Abstract
The diagnostic potential of the 4B7 and 6B12 monoclonal antibodies (MAbs) against human adenovirus hexon protein has been studied in various immunological tests, namely, the in-cell enzyme-linked immunosorbent assay (cell-ELISA), sandwich ELISA, and the immunofluorescence antibody technique (IFA). It was shown that the sensitivities of cell-ELISA, sandwich ELISA, and IFA were 96, 86, and 84%, respectively, compared to PCR. Thus, 4B7 and 6B12 MAbs are promising immunoreagents for the construction of various diagnostic kits to use in laboratory practice for adenovirus detection in clinical samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Fifty-million cases of infectious diseases are annually registered in Russia. Up to 95% of them are acute respiratory virus infections (ARVI) [1]. The most important role in the development of respiratory pathology is played by the influenza virus, respiratory syncytial virus (RSV), and adenovirus (AdV). AdVs that cause infections are observed throughout the year [2]; they lead to morbidity and outbreaks of epidemics, mainly in organized children’s groups [3] and among conscripts [4, 5]. The upper respiratory tract is the first to be affected upon the respiratory form of AdV infection; the development of aggravations in the form of bronchitis, bronchiolitis, pneumonia, encephalitis, meningitis, and myocarditis is possible, especially in patients with immunosuppression [6, 7]. Lethal cases have been described as a result of AdV pneumonia in children and among the military personnel of the armies of Russia, Canada, and the United States. The reasons for the emergence of such severe infections and their mortality still remain unclear.
To date, 80 types of human AdVs are known. They contain variable antigens, which determines the variety of clinical forms of AdV infections, complicates the clinical diagnosis, and requires expensive laboratory tests. The use of accurate diagnostic tests allows timely diagnostics, which is especially relevant for at-risk patients.
Currently, the polymerase chain reaction (PCR), enzyme-linked immonosorbent assay (ELISA), and IFA with the use of polyclonal AdV-specific sera are commonly used for the rapid diagnostics of AdV infections. The sensitivity of laboratory diagnosis of these diseases is 83.0% for PCR, 44.6% for IFA, and 48.7% for ELISA [5]. Despite the cost effectiveness and reliability of the PCR diagnostics, it has a number of limitations, primarily related to high requirements for the laboratory technical equipment and staff qualification. In view of this, conventional immunological methods of laboratory diagnostics remain in demand. Their efficiency can be improved by including high-specific monoclonal antibodies (MAbs) in diagnostic test systems.
Highly specific 4B7 and 6B12 MAbs to the AdV hexon protein have been obtained in the Laboratory of Biotechnology of the Research Institute of Influenza. Based on the data of studies on laboratory AdV strains of various types, the 4B7 and 6B12 MAbs were selected as the most promising for use in ELISA variants and IFA, respectively [8].
The goal of the present work was to assess the diagnostic potential of the obtained MAbs in various immunological reactions aimed at detecting AdV in clinical samples.
MATERIALS AND METHODS
Monoclonal Antibodies
As noted, the 4B7 and 6B12 MAbs targeted at AdV hexon were previously obtained in the Laboratory of Biotechnology of the Research Institute of Influenza. The methods for obtaining, as well as the properties and selection criteria for MAbs, were described in detail in [8]. The highest specificity in ELISA was found for the 4B7 MAb; its titer in ELISA with purified type 6 AdV was 10–6, while the nonspecific interaction with the purified concentrate of heterologous RSV were not observed. The 6B12 MAb showed the highest activity in IFA against various AdV types (3, 4, 6 and 19) with the complete absence of nonspecific reactions with heterologous respiratory viruses.
Cell Culture
The A-549 cell line (human lung carcinoma) was provided by the Collection of the Research Institute of Influenza.
Samples for Analysis
Nasopharingeal swabs from 66 patients with ARVI symptoms were analyzed for the respiratory viruses by real time PCR (qPCR). AdV was detected in 63 samples, and in 2 of them RSV and rhynovirus were found in addition to AdV. Sanger sequencing identified AdV types in 31 analyzed samples; 30 contained AdV type 4, and 1 sample, adenovirus type 7 [9]. Three clinical samples were used as negative controls: one contained influenza A virus and another had RSV; a third sample did not contain ARVI pathogens according to qPCR.
