Abstract
It has been shown that when a fermented protein feed additive is added to the diet of broiler chickens, the presence of additional protein fragments was noted in the proteomes of the breast (pectoralis) and leg (femoralis) muscles of poultry. These proteins (actin, troponin, myosin heavy and light chains) are biomarkers of tenderness and water-holding capacity of meat (type M creatine kinase). The presence of additional isoforms of various complexes of the electron transport chain (cytochrome bc1 complex, or complex III of the electron transport chain), characterizing the effectiveness of this diet, was also noted. In addition, the introduction of fermented protein feed additive into the diet of poultry leads to an increase in the antioxidant capacity of the tissues of the breast and leg muscles of broilers and a decrease in the fat content in the tissues of the leg muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The quality and safety of poultry products largely depend both on the technology of growing and keeping poultry and on feeding rations. The balanced feeding of broiler chickens (chickens of certain crosses with a high productivity of gaining muscle mass over a short period) involves the use of diets containing harmless and complete feeds and additives that allow the genetic potential of the bird and obtain highly nutritious and safe food products to be fully realized.
Recently, the substitution of traditional protein sources in the diet of poultry with hydrolysates of secondary livestock products [1, 2], including poultry [3–5], has become increasingly popular. Attention to such approaches is due to the fact that the peptides of hydrolysates can exhibit both functional (regulatory) and biological (antimicrobial, antioxidant, antihypertensive, and immunomodulatory) activities.
Since the juiciness, tenderness, smell, and taste of chicken meat depend on various factors (genetic characteristics, conditions for feeding and keeping poultry, processing and storage of carcasses) [6], knowledge of the growth dynamics, morphology, and biochemical composition of muscle tissue, taking into account the species, breed, gender, husbandry, and feeding of poultry is of great scientific and practical interest. However, traditional methods for measuring meat quality, such as analysis of texture, color differences, water-holding capacity (WHC), and taste cannot fully achieve the goal of controlling and predicting meat quality [7–9]. Therefore, recently, more and more attention has been paid to the search and study of biomarkers that determine the qualitative characteristics of meat using proteomic technologies [10–18]. Proteomics is becoming an important and promising tool in meat science and allows researchers to gain deeper knowledge about the molecular mechanisms that affect meat quality.
Although proteomic studies have been successfully used for searching for biomarkers and studying the molecular mechanisms associated with meat quality in domestic animals such as pigs [19], cows [20], and sheep [21], reports of such studies in poultry are very limited. Thus, proteomic studies were carried out to determine the effect of diet on the growth and quality of poultry meat [10, 11]. The researchers found the effect of dietary amino acid deficiency on the muscle proteome and explained changes in proteomes during the growth of egg-producing chickens [11]. As a result of the study of turkey proteomes, differences in fast and normally proceeding glycolysis in the tissues of the breast muscles were found and their relationship with meat quality was established [12]. The proteomic analysis of the muscles of native and commercial broiler chickens [13] was carried out for the determination of the relationship between protein composition and meat tenderness. The results showed that glycolytic enzymes such as pyruvate kinase, phosphoglycerate mutase, and triose phosphate isomerase are associated with meat quality. Proteomic characterization of sarcoplasmic proteins in the breast muscles was carried out for two different chicken genotypes, including commercial Ross 708 broilers and Leghorn chicks (Hyline W-36) [15]. The results showed that glycogen phosphorylase, enolase, creatine kinase, fructose-bisphosphate aldolase, and glyceraldehyde-3-phosphate dehydrogenase differed between the two strains during the period of breast muscle growth. In [16], the authors studied the proteomic composition and differential expression of proteins extracted from the muscles of young chickens with different growth rates and different WHC. Proteins identified in different groups of chickens by two-dimensional electrophoresis and mass spectrometry included such metabolic enzymes as creatine kinase and pyruvate kinase. These studies have highlighted the potential of proteomic methods to elucidate the biochemical basis for color variation, WHC, and meat texture in broiler chickens.
Objective—To perform comparative research of the proteomes of the white and red meat of broiler chickens grown on different diets and the analysis of protein biomarkers of the quality of poultry meat.
MATERIALS AND METHODS
Feed additives. Poultry by-products were used as feed additives of animal origin for feeding broilers. These by-products include blood, intestines, and feathers, subjected to short-term hydrothermal hydrolysis (hydrolyzed feed additive—HFA) [22], followed by enzymatic hydrolysis by proteases (fermented feed additive—FFA) [23] and fishmeal—FM (MK-0378, manufacturer Kapitan Nazin, Russia).
