoprotein A-I stabilizes ATP-binding cassette transporter A1 and modulates Toll-like receptor 4 signaling in human macrophages, FASEB J., 26, 2019-2030, https://doi.org/10.1096/fj.11-193946.
Shavva, V. S., Mogilenko, D. A., Nekrasova, E. V., Trulioff, A. S., Kudriavtsev, I. V., et al. (2018) Tumor necrosis factor alpha stimulates endogenous apolipoprotein A-I expression and secretion by human monocytes and macrophages: role of MAP-kinases, NF-κB, and nuclear receptors PPARα and LXRs, Mol. Cell. Biochem., 448, 211-223, https://doi.org/10.1007/s11010-018-3327-7.
Bogomolova, A. M., Shavva, V. S., Nikitin, A. A., Nekrasova, E. V., Dizhe, E. B., et al. (2019) Hypoxia as a factor involved in the regulation of the apoA-1, ABCA1, and complement C3 gene expression in human macrophages, Biochemistry (Moscow), 84, 529-539, https://doi.org/10.1134/S0006297919050079.
Major, A. S., Dove, D. E., Ishiguro, H., Su, Y. R., Brown, A. M., et al. (2001) Increased cholesterol efflux in apolipoprotein AI (ApoAI)-producing macrophages as a mechanism for reduced atherosclerosis in ApoAI((–/–)) mice, Arterioscler. Thromb. Vasc. Biol., 21, 1790-1795, https://doi.org/10.1161/hq1101.097798.
Ishiguro, H., Yoshida, H., Major, A. S., Zhu, T., Babaev, V. R., et al. (2001) Retrovirus-mediated expression of apolipoprotein A-I in the macrophage protects against atherosclerosis in vivo, J. Biol. Chem., 276, 36742-36748, https://doi.org/10.1074/jbc.M106027200.
Su, Y. R., Ishiguro, H., Major, A. S., Dove, D. E., Zhang, W., et al. (2003) Macrophage apolipoprotein A-I expression protects against atherosclerosis in ApoE-deficient mice and up-regulates ABC transporters, Mol. Ther., 8, 576-583, https://doi.org/10.1016/s1525-0016(03)00214-4.
Su, Y. R., Blakemore, J. L., Zhang, Y., Linton, M. F., and Fazio, S. (2008) Lentiviral transduction of apoAI into hematopoietic progenitor cells and macrophages: applications to cell therapy of atherosclerosis, Arterioscler. Thromb. Vasc. Biol., 28, 1439-1446, https://doi.org/10.1161/ATVBAHA.107.160093.
Higuchi, K., Law, S. W., Hoeg, J. M., Schumacher, U. K., Meglin, N., and Brewer, H. B. Jr. (1988) Tissue-specific expression of apolipoprotein A-I (ApoA-I) is regulated by the 5′-flanking region of the human ApoA-I gene, J. Biol. Chem., 263, 18530-18536.
Widom, R. L., Ladias, J. A., Kouidou, S., and Karathanasis, S. K. (1991) Synergistic interactions between transcription factors control expression of the apolipoprotein AI gene in liver cells, Mol. Cell. Biol., 11, 677-687, https://doi.org/10.1128/mcb.11.2.677.
Mogilenko, D. A., Shavva, V. S., Dizhe, E. B., and Orlov, S. V. (2019) Characterization of distal and proximal alternative promoters of the human ApoA-I gene, Mol. Biol. (Mosk.), 53, 485-496, https://doi.org/10.1134/S0026898419030121.
Huuskonen, J., Vishnu, M., Chau, P., Fielding, P. E., and Fielding, C. J. (2006) Liver X receptor inhibits the synthesis and secretion of apolipoprotein A1 by human liver-derived cells, Biochemistry, 45, 15068-15074, https://doi.org/10.1021/bi061378y.
Ge, R., Rhee, M., Malik, S., and Karathanasis, S. K. (1994) Transcriptional repression of apolipoprotein AI gene expression by orphan receptor ARP-1, J. Biol. Chem., 269, 13185-13192.
Shavva, V. S., Mogilenko, D. A., Bogomolova, A. M., Nikitin, A. A., Dizhe, E. B., et al. (2016) PPARγ represses apolipoprotein A-I gene but impedes TNFalpha-mediated ApoA-I downregulation in HepG2 cells, J. Cell. Biochem., 117, 2010-2022, https://doi.org/10.1002/jcb.25498.
Chan, J., Nakabayashi, H., and Wong, N. C. (1993) HNF4 increases activity of the rat ApoA1 gene, Nucleic Acids Res., 21, 1205-1211, https://doi.org/10.1093/nar/21.5.1205.
Rottman, J. N., Widom, R. L., Nadal-Ginard, B., Mahdavi, V., and Karathanasis, S. K. (1991) A retinoic acid-responsive element in the apolipoprotein AI gene distinguishesbetween two different retinoic acid response pathways, Mol. Cell. Biol., 11, 3814-3820, https://doi.org/10.1128/mcb.11.7.3814.