Cell-ELISA
The analysis was carried out according to the previously published protocol [10]. The A-549 cell culture was contaminated with clinical samples (63 of which contained AdV according to PCR, while the other 3 did not), and 4 consecutive passages were performed. The AdV reproduction was detected either visually using an Axiovert 40С light microscope (Karl Zeiss, Germany) by the presence of a typical cytopathogenic effect (CPE), or semi-quantitatively by cell-ELISA using the 4B7 MAb conjugated with horse radish peroxidase (4B7-HRP) and tetramethylbenzidine (TMB) as a substrate. Optical density was measured at a wavelength of 450 nm (OD450). Cell-ELISA results were taken into consideration at each stage of passage of clinical samples, regardless of the presence or absence of CPE.
Sandwich ELISA
This method was used to analyze the same clinical samples (n = 66) as cell-ELISA. The initial samples were studied together with the virus-containing culture liquid (VCL) obtained after passing the samples through the A-549 culture and at each passage stage (no more than four passages). The 4B7 MAb at a concentration of 5 μg/mL in carbonate–bicarbonate buffer (pH 9.5) was adsorbed on a 96-well plate (Medpolimer, Russia) for 16 h at 4°C. The plate was then washed with phosphate-buffered saline with 0.5% Tween-20 (PBST), after which the analyzed samples were added (100 μL/well) and incubated for 2 h at 37°C. The plate was washed again with PBST, the 4B7-HRP conjugate in PBST was introduced (1 : 4000, 100 μL/well), and the mixture was incubated for 1 h at 37°C. After washing and reaction with TMB, OD450 was measured and AdV was determined in the wells.
Immunofluorescence Antibody Technique (IFA)
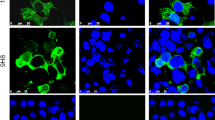
Forty-eight clinical samples were analyzed: 45 of them were AdV-positive at the first passage according to cell-ELISA and 3 were AdV-negative according to PCR. The A-549 cell culture was contaminated with clinical samples, incubated for 48 h, and then fixed with chilled acetone. The treated cells were incubated with the 6B12 MAb conjugated with fluorescein isothiocyanate (FITC) and analyzed under an Axioskop 40 fluorescent microscope (Karl Zeiss, Germany) at 400 × magnification.
RESULTS AND DISCUSSION
Detection of AdV by cell-ELISA
The A-549 cell culture was infected with 66 clinical samples, 63 of which were AdV-positive according to PCR. Specificity was assessed using three clinical samples: one of them contained influenza A virus, another one carried RSV, and the third was negative for ARVI pathogens according to PCR. The 4B7-HRP MAb was used to detect AdV by cell-ELISA. In addition, the AdV reproduction in the cell culture was assessed visually (by the presence of a typical CPE in consent with the standard for traditional isolation of viruses in cell culture). The samples with negative results in cell-ELISA after the first passage were subjected to another 3 blind consecutive passages through the A-549 cell culture. The AdV reproduction after each passage was assessed visually and by cell-ELISA. The results of the sensitivity and specificity of cell-ELISA using 4B7-HRP and conventional virus isolation in cell culture after the 4th passage compared to PCR data are shown in Table 1.
The study of clinical samples by cell-ELISA detected AdV in 61 out of 66 analyzed samples, while the virological method detected the virus in only 47 samples. The sensitivity of cell-ELISA with the 4B7-HRP MAb conjugate was 96% of that of PCR, which is much higher than the sensitivity of the classical technique for the virus isolation in cell culture (74%). The specificity of both methods was 100%.
Detection of AdV by Sandwich ELISA
The same clinical samples (n = 66) as for the cell-ELISA were analyzed by sandwich ELISA. The results were recorded at each stage of the passage of clinical samples, regardless of the presence of CPE. The sensitivity and specificity of sandwich ELISA using the 4b7 MAb and 4B7-HRP conjugate in comparison with PCR are represented in Table 1.
