Abstract
As a result of cloning of the inuA, inu1, aglC, and fopA genes encoding endoinulinase (endoINU), exoinulinase (exoINU), α-galactosidase C (AGLС) and sucrase (SUC), respectively, into the recipient strain Penicillium verruculosum B1-537 (ΔniaD), recombinant producer strains were obtained that are capable of producing target recombinant enzymes with a high yield (32‒50% of the total extracellular protein). Enzyme preparations of endoINU, exoINU, AGLC, and SUC were obtained and characterized. Using chromatographic methods, endoINU, exoINU, SUC, and AGLC with a molecular weights of 62, 56, 67, and 76 kDa, respectively, were isolated in a homogeneous form (according to polyacrylamide gel electrophoresis). The homogeneous endoINU had a high specific activity against Jerusalem artichoke inulin (56 U/mg). ExoINU was active towards inulin (17 U/mg), sucrose (850 U/mg), raffinose (41 U/mg), and stachyose (15 U/mg). SUC decomposed sucrose (10.5 U/mg), raffinose, and stachyose (3.8 and 1.4 U/mg, respectively). AGLC had raffinase and stachyase activities (31 U/mg and 30 U/mg, respectively), exhibited no activity towards sucrose, but had a high level of activity towards the synthetic substrate, p-nitrophenyl-α-D-galactoside (311 U/mg). The kinetic parameters (kcat and Km) of the hydrolysis of the corresponding substrates by homogeneous enzymes were determined. The temperature optimum was 50‒55°C for endoINU, 55‒65°C for exoINU, 65°C for AGLC, and 35°C for SUC. EndoINU, exoINU, AGLC and SUC exhibited its maximum activity at pH 6.5, 4.5, 4.5‒5.0, and 5.5‒6.0, respectively. The thermal stability of the enzymes was studied at different temperatures. EndoINU exhaustively hydrolyzed inulin with the formation of fructooligosaccharides with a degree of polymerization of 3‒8. ExoINU quantitatively converted inulin into glucose-fructose syrup (GFS) with a Glu : Fru ratio of 1 : 3, and sucrose into GFS with a Glu : Fru ratio of about 1 : 0.63 (SUC provided the same results in the sucrose hydrolysis). Soy galactooligosaccharides (raffinose and stachyose) were converted to sucrose and monosaccharides (glucose, galactose, and fructose) under the action of AGLC. The combined action of SUC, and AGLC resulted in a complete conversion of raffinose, stachyose and sucrose to monosaccharides. The same results were achieved using ExoINU. This enzyme can be considered promising for biotechnological applications due to its broad substrate specificity, which allows it be used both for the production of GFS from inulin and sucrose, and for the destruction of soybean galactooligosaccharides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Enzymes find broad application in food industry to process agricultural products [1, 2]. Among them, a group of carbohydrases should be highlighted that have similar substrate specificities and hydrolyze α- and β-glycosidic bonds in various oligo- and polysaccharides. This group also includes endoinulinase (endoINU), exoinulinase (exoINU), sucrase (SUC), and α-galactosidase C (AGLC).
EndoINU (2,1-β-D-fructanfructanohydrolase, EC 3.2.1.7, glycoside hydrolase family 32 (GH32)) randomly cleaves the internal bonds of polyfructan (inulin) from Jerusalem artichoke, chicory, agave, and other inulin-containing plants to form fructooligosaccharides (FOSs) as main products that are used to obtain functional products and medicines [3].
ExoINU (2,1-β-D-fructanfructohydrolase, EC 3.2.1.80, GH32) processively hydrolyzes the terminal β-2,1-fructoside bonds of inulin, degrades sucrose, and is used in the production of fructose and glucose–fructose syrups (GFS) with high fructose content from Jerusalem artichoke, chicory, agave and other inulin-containing plants. Sugars obtained using the exoINU activity can serve as raw materials for obtaining a wide range of products of microbial synthesis: ethanol, butanol, lactic, citric and fumaric acids, etc. [3, 4].
Sucrase (invertase, β-fructofuranosidase, EC 3.2.1.26, GH32) hydrolyzes 1,2-β-D-glycosidic bonds in sucrose producing glucose and fructose, and also cleaves 1,2-β-glycosidic bonds in galactooligosaccharides. SUCs are used for the hydrolysis (inversion) of sucrose and the production of GFS [5].