Martin, C., Duez, H., Blanquart, C., Berezowski, V., Poulain, P., et al. (2001) Statin-induced inhibition of the Rho-signaling pathway activates PPARα and induces HDL apoA-I, J. Clin. Invest., 107, 1423-1432, https://doi.org/10.1172/JCI10852.
Harnish, D. C., Malik, S., Kilbourne, E., Costa, R., and Karathanasis, S. K. (1996) Control of apolipoprotein AI gene expression through synergistic interactions between hepatocyte nuclear factors 3 and 4, J. Biol. Chem., 271, 13621-13628, https://doi.org/10.1074/jbc.271.23.13621.
Shavva, V. S., Bogomolova, A. M., Nikitin, A. A., Dizhe, E. B., Oleinikova, G. N., et al. (2017) FOXO1 and LXRα downregulate the apolipoprotein A-I gene expression during hydrogen peroxide-induced oxidative stress in HepG2 cells, Cell Stress Chaperones, 22, 123-134, https://doi.org/10.1007/s12192-016-0749-6.
Shavva, V. S., Bogomolova, A. M., Nikitin, A. A., Dizhe, E. B., Tanyanskiy, D. A., et al. (2017) Insulin-mediated downregulation of apolipoprotein A-I gene in human hepatoma cell line HepG2: the role of interaction between FOXO1 and LXRβ transcription factors, J. Cell. Biochem., 118, 382-396, https://doi.org/10.1002/jcb.25651.
Libby, P., and Theroux, P. (2005) Pathophysiology of coronary artery disease, Circulation, 111, 3481-3488, https://doi.org/10.1161/CIRCULATIONAHA.105.537878.
Parthasarathy, S., Raghavamenon, A., Garelnabi, M. O., and Santanam, N. (2010) Oxidized low density lipoprotein, Methods Mol. Biol., 610, 403-417, https://doi.org/10.1007/978-1-60327-029-8_24.
Feng, Y., Cai, Z. R., Tang, Y., Hu, G., Lu, J., et al. (2014) TLR4/NF-κB signaling pathway-mediated and oxLDL-induced up-regulation of LOX-1, MCP-1, and VCAM-1 expressions in human umbilical vein endothelial cells, Genet. Mol. Res., 13, 680-695, https://doi.org/10.4238/2014.
Yu, X.-H., Fu, Y.-C., Zhang, D.-W., Yin, K., and Tang, C-K. (2013) Foam cells in atherosclerosis, Clin. Chim. Acta, 424, 245-252, https://doi.org/10.1016/j.cca.2013.06.006.
Chistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V., and Orekhov, A. N. (2017) Mechanisms of foam cell formation in atherosclerosis, J. Mol. Med. (Berl)., 95, 1153-1165, https://doi.org/10.1007/s00109-017-1575-8.
Chávez-Sánchez, L., Garza-Reyes, M. G., Espinosa-Luna, J. E., Chávez-Rueda, K., Legorreta-Haquet, M. V., and Blanco-Favela, F. (2014) The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans, Hum. Immunol., 75, 322-329, https://doi.org/10.1016/j.humimm.2014.01.012.
Stewart, C. R., Stuart, L. M., Wilkinson, K., van Gils, J. M., Deng, J., et al. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer, Nat. Immunol., 11, 155-161, https://doi.org/10.1038/ni.1836.
Yang, K., Liu, X., Liu, Y., Wang, X., Cao, L., et al. (2017) DC-SIGN and Toll-like receptor 4 mediate oxidized low-density lipoprotein-induced inflammatory responses in macrophages, Sci. Rep., 3296, 1-11, https://doi.org/10.1038/s41598-017-03740-7.
Kannan, Y., Sundaram, K., Narasimhulu, C. A., Parthasarathy, S., and Wewers, M. D. (2012) Oxidatively modified low density lipoprotein (LDL) inhibits TLR2 and TLR4 cytokine responses in human monocytes but not in macrophages, J. Biol. Chem., 287, 23479-23488, https://doi.org/10.1074/jbc.M111.320960.
Bzowska, M., Nogieć, A., Skrzeczyńska-Moncznik, J., Mickowska, B., Guzik, K., and Pryjma, J. (2012) Oxidized LDLs inhibit TLR-induced IL-10 production by monocytes: a new aspect of pathogen-accelerated atherosclerosis, Inflammation, 35, 1567-1584, https://doi.org/10.1007/s10753-012-9472-3.
Bennett, S, and Breit, S. N. (1994) Variables in the isolation and culture of human monocytes that are of particular relevance to studies of HIV, J. Leukoc. Biol., 56, 236-240, https://doi.org/10.1002/jlb.56.3.236.
Khan, B. V., Parthasarathy, S. S., Alexander, R. W., and Medford, R. M. (1995) Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells, J. Clin. Invest., 95, 1262-1270, https://doi.org/10.1172/JCI117776.