The use in sandwich ELISA of the 4B7 MAb at the capture stage and 4B7-HRP at the detection stage made it possible to identify AdV in 35 initial clinical samples and 19 VCL samples. The sensitivity of the method was 86 with 100% specificity.
Detection of AdV in IFA
For this analysis, 45 clinical samples were used that were AdV-positive at the first passage in the cell-ELISA test, and 3 AdV-negative samples according to PCR. The A-549 cell culture contaminated with clinical samples was fixed 48 h after the infection and examined by IFA using the 6B12-FITC MAb conjugate (Fig. 1). As a result, AdV was found in 38 of 45 tested samples. Thus, the IFA sensitivity was 84% compared to PCR.
Infectious diseases of viral etiology remain one of the most serious problems in public health. AdV, RSV, rhinoviruses, and others, are detected as the main causative agents in noninfluenza seasons [2]. Typically, AdV attacks the upper or lower respiratory tract, pharynx, conjunctiva, or gastrointestinal tract. AdV-induced outbreaks can occur throughout the year not only among immunocompromised persons, but also among healthy children and adults, especially in closed groups: military units, nursing homes, specialized medical institutions, and summer camps. The percentage of AdV among children and young people of military age in the total number of ARVI cases can reach 30 and 64.6%, respectively [3, 11]. An increase in the frequency of pneumonia, including that with a severe course and fatal outcomes in patients without impaired immunity is observed in the foci of these outbreaks [12]. The variety of clinical forms of AdV infections makes it necessary to develop effective tools for differential diagnostics of this disease. The effective identification of the pathogen is mandatory to prevent spreading infection and to conduct timely therapy and therefore, to avoid the development of severe forms of the disease.
Currently, differential diagnostics of AdV infection by ELISA and FAT using polyclonal virus-specific sera is popular in Russia. The main disadvantage of these methods is the unstable antibody composition, which depends on the peculiarities of the producer’s immune response, while obtaining a new batch of immune serum requires a highly purified antigen, a complete immunization cycle and quality control of the final drug. The sensitivity of the above methods for diagnosing AdV infection is 44.6% for IFA and 48.7% for ELISA compared to PCR [5]. In this regard, the problem of obtaining standardized antibody preparations, high-specific to antigenic determinants of AdV pathogenic for human is extremely urgent.
The current level of biotechnology development makes it possible to produce MAbs on an industrial scale; therefore, MAbs can be included in new generation test systems, which will improve the quality and increase the availability of laboratory diagnostics of AdV infection.
Highly specific 4B7 and 6B12 MAbs to the AdV hexon have been obtained at the Laboratory of Biotechnology of the Research Institute of Influenza. Their diagnostic potential was studied in various immunological and virological reactions with clinical samples obtained from patients hospitalized with ARVI symptoms in 2014–2018.
According to molecular genetic analysis the AdV-positive samples were studied by cell-ELISA using the 4B7 MAb. This method, together with the use of specific MAbs, is widespread for identification of respiratory viruses in cell cultures contaminated with clinical samples from ARVI patients, both in Russia and abroad. As an example, cell-ELISA with specific MAbs to the influenza virus nucleoprotein has been recommended by WHO as a test for serological diagnosis of influenza, including the identification of virus strains with weak hemagglutination activity [13]. According to the published data, the sensitivity of cell-ELISA in the identification of ARVI pathogens ranges from 78 to 94% for different respiratory viruses, which is much higher than that of the traditional method of virus isolation in cell cultures [14].
In our study of clinical samples by cell-ELISA with the 4B7 MAb, the sensitivity and specificity reached 96 and 100%, respectively, compared to PCR, which largely exceeds the similar characteristics of the traditional method of AdV isolation in cell cultures (about 50% compared to PCR) [5]. The use of 4B7 in cell-ELISA not only reduces the isolation time of the etiological agent in cell culture to 3 days, but also allows identification of AdV without the characteristic CPE. The developed cell-ELISA variant can be used to screen clinical samples for the presence of AdV.