HFA was obtained by hydrolysis of by-products of poultry processing (feathers, intestines) in a high-temperature treatment apparatus at 140–190°C for 60–90 s [22]. FFA was obtained by fermentation of by-products of poultry processing after hydrothermal treatment [23]. Raw feather materials were hydrolyzed using enzyme preparations Protozyme C (Biopreparat, Russia) and Novo-Pro D (Novozymes, Denmark) at the rate of 5–15 proteolytic activity units (IU) per g of protein at hydromodulus 1 : 4, at a temperature of 55–58°C for 4 h. For the fermentation of intestinal raw materials, neutrase and alkalase enzyme preparations (Novozymes, Denmark) at a dose of 5–10 U/g protein at hydromodulus 1 : 1, temperature of 50–52°С, and hydrolysis duration of 2–4 h were used.
In vivo research. The experiment on the inclusion of various feed additives in the diet of broiler chickens was carried out in the vivarium of the breeding and genetic center of the Zagorsk Experimental Breeding Farm, All-Russian Research and Technological Poultry Institute, Russian Academy of Sciences (Russia). For the experiment, broiler chickens of the Smena 9 cross with an average live weight of 43 ± 0.8 g were selected. Four groups of 35 animals each were randomly formed.
The control group (no. 1) received a regular diet without the addition of feed additives of animal origin (Table 1), and the control group no. 4 received a regular diet with addition of fishmeal. Experimental groups (nos. 2 and 3) received experimental feed additives in addition to the regular diet until the end of the growing period (Table 2). In experimental group no. 2, we used hydrolyzate of by-products of poultry processing obtained by short-term high-temperature treatment (HFA, hydrolyzed feed additive) as a feed additive. Fermented hydrolyzate of poultry by-products (FFA) was used in experimental group no. 3. The weight of protein consumed in the composition of protein supplements was 1.6 kg per 1 kg of poultry live weight.
At the age of 38 days, three broilers with average live weight were selected from each group. In order to assess the meat quality, chicken carcasses after bleeding were subjected to anatomical cutting according to the procedure in [24]. After anatomical cutting, samples of the muscle tissue of the leg and breast of broiler chickens were taken, their physicochemical properties (pH, protein content, fat, moisture and ash, WHC), protein composition, and antioxidant capacity were analyzed.
The protein content was determined according to ISO 5983-1:2005; fat according to ISO 6492:1999; moisture according to ISO 6496:1999; ash according to ISO 5985:2002 and pH according to ISO 2917:1974. WHC was determined according to the Grau–Hamm method modified by Zhuravskaya [25].
Proteomic analysis of broiler muscle tissue extracts. Analysis was carried out by two-dimensional electrophoresis following mass-specrtometry protein identification. We used muscle tissue samples of the breast and leg of broilers from four experimental groups (Table 1).
In order to obtain protein extracts, muscle tissue from the leg and chest of broilers were ground in a blender (Selecline, China) for 5 min until a homogeneous state was reached. Then, a sample of ground muscle tissue (100 mg) was homogenized using a Potter homogenizer in a 400 µL lysis buffer of the following composition (%): dithiothreitol (DTT, Merck, Germany)—1; 3-(3-cholamidopropyl) dimethylammonium-3-propanesulfonate (CHAPS, VWR Chemicals, United States)—4; ampholines 3/10 (Serva Electrophoresis, Germany)—5; 7 M urea and 2 M thiourea (GE Healthcare, United States). The resulting extract was centrifuged for 10 min at 800 g, the supernatant was collected and stored at –73°С until analysis.
Two-dimensional electrophoresis (2-DE). 2-DE electrophoresis was performed according to O’Farrell with isoelectric focusing in an ampholine gradient with a pH of 3–10 (Serva Electrophoresis, Germany), as described earlier in [26], on a PROTEAN II xi 2-D Cell system (Bio -Rad, the United States). The sample amount was 70 μg of protein per tube. Electrophoresis of the samples obtained after isoelectric focusing was carried out in a gradient acrylamide gel (7.5–25%) in the presence of sodium dodecyl sulfate (SDS) at a voltage of 300 V. Before applying to the second dimension, the samples were incubated for 20 min in a solution containing dithiothreitol (urea, 6 M; SDS-Na, 2%; DTT, 10 mM; Tris-HCl, 0.5 M; pH 6.8) to prevent oxidation of sulfhydryl groups of proteins. For visual analysis of the distribution of protein components and mass spectrometric analysis, the gels were stained with a solution of AgNO3 or Brilliant Blue R Staining Solution (Sigma, United States).