α-Galactosidase (EC 3.2.1.22, GH36С) hydrolyzes terminal nonreducing α-D-galactosidic bonds in natural galactoologosaccharides (among which raffinose (α-D-Gal-p-(1→6)-α-D-Glu-p-(1→2)-β-D-Fru-f) and stachyose (α-D-Gal-p-(1→6)-α-D-Gal-p-(1→6)-α-D-Glu-p-(1→2)-β-D-Fru-f) are the most widespread), as well as synthetic substrates. Galactoologosaccharides are found in soybeans and other legumes used as feed ingredients for animals and birds; they are not practically assimilated and cause flatulence, reduced nutrient absorption, and intestinal hypertrophy. In this regard, to improve the nutritional value of feed, it is necessary to remove or decompose galactooligosaccharides, for which AGLC is used [6, 7].
The goal of the present work was to create highly active producer strains of exoINU, endoINU, SUC, and AGLC based on the Penicillium verruculosum B1-537 (ΔniaD) recipient strain. It seemed appropriate to compare the activities, compositions, and properties of enzyme preparations obtained using these producer strains, as well as the characteristics of enzymes isolated in a homologous state, and to evaluate their potential in various fields of biotechnology.
MATERIALS AND METHODS
Reagents
As substrates, the following compounds were used: inulin from Jerusalem artichoke (Reakhim, Russia), sucrose, raffinose, stachyose and n-nitrophenyl-α-D-Gal (pNPG) (Sigma-Aldrich, United States).
To prepare buffer solutions and fermentation media, chemically pure, pure for analysis and especially pure reagents were used (Helicon (Russia), Reakhim), as well as Pharmacia (Sweden) and Sigma-Aldrich products.
Genetic Constructs
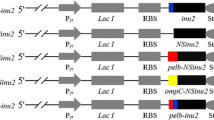
All genetic constructs were designed according to the same pattern and consisted of sequentially linked nucleotide sequences corresponding to the promoter region of the P. verruculosum cellobiohydrolase gene cbh1, a full-length target gene plus the terminal area of this gene. Regulatory segments of the cbh1 gene were amplified previously [8] and served as the basis for creating the main vectors of the pCBHI series for cloning and expression of heterologous genes [8–12]. The nucleotide sequences of the full-length target genes for INU A (inuA), exoINU (inu1), SUC (forpA) and AGLC (aglC) were amplified by PCR using DNA of Aspergillus sp. strains as a template. The genomic DNA of these strains was isolated using the QIAGEN kit according to standard protocols. The amplified target genes were inserted into the pCBHI vector by ligation-independent cloning [13]. In this way, four constructs for the recipient strain transformation were obtained and the absence of mutations in them was confirmed by bidirectional Sanger sequencing [14].
Recipient Strain Transformation
Transformation of the P. verruculosum 537 (ΔniaD) strain and obtaining protoplasts were conducted by the previously described method [8]. For co-transformation, the pSTA10 plasmid bearing the nitrate reductase gene was used, which provided the complementation of the defective niaD gene in the recipient strain. This allowed transformants to be selected on a medium with sodium nitrate.
The resulting transformants were analyzed for the presence and level of biosynthesis of the target proteins, as well as for the concentration of extracellular protein in the culture liquid (CL) after fermentation in Erlenmeyer flasks [15]. The most productive strains in terms of the target activity were selected and used for obtaining dry enzyme preparations by fermentation in 1-L fermenters (Prointech, Russia) [15]. Dry enzyme preparations were produced by lyophilization (Virtis Benchtop 9L Pro dryer, SP Scientific, United States). A dry preparation obtained from the P. verruculosum 537 (ΔniaD) recipient strain was used as a control.
Analysis of Enzyme Activities
The activities of enzyme preparations and homogenous enzymes against inulin, raffinose, stachyose, and sucrose were determined by the initial rate of hydrolysis of these substrates at their concentration in the reaction mixture of 5 g/L, temperature 50°C and pH 5.0 with a reaction time of 5 min. The activity was expressed in international units; 1 unit (U) corresponded to the amount of the enzyme catalyzing the formation of 1 μmol of the product (reducing sugars, RS) per 1 min of action on the corresponding substrate. RS were determined by the Somogyi–Nelson method [16].