Another trend in the use of MAbs in the ARVI diagnosis is the development of IFA for the analysis of nasopharyngeal swabs and cell cultures contaminated with clinical samples from patients [17]. The so-called shell vial culture [18] is quite popular in foreign research laboratories; it allows detection of virus reproduction in a cell culture as early as 16 h after the test material introduction. The analysis time is reduced and the sensitivity is increased due to both preliminary centrifugation of cell cultures with added clinical material and the use of specific MAbs [19].
CONCLUSIONS
Only specialized laboratories are able to carry out virological assays using cell cultures. Sandwich ELISA is widely used to detect respiratory viruses in clinical samples [15, 16]. It was shown that using the 4B7 MAbs at the capture stage and the 4B7-HRP conjugate at the detection stage, the sensitivity of sandwich-ELISA was 86%, and its specificity was 100% relative to PCR. At present, there are no commercial ELISA kits for detecting respiratory AdV. Thus, the 4B7 MAbs can be regarded as a promising component for inclusion in Russian AdV kits.
The sensitivity and specificity of the proposed method for AdV identification in contaminated clinical samples by IFA with the 6B12-FITC conjugate was 84% and 100%, respectively, compared to PCR. These results are comparable to the sensitivity of foreign kits for IFA diagnostics of ARVI (~90%) and significantly exceeds that of IFA using polyclonal antibodies widespread in Russia (≤50%) [5].
The analysis of the study convincingly showed that the 4B7 and 6B12 MAbs are promising tools for the construction of various immunological kits and their further use in the laboratory practice for the diagnosis of adenovirus infection.
REFERENCES
WHO Regional Office for Europe. WHO Regional Office for Europe Guidelines for Sentinel Influenza Surveillance in Humans. http:// www.euro.who.int/__data/assets/pdf_file/0003/90444/e92738R.pdf?ua=1. Accessed June 28, 2019.
Walter, J.M. and Wunderink, R.G., Severe respiratory viral infections: new evidence and changing paradigms, Infect. Dis. Clin. North Am., 2017, vol. 31, no. 3, pp. 455–474. https://doi.org/10.1016/j.idc.2017.05.004
Yatsyshina, S.B., Ageeva, M.R., Vorob’eva, N.S., et al., Adenoviruses in the etiological structure of acute respiratory viral infections in Moscow in 2004–2014, Zh. mikrobiol., epidemiol. immunobiol., 2015, vol. 21, no. 5, pp. 50–57.
O’Shea, M.K. and Wilson, D., Respiratory infections in the military, J.R. Army Med. Corps., 2013, vol. 159, pp. 181–189. https://doi.org/10.1136/jramc-2013-000110
L’vov, N.I., Adenovirus infection in military personnel: clinic, diagnosis, and treatment, Extended Abstract of Doctoral (Med.) Dissertation, St. Petersburg: Voen.-Med. Akad. im. S.M. Kirova, Min. Oborony Ross. Fed., 2016.
Rautonen, J., Koskiniemi, M., and Vaheri, A., Prognostic factors in childhood acute encephalitis. Pediatr. Infect. Dis. J., 1991, vol. 10, no. 6, pp. 441–446. https://doi.org/10.1097/00006454-199106000-00005
Wold, W. and Ison, M., Adenoviruses, in Fields Virology, Knipe, D.M. and Howley, P., Eds., Philadelphia: Wolters Kluwer Health/Lippincott Williams and Wilkins, 2013, 6th ed., vol. 1, pp. 1732–1767.
Timoshicheva, T.A., Zabrodskaya, Y.A., Ramsay, E., et al., Use of hexon as an antigen for the production of monoclonal antibodies capable of detecting multiple adenovirus types, Biologicals, 2019, vol. 58, pp. 44–49. https://doi.org/10.1016/j.biologicals.2019.01.006
Amosova, I.V., Timoshicheva, T.A., Egorova, A.A., et al., Molecular genetic analysis of modern adenovirus strains, Sovr. Med., 2018, vol. 3, no. 11, pp. 13–16.