To obtain protein maps, an Infinity1000/26MX gel-documentation system (Vilber Lourmat, France) was used. Protein maps were analyzed using ImageMaster 2D Platinum, v.7 software (GE Healthcare, United States).
Mass spectrometric analysis of proteins. For mass spectrometric analysis, gel pieces 3–4 mm3 in size, corresponding to protein stains were cut out and washed twice to remove the dye in 100 μL of 40% acetonitrile solution in 0.1 M NH4HCO3 for 20 min at 37°C. After removal of the solution, 100 μL of acetonitrile was added to dehydrate the gel. After removing acetonitrile and drying the gel, a modified trypsin solution (Promega, United States) in 0.05 M NH4HCO3 with a concentration of 15 μg/mL was added. Hydrolysis was carried out for 8 h at 37°C, then 0.5% trifluoroacetic acid (TFA) in 10% aqueous acetonitrile solution was added. The solution containing the protein hydrolyzate was used for mass spectrometric analysis. A solution of 2,5-dihydroxybenzoic acid (Aldrich, United States) with a concentration of 10 mg/mL in 20% aqueous acetonitrile and 0.5% TFA was used as a matrix.
Mass spectra were obtained using a MALDI time-of-flight/ Ultraflex II time-of-flight mass spectrometer (Bruker, Germany) equipped with a UV laser in the positive ion mode using a reflectron. The accuracy of the measured masses of monoisotopes after additional calibration by the trypsin autolysis peaks was 0.005% (50 ppm). The spectra were obtained in the mass range 700–4500 m/z, choosing the optimal laser power to achieve the best resolution. A MALDI TOF/TOF-MS was used to obtain fragmentation spectra, the measurement accuracy of fragment ions was no lower than 1 Da.
Mass spectra were processed using the FlexAnalysis 3.3 software package (Bruker Daltonics, Germany). Protein identification was performed using the Mascot program (www.matrixscience.com). For the identification, a search was carried out in the NCBI database (www.ncbi.nlm.nih.gov) using the “peptide fingerprint” option among the proteins of all organisms with the accuracy described above, taking into account the possible oxidation of methionine by atmospheric oxygen and the possible modification of cysteine by acrylamide gel. Candidate proteins with a score >76 in the NCBI database were considered reliable (p < 0.05), proteins with a score >50 were considered probable. The search using the pooled results was carried out using the Biotools 3.2 software (Bruker Daltonics, Germany).
In vitro determination of antioxidant capacity (AOC) in muscle tissue homogenates of the leg and breast of broiler chickens. For the determination of the AOC of the hydrophilic components of tissue extracts, 200 mg of breast muscle or leg tissue was placed in a plastic tube, and 8 mL of 11.5% potassium chloride solution was added. Homogenization was carried out for 5 min at a temperature of 4°C in a Silent Crusher S homogenizer equipped with a 7F nozzle (Heildolph, Germany) at a speed of 75 000 rpm. The homogenate was centrifuged for 20 min at 30 000 g and 4°C. The supernatant was separated and diluted in 50 mM phosphate buffer pH 7.4 for 15–25 times.
Analysis of the antioxidant capacity of the homogenized samples of the breast muscle and poultry leg meat was measured in relation to a peroxyl radical. The peroxyl radical was generated directly in the reaction medium during the thermal decomposition of the azo compound 2,2'-azobis(2-methylpropionamidine) dihydrochloride (AAPH, Sigma, United States), which was initiated by incubation at 37°С for 10 min [27]. The AOC of the hydrophilic components of the poultry muscle tissue extracts towards the peroxyl radical was expressed in μM of Trolox equivalents (TEs) per g of tissue. Fluorescence reduction kinetics was recorded for 1 h with a measurement interval of 60 s using BioTek Synergy 2 photometer-fluorimeter (BioTek, United States) in the fluorescence intensity recording mode (excitation wavelength, 485 nm; emission wavelength, 528 nm).