The activity towards pNPG (at its concentration of 0.9 mM) was determined by the rate of the p-nitrophenol formation in 10 min at 50°C and рН 5.0. The reaction was stopped by the addition of 1 M Na2CO3. The amount of the enzyme catalyzing the formation of 1 μmol of p-nitrophenol per 1 min was taken as a unit of activity [16].
Protein Content
This component was determined by the Lowry method using bovine serum albumin as a standard [17].
Polyacrylamide Gel Electrophoresis
PAGE under denaturing conditions was carried out in a Miniprotean Tetra cell with a Model 300Xi power supply (Bio-Rad Laboratories, United States) according to the manufacturer’s protocol. Proteins were gel stained with Coomassie-Brilliant Blue R-250 (Ferrak, Germany). A commercially available mixture (#26612, Thermo Fisher Scientific) was used as a protein molecular weight marker.
Identification of Enzymes
Enzymes were identified by peptide mapping after hydrolysis with trypsin (Promega, United States) of a protein band in the gel after PAGE. MALDI mass spectrometry of the trypsin hydrolysate was carried out on an UltrafleXtreme time-of-flight mass spectrometer (Bruker Daltonik GmbH, Germany) at the Industrial Biotechnologies Common Use Center of the Fundamentals of Biotechnology Federal Research Center (Russian Academy of Sciences). The resulting mass spectra of the trypsin-treated peptides were analyzed using the MASCOT program (http://www.matrixscience.com), PeptideMass program (http://expasy.org/tools/peptide-mass.html) and the known target enzyme sequences.
The Composition of Enzyme Preparations
To determine the composition of the enzyme preparations, the resulting electrophoregrams were subjected to densitometry using the GelAnalyzer2010a software.
pH Profile of Activity
This profile for homogenous enzymes and enzyme preparations was analyzed by measuring their activity in the pH range of 2.5–7.5 with a step of 0.5 pH units at 50°C using the following substrates: inulin for exo- and endoINU; sucrose for SUC and pNPG for AGLC. Citrate buffer (0.1 M) with different set pH values was used. The results were reflected in a percentage of the maximum activity at optimal pH.
Temperature Profile of Activity
The temperature profile was studied in homogenous enzymes and enzyme preparations by measuring their activity towards appropriate substrates (see previous subsection) at various temperatures from 4 to 80°C with a step of 5–10°C at pH 5.0. The results were expressed as a percentage of the maximum activity at the optimal temperature.
The Stability of the Enzymes
To analyze their stability, homogenous enzymes and enzyme preparations were incubated at various temperatures and pH 5.0 with sampling of the reaction mixture at specified time intervals (15–30 min). The appropriate substrates indicated above were used to assess enzyme activities.
The results were reflected as the dependency of the residual activity (% of the initial value) on the incubation time at a certain temperature.
Kinetic Parameters
The kinetic constants of the enzymes were determined using the following substrate concentrations in the reaction mixture: inulin, 1–10 g/L; sucrose, 0.018–0.090 M; and pNPG, 0.45–8.10 M. The enzyme concentrations were as follows: endoINU, 0.034 mg/mL; exoINU, 0.28 mg/mL; SUC, 0.36 mg/mL; and AGLC, 0.12 mg/mL. The experiments were carried out at 50°C and pH 5.0. The Michaelis–Menten constants were obtained by processing the experimental data in the Lineweaver–Burk coordinates.
Enzymatic Hydrolysis of Inulin, Sucrose, and Soybean Galactooligosaccharides
Inulin (100 g/L) was hydrolyzed at 50°C and pH 5.0 (in 0.1 M acetate buffer) on a magnetic stirrer (250 rpm). In preliminary experiments, the optimal enzyme doses were selected: 5 U/g of substrate for endoINU and 0.5 U/g of substrate for exoINU.
Hydrolysis of sucrose (200 g/L) was performed at 50°C and рН 5.0; the latter parameter was controlled by citric acid at constant stirring on a magnetic stirrer (250 rpm). As shown in preliminary experiments, the optimal dose for SUC is 25 U/g.