Amosova, I.V., Timoshicheva, T.A., Sverlova, M.V., et al., The use of microcultural enzyme immunoassay and a modified immunofluorescence method for the diagnosis of adenovirus infection, Klin. Lab. Diagn., 2017, vol. 62, no. 4, pp. 230–235. https://doi.org/10.18821/0869-2084-2017-62-4-230-235
L’vov, N.I., Pisareva, M.M., Mal’tsev, O.V., et al., Peculiarities of the etiological structure of SARS in certain age and occupational groups of the population of St. Petersburg during the epidemic season of 2013–2014, Zh. Infektol., 2014, vol. 6, no. 3, pp. 62–70. https://doi.org/10.22625/2072-6732-2014-6-3-62-70
Ivanov, V.V., Kharitonov, M.A., Grozovsky, Yu.R., et al., Severe virus-associated pneumonia in military personnel, Vestn. Ross. Voen.-Med. Akad., 2015, vol. 1, no. 49, pp. 146–152.
Van Baalen, C.A., Els, C., Sprong, L., et al., Detection of nonhemagglutinating influenza A(H3) viruses by enzyme-linked immunosorbent assay in quantitative influenza virus culture, J. Clin. Microbiol., 2014, vol. 52, no. 5, pp. 1672–1677. https://doi.org/10.1128/JCM.03575-13
Rabalais, G.P., Stout, G.G., Ladd, K.L., et al., Rapid diagnosis of respiratory viral infections by using a shell vial assay and monoclonal antibody pool, J. Clin. Microbiol., 1992, vol. 30, no. 6, pp. 1505–1508. https://doi.org/10.1128/JCM.30.6.1505-1508.1992
Chen, L., Ruan, F., Sun, Y., et al., Establishment of sandwich ELISA for detecting the H7 subtype influenza A virus, J. Med. Virol., 2019, vol. 91, no. 6, pp. 1168–1171. https://doi.org/10.1002/jmv.25408
González, L.A., Vázquez, Y., Mora, J.E., et al., Evaluation of monoclonal antibodies that detect conserved proteins from respiratory syncytial virus, metapneumovirus and adenovirus in human samples, J. Virol. Methods, 2018, vol. 254, pp. 51–64. https://doi.org/10.1016/j.jviromet.2018.01.011
Corvalán, L.P., Arias, G.B., Morales, P.S., et al., Inmunofluorescencia indirecta versus reacción de polimerasa en cadena para el diagnóstico de virus respiratorios en niños ingresados en un hospital de la Región Metropolitana, Rev. Chil. Infectol., 2019, vol. 36, no. 1, pp. 26–31. https://doi.org/10.4067/S0716-10182019000100026
Maitreyi, R.S., Broor, S., Kabra, S.K., et al., Rapid detection of respiratory viruses by centrifugation enhanced cultures from children with acute lower respiratory tract infections, J. Clin. Virol., 2000, vol. 16, no. 1, pp. 41–47. https://doi.org/10.1016/s1386-6532(99)00075-x
Kowalski, R.P., Karenchak, L.M., Romanowski, E.G., et al., Evaluation of the shell vial technique for detection of ocular adenovirus. Community Ophthalmologists of Pittsburgh, Pennsylvania, Ophthalmology, 1999, vol. 106, no. 7, pp. 1324–1327. https://doi.org/10.1016/s0161-6420(99)00718-6
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors outside the scope of people’s normal professional activities.
Additional information
Translated by I. Gordon
Abbreviations: Adv, adenovirus; ARVI, acute respiratory virus infection(s); cell-ELISA, micro-culture ELISA; CPE, cytopathogenic effect; ELISA, enzyme-linked immunosorbent assay; FITC, fluorescein isothiocyanate; IFA, fluorescence with fluorochrome-labeled antibodies; MAb, monoclonal antibody; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; TMB, tetramethylbenzidine; VCL, virus-containing culture liquid.
Rights and permissions
About this article
Cite this article
Timoshicheva, T.A., Amosova, I.V. & Grudinin, M.P. The Diagnostic Potential of Monoclonal Antibodies to Adenovirus. Appl Biochem Microbiol 58, 873–877 (2022). https://doi.org/10.1134/S0003683822070079
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822070079