RESULTS AND DISCUSSION
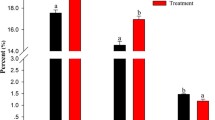
Physiochemical properties of breast and leg muscles. The results of assessing the effect of feeding protein feed additives on the physicochemical composition of the breast and leg muscles of broiler chickens are presented in Table 3. According to the pH of the breast and leg muscle tissues after slaughter and anatomical cutting of carcasses no fundamental differences were found in the groups. In groups 2–4 of broiler chickens, a general pattern of reduced total moisture was observed compared to control group 1, in breast muscles the moisture content was lower than the controls in groups 2 and 3, and the moisture content in leg muscles was lower than in groups 2 and 4. The content of fat in the breast muscles of the experimental poultry in groups 2–4 was significantly lower (by about 28%) relative to control group 1. Also, the fat content in the leg muscles of experimental group 3 was lower than the control by 16%. At the same time, in groups 2 and 4, the fat content in the leg muscles was higher by 26 and 46%, respectively, compared with control group 1.
An increase in the content of crude protein in the breast muscles of broiler chickens of three groups (groups 2, 3, and 4) by about 3.4% was shown in comparison with the control (group 1). At the same time, the total protein content in the leg muscles of chickens in experimental group 3 exceeded this indicator in all other groups by 4.3%, which, probably, was a consequence of an increase in protein assimilation in chickens of this group that received the FFA protein feed supplement (Table 3).
The WHC of breast and leg muscle tissue in experimental group 3 was the highest among all groups and exceeded that of groups 1 and 4 by 8.4% for the breast muscles and by 4.7% for the leg muscles. The WHC value of the breast muscles of the poultry that received the diet with HFA (group 2) was also higher than the values of groups 1 and 4 by 3.2%. However, the WHC value of the leg muscles of the poultry from group 2 was close to those in control group 1, and was somewhat inferior to the values in group 4.
Poultry meat is the most complete and dietary product compared to the meat of other farm animals, as it contains more complete and less hard-to-digest proteins (collagen and elastin), which determines its high nutritional value. An increase in the protein content in the muscle tissue of group 3 broiler chickens increased the nutritional value of poultry meat from this experimental group. At the same time, the indicators of WHC of breast and leg muscle tissues in this experimental group were the highest, which also indicated an increase in the meat quality of poultry receiving FFA.
Thus, it has been shown that the inclusion of the experimental feed protein supplement FFA in the diet of broiler chickens had the most significant effect on the physicochemical composition of muscle tissue, compared with HFA and FM, which in turn had a positive effect on the nutritional value and quality of meat.
Now, for the study the quality of meat and search for biomarkers that characterize its palatability traits, such as tenderness and WHC, proteomic methods are actively used [28, 29]. In addition, proteomics allows us to determine the modification of muscle tissue proteins, namely, phosphorylation, oxidation, degradation, and denaturation [30]. Therefore, the protein composition of poultry muscle tissue samples from different groups was studied and their comparative analysis was carried out.
Fractional composition of protein extracts of breast and leg muscles of poultry. During the study, proteomic maps of breast (pectoralis muscle) and leg (femoralis muscle) tissue samples of broiler muscles (white and red poultry meat, respectively) at different feeding rations were obtained (Figs. 1 and 2).
The protein fractions were characterized by different pI isoelectric points and different molecular weights. The fractionation of samples by two-dimensional electrophoresis allowed us to identify up to 150 protein fractions (the main ones are shown in Table 4). Most of the tryptic spectra of proteins corresponded to sequences found in databases for chicken (Gallus gallus), but for some proteins homologues have been found in other species. Among the identified proteins, more than half (61%) were structural function proteins, including fibrillar proteins: actin, myosin, tropomyosin, and troponin; vimentin and parvalbumin were also identified. Changes in the spectrum of structural proteins in samples were mainly due to amino acid substitutions or modifications of these forms relative to protein sequences that exist in databases. This was probably due to the genetic characteristics of the poultry used in the study.
In addition, enzymes involved in glycolysis have been identified: enolase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate mutase (PGM), lactate dehydrogenase (LDH), triose phosphate isomerase, and other enzymes: ATP synthase, malate dehydrogenase, creatine kinase (CK), as well as various proteins of the molecular chaperone family (HSP70 and HSP71).