Extruded soybean meal (ESM; Food Industries, Russia) was obtained using a twin-screw extruder (Werner & Pfleiderer Continua, Germany) at 120°C and used as a source of galactooligosaccharides. The preliminary experiments showed the effectiveness of enzymatic treatment under the following conditions: 40°C; рН 5.0; hydromodule 1 : 1; and periodic mixing. An enzyme content of 5 mg of protein per 1 g of ESM (dry weight) was optimal for both exo- and endoINU.
Analysis of Carbohydrates
The hydrolysis products of inulin, sucrose, and galactooligosaccharides were analyzed by HPLC (Agilent Technologies, United States) with an ESA Coulochem III electrochemical detector (Conquer Scientific, United States) and an online registration system. An immobile phase was represented by a Carbopak PA100 anion-exchange column (Thermo Fischer Scientific); 100 mM NaOH served as a mobile phase. Elution was performed with a sodium acetate concentration gradient from 0 to 500 mM within 20 min. Calibration plots were built using glucose, galactose, fructose, sucrose, raffinose, and stachyose (Sigma-Aldrich and Merck, Germany). The eluent solutions were passed through a membrane filter with a pore diameter of 0.45 μm (Millipore, United States) and thoroughly degassed. Before chromatography, the samples were preliminarily centrifuged at 14 000 g for 4 min.
Isolation of Homogenous Enzymes
Target proteins were isolated by liquid chromatography using NGC Chromatography Systems (Bio-Rad Laboratories) with a UV detector. Protein (100 mg in 10 mL) was precipitated with a (NH4)2SO4 solution (80% of saturation), desalted on a BioGel Р4 column (Bio-Rad Laboratories) and applied to a Source 15Q column (10 mL, Pharmacia), equilibrated with 0.02 M Tris-HCl buffer, pH 6.8. Carrier-bound proteins were eluted by a NaCl concentration linear gradient (0 → 0.4 M) with a flow rate of 5 mL/min. The target enzyme activity was analyzed in fractions, after which they were subjected to PAGE.
Hydrophobic chromatography on a Source 15 Isopropyl column (10 mL, Pharmacia) of the fractions containing the target enzymes was carried out. A sample was preloaded with ammonium sulfate up to a concentration of 1.7 M and loaded on a column equilibrated with 0.05 M Na-acetate buffer, pH 5.0, containing 1.7 M (NH4)2SO4. Carrier-bound proteins were eluted with a 1.7 → 0 M ammonium sulfate reverse gradient at a flow rate of 1.5 mL/min. The target enzyme activity was determined in fractions and they were subjected to PAGE.
RESULTS AND DISCUSSION
Obtaining Producer Strains
The P. verruculosum B1-537 (ΔniaD) strain was used as a recipient in obtaining recombinant strains that produce target enzymes. To provide the expression of the target genes, genetic constructs were used that contained target coding sequences linked with a strong inducible promoter and terminator of the gene for cellobiohydrolase 1 (cbh1), one of the main enzymes synthesized by P. verruculosum [9].
A highly active producer of endoINU, P. verruculosum-endoINU, was created as a result of transformation of the recipient strain with a plasmid containing the heterologous Aspergillus niger inuА gene encoding endoINU (62 kDa, pI 2.8). Cultivation of this strain in laboratory 1-L fermenters resulted in a yield of inulinase activity of 400 U/mL (determined using Jerusalem artichoke inulin as a substrate).
Transformation of the recipient strain with a plasmid containing the heterologous A. awamori exoINU-1 gene (56 kDa, pI 4.3) led to the creation of a P. verruculosum-exoINU strain producing this enzyme. The cultivation of the strain in 1-L laboratory fermenters resulted in the release 2500 U/mL of inulinase activity towards Jerusalem artichoke inulin.
Using plasmids containing a heterologous gene for A. niger AGLC (76 kDa, pI 4.8), a high-active P. verruculosum-AGLC strain was created, which produced α-galactosidase activity in an amount of about 28 000 U/mL of CL (determined with pNPG as a substrate) when cultured in 1-L laboratory fermenters.