Jialal, I., and Chait, A. (1989) Differences in the metabolism of oxidatively modified low density lipoprotein and acetylated low density lipoprotein by human endothelial cells: inhibition of cholesterol esterification by oxidatively modified low density lipoprotein, J. Lipid Res., 30, 1561-1568.
Scoccia, A. E., Molinuevo, M. S., McCarthy, A. D., and Cortizo, A. M. (2001) A simple method to assess the oxidative susceptibility of low density lipoproteins, BMC Clin. Pathol., 1, 1, https://doi.org/10.1186/1472-6890-1-1.
Esterbauer, H., and Cheeseman, K. H. (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal, Methods Enzymol., 186, 407-421, https://doi.org/10.1016/0076-6879(90)86134-h.
Mogilenko, D. A., Dizhe, E. B., Shavva, V. S., Lapikov, I. A., Orlov, S. V., and Perevozchikov, A. P. (2009) Role of the nuclear receptors HNF4α, PPARα, and LXRs in the TNFα-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells, Biochemistry, 48, 11950-11960, https://doi.org/10.1021/bi9015742.
Mogilenko, D. A., Kudriavtsev, I. V., Shavva, V. S., Dizhe, E. B., Vilenskaya, E. G., et al. (2013) Peroxisome proliferator-activated receptor alpha positively regulates complement C3 expression but inhibits tumor necrosis factor α-mediated activation of C3 gene in mammalian hepatic derived cells, J. Biol. Chem., 288, 1726-1738, https://doi.org/10.1074/jbc.M112.437525.
Yao, S., Miao, C., Tian, H., Sang, H., Yang, N., et al. (2014) Endoplasmic reticulum stress promotes macrophage-derived foam cell formation by up-regulating cluster of differentiation 36 (CD36) expression, J. Biol. Chem., 289, 4032-4042, https://doi.org/10.1074/jbc.M113.524512.
Sanda, G. M., Deleanu, M., Toma, L., Stancu, C. S., Simionescu, M., and Sima, A. V. (2017) Oxidized LDL-exposed human macrophages display increased MMP-9 expression and secretion mediated by endoplasmic reticulum stress, J. Cell. Biochem., 118, 661-669, https://doi.org/10.1002/jcb.25637.
Bae, Y. S., Lee, J. H., Choi, S. H., Kim, S., Almazan, F., et al. (2009) Macrophages generate reactive oxygen species in response to minimally oxidized low-density lipoprotein: toll-like receptor 4- and spleen tyrosine kinase-dependent activation of NADPH oxidase 2, Circ. Res., 104, 210-218, https://doi.org/10.1161/CIRCRESAHA.108.181040.
Park, Y. M. (2014) CD36, a scavenger receptor implicated in atherosclerosis, Exp. Mol. Med., 46, e99, https://doi.org/10.1038/emm.2014.38.
Yang, H. Y., Bian, Y. F., Zhang, H. P., Gao, F., Xiao, C. S., et al. (2015) LOX-1 is implicated in oxidized low-density lipoprotein-induced oxidative stress of macrophages in atherosclerosis, Mol. Med. Rep., 12, 5335-5341, https://doi.org/10.3892/mmr.2015.4066.
Chawla, A., Boisvert, W. A., Lee, C. H., Laffitte, B. A., Barak, Y., et al. (2001) A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis, Mol. Cell, 7, 161-171, https://doi.org/10.1016/s1097-2765(01)00164-2.
Hopkins, P. N. (2013) Molecular biology of atherosclerosis, Physiol. Rev., 93, 1317-1542, https://doi.org/10.1152/physrev.00004.2012.
Eichelbaum, K., and Krijgsveld, J. (2014) Rapid temporal dynamics of transcription, protein synthesis, and secretion during macrophage activation, Mol. Cell. Proteomics, 13, 792-810, https://doi.org/10.1074/mcp.M113.030916.
Clerck, A., and Sugden, P. H. (1998) The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs), FEBS Lett., 426, 93-96, https://doi.org/10.1016/s0014-5793(98)00324-x.
Morishima, A., Ohkubo, N., Maeda, N., Miki, T., and Mitsuda, N. (2003) NFkappaB regulates plasma apolipoprotein A-I and high density lipoprotein cholesterol through inhibition of peroxisome proliferator-activated receptor alpha, J. Biol. Chem., 278, 38188-38193, https://doi.org/10.1074/jbc.M306336200.
Funding
This work was financially supported by the Russian Science Foundation (project no. 17-15-01326).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. All procedures with the participation of human subjects were performed in accordance with the ethical standards of the Institutional and National Ethics Committees and the Helsinki Declaration of 1964 and its following revisions.
Rights and permissions
About this article
Cite this article
Nekrasova, E.V., Larionova, E.E., Danko, K. et al. Regulation of Apolipoprotein A-I Gene Expression in Human Macrophages by Oxidized Low-Density Lipoprotein. Biochemistry Moscow 86, 1201–1213 (2021). https://doi.org/10.1134/S0006297921100047
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921100047