Finally, using a plasmid with a heterologous A. oryzae gene for SUC (67 kDa, pI 6.2), a highly productive P. verruculosum-SUC strain was constructed, which produced 400 U/mL of sucrase activity when cultured in laboratory fermenters.
Activity of Enzyme Preparations
Laboratory enzyme preparations were represented by lyophilized CLs of the corresponding producer strains obtained by cultivation in 1-L fermenters. The activity of dry CLs in terms of endoINU, exoINU, SUC, and AGLC, as well as that of the dry CL of P. verruculosum 537 (ΔniaD), a recipient strain, used as a control, is shown in Table 1.
The endoINU enzyme preparation exhibited high hydrolytic activity against Jerusalem artichoke inulin as a substrate and low activity against sucrose, galactooligosaccharides, and pNPG.
In comparison with other enzyme preparations, exoINU had the highest activity towards Jerusalem artichoke inulin, sucrose, raffinose, and stachyose, and low activity against pNPG.
The SUC enzyme preparation showed low activity in inulin hydrolysis, high activity towards sucrose, and decreasing activity in the series raffinose → stachyose → pNPG.
The AGLC enzyme preparation was highly and almost equally active in relation to raffinose and stachyose, extremely active against pNPG and had very low activity towards inulin and sucrose.
We note that the control enzyme preparation obtained from the recipient strain exhibited low activity towards substrates used for the analysis of recombinant enzyme preparations.
Table 2 shows the specific activities of the resulting enzyme preparations (U/mg of protein). The highest activity towards Jerusalem artichoke inulin was demonstrated by the exoINU preparation; the endoINU enzyme preparation had lower activity (by 23%). Among the studied enzyme preparations, the greatest sucrase specific activity was observed in exoINU; it significantly exceeded that of SUC. In addition, the exoINU enzyme preparation showed a considerably higher specific activity towards raffinose and stachyose than the AGLC enzyme preparation. Among the studied enzyme preparations, the latter had the highest specific activity against the pNPG synthetic substrate.
The Compositions of Enzyme Preparations
Figure 1 shows the results of the PAGE analysis of enzyme preparations endoINU, exoINU, SUC, and AGLC, as well as a control enzyme preparation from the recipient strain. Major bands corresponding in MW to the target recombinant proteins can be seen on the electrophoregrams of the recombinant enzyme preparations. We note that these MW values strongly differ from those in the recipient strain electrophoregram.
Electrophoresis of the following enzyme preparations: (1) exoINU, (2) endoINU, (3) AGLC, (4) SUC, and (5) control (enzyme preparation produced by the P. verruculosum B1-537 (ΔniaD) recipient strain). M, protein MW markers. Target recombinant enzymes on tracks 1–4 are marked by arrows. Track 5: a, β-glucosidase; b, CBH1 (heavy form); c, CBH1 (light form); d, CBH2 (heavy form); e, CBH2 (light form); and f, endoglucanase-2.
Protein bands corresponding to the target enzymes were excised, treated with trypsin, and the resulting hydrolysates were analyzed by MALDI-TOF mass spectrometry. It was found that the electrophoregram bands (Fig. 1) corresponding to proteins with MW of 62 kDa, 56, 67, and 76 kDa are endoINU, exoINU, SUC, and AGLC, respectively (data not shown).
Data on the composition of the dry recombinant and control enzyme preparations obtained by densitometry of PAGE electrophoregrams are shown in Table 3. It is important that the control preparation of the recipient strain contained a significant amount (60% of the total protein) of cellobiohydrolases (CBH1 and CBH2), endoglucanases (EG, 12%), and other enzymes (28%). The recombinant strains had strongly different enzyme composition due to the presence of the target recombinant proteins. As an example, the corresponding enzyme preparations contained the following amounts of the enzymes: endoINU, about 40% of the total protein; exoINU, 50%; SUC, about 30%; and AGLC, 40%. The content of their own enzymes inherited by the recombinant strains from the recipient strain was reduced (in total): CBH, up to 40–45%; EG, up to 6–8%; and other enzymes, up to 3–20%. The change in the composition of the recombinant enzyme preparations in terms of the occurrence of a significant amount of target enzymes correlated well with the level of their activities towards specific substrates.
Isolation and Properties of Homogenous Enzymes
Target homogenous enzymes were isolated from the corresponding enzyme preparations. At the first stage, the desalted enzyme preparations were fractionated on the Source 15Q anion-exchanger. Fractions with target activities were processed by hydrophobic chromatography on a Source 15 Isopropyl column; as a result, the target enzymes were obtained in a homogenous state (Fig. 2).
The specific activity of the homogenous enzymes against various substrates is shown in Table 4. The endoINU specific activity towards Jerusalem artichoke inulin was about 3 times higher than that of exoINU. When using sucrose as a substrate, the specific activity of exoINU was 50 times as high as that against inulin. It is important that the specific activity of homogenous SUC towards sucrose was 13 times lower than that of exoINU. The specific activity of exoINU against raffinose and stachyose was comparable with that of AGLC. We also note the high specific activity of AGLC with respect to pNPG. In general, the data on the specific activities of the homogenous enzymes were in full agreement with the results in Tables 1 and 2 that give the activities of the corresponding enzyme preparations.
The kinetic parameters of the hydrolysis of the appropriate substrates by the homogenous enzymes were determined (Table 5). When using inulin as a substrate, the kcat/Km ratio, which characterizes the efficiency of the potential substrate hydrolysis, was an order of magnitude higher for exoINU than for endoINU. When sucrose was used, kcat/Km for exoINU more than 300 times exceeded the value for SUC. Special attention should be paid to the high kcat/Km value characteristic of AGLC using pNPG as a substrate.
The temperature optimum (Тopt) for endoINU was 50–55°C (Table 6); the temperature range in which the activity of this enzyme was at least 80% of the maximum (Т80) was 40–60°C. For exoINU, the temperature optimum was observed at 55–65°С and Т80 within 45‒70°C; the corresponding numbers for AGLC were 60°C and 45‒62°C. The lowest Тopt was noted for SUC (35°C) with Т80 = 30‒40°C.
All the studied enzymes exhibited the maximum hydrolytic activity in a neutral or slightly acidic medium. The highest activity of endoINU was observed at pH 6.5 with a рН80 (the pH range, in which enzyme activity was at least 80% of its maximum) of 6.0–7.0. The pH optimum for exoINU was 4.5 with рН80 4.0‒5.5; the corresponding parameters for AGLC and SUC were 4.5–5.0 (рН80 4.0–6.0) and 5.5–6.0 (рН80 5.0‒6.5).
We note that the values of temperature and pH optima, as well as Т80 and рН80, were the same for homogenous enzymes and enzyme preparations.
Thus, the studied homogenous enzymes and the corresponding enzyme preparations have close ranges of Т80 and рН80 (except for SUC, whose temperature optimum was much lower than that of other enzymes).
We analyzed the stability of the homogenous enzymes at various temperatures (Table 7). EndoINU manifested relatively high stability; upon incubation at 40°C and 50°C, its activity was almost unchanged for 180 min and decreased up to 40% and 30% of the initial value only at 60 and 70°C, respectively. The exoINU activity remained practically at the same level (90–100% of the initial value) for 180 min at 40–50°C and decreased to 65 and 10% at 60 and 70°C, respectively. AGLC turned to be less stable; its activity was reduced to 30–20% of the starting value when kept at 40–50°C for 180 min; at 60 and 70°C, 7% of the initial activity and complete inactivation, respectively, were observed. SUC had extremely low stability: 15 min at 40°C resulted in a residual activity of 30%, at 50°C, 5%, and at higher temperatures the enzyme was unstable.
It should be noted that the thermal stability characteristics were similar in the homogenous enzymes and the corresponding enzyme preparations.
Complete substrate hydrolysis was observed after treatment of Jerusalem artichoke inulin (100 g/L) with homogenous endoINU (5 U/g of substrate) for 3 h. According to HPLC data, the products of this hydrolytic reaction were FOSs with a degree of polymerization of 3–8 (predominantly) and FOSs with a higher MW (to a lesser extent). In addition, sucrose (at a low concentration), fructose, and glucose were also identified in the reaction mixture (Fig. 3). The initial substrate contained FOSs, sucrose, fructose, and glucose at low concentrations.
Inulin (100 g/L) was quantitatively hydrolyzed by homogenous exoINU (0.5 U/g of substrate) for 3 h at 50°C and рН 5.0; GFS was formed with a Glu:Fru ratio of about 1 : 3 (HPLC, data not shown). Under the same conditions, using sucrose as a substrate (200 g/L), exoINU (25 U of sucrase activity per 1 g of substrate) quantitatively converted sucrose to GFS with a Glu : Fru ratio of about 1 : 0.63 (HPLC, data not shown). Therefore, GFS derived from inulin was significantly more enriched in fructose compared to the product obtained from sucrose.
Like exoINU, homogenous SUC quantitatively converted sucrose (200 g/L, 25 U of sucrase activity per 1 g of substrate, 50°C, рН 5.0) for 3 h to produce GFS similar in composition to that obtained under the action of exoINU (due to its lower thermal stability, the reaction with SUC was performed at a lower temperature than for exoINU; moreover, the SUC consumption was about 11 times greater than that of exoINU due to differences in the specific activities of these two enzymes (Table 4).
The capacity of homogenous enzymes for hydrolyzing soybean galactoologosaccharides was studied using extruded soybean meal (ESM) as a substrate; the enzymatic treatment was carried out for 6 h at 40°C and рН 5.0 with a concentration of each enzyme of 5 mg/g of substrate. The reaction products were analyzed by HPLC.
The initial ESM sample contained stachyose, raffinose, and sucrose, as well as monosaccharides (glucose and fructose) (Table 8). Treatment with AGLC resulted in almost complete decomposition of raffinose and stachyose, as well as in the formation of monosaccharides (glucose, fructose, and galactose) and a large amount of sucrose (AGLC has no sucrase activity, Table 4). The combined use of AGLC and SUC led to complete conversion to monosaccharides of raffinose, stachyose, and sucrose (Table 8). We note that the application of SUC alone provided the hydrolysis of sucrose, but did not change the contents of raffinose and stachyose in ESM, since SUC does not hydrolyze these oligosaccharides (Table 4). Finally, the ESM treatment with exoINU alone gave the same result as the combined use of AGLC and SUC: complete destruction of raffinose, stachyose, and sucrose to monosaccharides (Table 8).
CONCLUSIONS
It can be concluded that exoINU is the most valuable enzyme preparation in terms of biotechnological potential due to a broad specificity and high hydrolytic activity. Exoinulinase efficiently hydrolyzes inulin and also exhibits high α-galactosidase and sucrase activity. In this regard, exoINU can be used with equal success for obtaining glucose–fructose syrup from inulin, sucrose inversion, and for the destruction of soybean galactooligosaccharides and the control of anti-nutrients in feed for farm animals and poultry.
REFERENCES
Handbook of Food Enzymology, Whitaker, J.R., Voragen, A.G.J., and Wong, D.W.S., Eds., New York: CRC Press, 2002. https://doi.org/10.1201/9780203910450
Chi, Z.-M., Zhang, T., Cao, T.-S., et al., Biotechnological potential of inulin for bioprocesses, Bioresour. Technol., 2011, vol. 102, no. 6, pp. 4295–4303. https://doi.org/10.1016/j.biortech.2010.12.086
Volkov, P.V., Sinitsyna, O.A., Fedorova, E.A., et al., Isolation and properties of recombinant inulinases from Aspergillus. sp., Biochemistry (Moscow), 2012, vol. 77, no. 5, pp. 492–501. https://doi.org/10.1134/S0006297912050094
Kholyavka, M.G., Study of the physicochemical, structural and functional properties of inulinases and the patterns of formation of supramolecular complexes by them under conditions of various microenvironments, Doctoral (Biol.) Dissertation, Voronezh: Voronezh State University, 2018.
Quenmeau, Y., Jarosz, S., Lewandowski, B., and Fitremann, J., Sucrose chemistry and applications of sucrochemicals, Adv. Carbohydr. Chem. Biochem., 2007, vol. 61, pp. 217–292. https://doi.org/10.1016/S0065-2318(07)61005-1
Sinitsyna, O.A., Fedorova, E.A., Vakar, I.M., et al., Isolation and characterization of extracellular β-galactosidases from Penicillium canescens, Biochemistry (Moscow), 2008, vol. 73, no. 1, pp. 97–106. https://doi.org/10.1134/S000629790801015x
Soybean and Nutrition, El-Shemy, H.A., Ed., Croatia: InTech, 2011. https://doi.org/10.5772/1008
Sinitsyn, A.P., Sinitsyna, O.A., and Rozhkova, A.M., Production of industrial enzymes based on the expression system of the Penicillium verruculosum fungus, Biotekhnologiya, 2020, vol. 36, no. 6, pp. 17–34. https://doi.org/10.21519/0234-2758-2020-36-6-17-34
Sinitsyn, A.P., Rozhkova, A.M., Zorov, I.N., et al., Recombinant strain of the mycelial fungus Penicillium verruculosum (variants) and method for obtaining an enzyme preparation and its use (variants), RF Patent no. RU2646136C2, 2018.
Sinitsyn, A.P., Rozhkova, A.M., Sinitsyna, O.A., et al., Genetic construction to ensure the expression of target homologous and heterologous genes in cells of the filamentous fungus Penicillium verruculosum used as a host, a method for producing a strain of the fungus Penicillium verruculosum and a method for producing an enzyme preparation, RF Patent no. RU2378372C2, 2010.
Volkov, P.V., Gusakov, A.V., Rubtsova, E.A., et al., Properties of recombinant GH49 family dextranase heterologously expressed in two recipient strains of Penicillium species, Biochimie, 2019, vol. 157, pp. 123‒130. https://doi.org/10.1016/j.biochi.2018.11.010
Volkov, P.V., Rubtsova, E.A., Rozhkova, A.M., et al., Properties of recombinant endo-β-1,6-glucanase from Trichoderma harzianum and its application in the pustulan hydrolysis, Carbohydr. Res., 2021, vol. 499, p. 108211. https://doi.org/10.1016/j.carres.2020.108211
Aslanidis, C. and de Jong, P.J., Ligation-independent cloning of PCR products (LIC-PCR), Nucleic Acids Res., 1990, vol. 18, pp. 6069–6074. https://doi.org/10.1093/nar/18.20.6069
Sanger, F., Coulson, A.R., Barrell, B.G., et al., Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing, J. Mol. Biol., 1980, vol. 143, pp. 161–178. https://doi.org/10.1016/0022-2836(80)90196-5
Sinitsyn, A.P., Rubtsova, E.A., Shashkov, I.A., et al., Preparation and properties of new biocatalysts for destruction of plant non-starch polysaccharides, Katal. Prom-sti., 2017, vol. 17, no. 4, pp. 331–338. https://doi.org/10.18412/1816-0387-2017-4-331-338
Sinitsyn, A.P., Chernoglazov, V.M., and Gusakov, A.V., Research methods and properties of cellulolytic enzymes, Itogi Nauki Tekhn., Ser. Biotechnol., Moscow: VINITI, 1990, vol. 25, pp. 30–37.
Dawson, R.M.C., Elliott, D.C., Elliot, W.H., and Jones, K.M., Biochemist’s Handbook, Moscow: Mir, 1991.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (state assignment no. 0104-2019-0009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by I. Gordon
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations: AGLC, α-galactosidase C; CBH, cellobiohydrolase; CL, culture liquid; EG, endoglucanase; endoINU, endoinulinase; ESM, extruded soybean meal; exoINU, exoinulinase; FOS, fructooligosacharide; Fru, fructose; Gal, galactose; GFS, glucose-fructose syrup; GH, glycoside hydrolase family; Glu, glucose; HPLC, high-performance liquid chromatography; MALDI-TOF mass spectrometry, matrix-assisted laser desorption/ionization time-of-flight mass-spectrometry; MCC, microcrystalline cellulose; pNPG, p-nitrophenyl-α-D-galactoside; PAGE, polyacrylamide gel electrophoresis; RS, reducing sugars; SUC, sucrase.
Rights and permissions
About this article
Cite this article
Sinitsyna, O.A., Rubtsova, E.A., Osipov, D.O. et al. A Comparative Analysis of the Properties of Recombinant Endoinulinase, Exoinulinase, Sucrase, and Alpha-Galactosidase C. Appl Biochem Microbiol 59, 1008–1017 (2023). https://doi.org/10.1134/S0003683823070050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823070050