The performed proteomic analysis revealed the localization zones of important structural muscle proteins: actins, myosins, tropomyosins, and troponins (Figs. 1a and 2a). As can be seen in the figures, proteomic profiles in groups 1–4 were similar for breast (Fig. 1) and leg muscles (Fig. 2) samples. Thus, in each group of proteins there are “stable” forms the production of which is unchanged from sample to sample. Nevertheless, individual protein components were also identified, varying both quantitatively and qualitatively depending on the sample (Figs. 1 and 2).
Significant changes were shown in the production profiles of three groups of muscle contraction proteins (actins, myosins, and troponins) depending on the diet. Additional actin fractions found in the proteome breast muscles from group 2: cardiac alpha-actin 1 (ACTA1, KFW81934.1) and skeletal muscle alpha actin (NP_001026234.1) (Fig. 1b). Additional actin isoforms were present on the proteomic maps of leg muscles of poultry from group 3: skeletal muscle alpha actin (NP_001026234.1) and cardiac alpha actin 2 (AAX85445.1) (Fig. 2c). Additional isoforms of skeletal muscle alpha actin (NP_001026234.1) were also identified in the proteome of the leg muscles of poultry from group 4 (Fig. 2d).
The composition of the myosin fractions also differed on the proteomic maps: in the breast muscles of the poultry from group 2, an increased content of the myosin heavy chain fraction (AAB20215.1) was found compared to the samples from other groups. At the same time, additional isoforms of myosin light chains were identified in the breast muscles of poultry from group 4 (XP_015144626.1). As for the isoenzyme composition of troponins in different samples, additional isoforms of troponin C (NP_990781.1 and NP_990464.1, Fig. 1d) were found in the breast muscle of experimental group 4. Troponin I (NP_990748.1) and T (XP_015142062.1, NP_990253.1) fractions were identified in proteomes of leg muscles of poultry from groups 3 and 4 (Figs. 2c and 2d).
In the process of meat maturation, significant biochemical changes occur in the muscles that affect the final characteristics of meat quality. It is well known that under the action of proteolytic enzymes postmortem autolytic changes occur in muscles, resulting in the formation of fragments of the myofibrillar proteins actin, myosin, and troponin, which can be considered as molecular markers of the delicate structure of meat [31]. Many studies have shown that an increase in the intensity of protein spots of myosin light and heavy chains, ACTA1 actin, and troponin T in muscle proteomes was associated with meat tenderness [32, 33].
Thus, in the proteomes of the breast and leg muscles of poultry on diets with the addition of protein feed additives, the presence of additional fragments of structural proteins, such as actin, troponin, and myosin (heavy and light chains), was noted in comparison with the first control group of broilers.
It should be also noted that, in addition to an increase in the content of myosin heavy chains, an additional protein vimentin was identified in the breast muscle sample of broilers from group 2 (VIM, vimentin, NP_001041541.2), which is absent in other samples. The proteins vimentin and desmin play an important role in maintaining muscle cytoarchitecture and are considered reliable markers of regenerative processes in muscles. Recent studies have shown an increase in the expression of the VIM gene, as well as an increase in the content of the protein encoded by vimentin in the breast muscle (Pectoralis major) of fast-growing broilers with various myopathies leading to the development of such meat defects as the appearance of white striping parallel to muscle fibers and the Wooden breast syndrome [34]. Thus, vimentin may be a marker of abnormal development of muscle fibers of Pectoralis major during the early stages. Interestingly, in groups 2 and 4, which received fermented protein feeds with a higher content of amino acids, low molecular weight peptides and high digestibility (Table 2), vimentin was not found in the breast muscles. It is known that muscular dystrophy was observed when chickens are fed diets with an insufficient content of vitamin E, selenium, and sulfur-containing amino acids [34]. Inclusion in the diet of methionine, cysteine, or an increase in vitamin E contributed to the prevention of muscular dystrophy in chickens, and the combination of antioxidants and omega-3 fatty acids was more effective in combating this meat defect. Analysis of the antioxidant capacity of feed additives (Table 2) used in this study showed that the content of antioxidants was significantly higher in the protein supplement FFA (1980 µM TE/g), compared with HFA (about 400 µM TE/g) and FM (about 150 µM TE/g).
The second widely represented group in the muscle proteome samples was composed of enzymes (Table 4). The main soluble protein components of the proteome were α- and β-enolase, lactate dehydrogenase A, phosphoglycerate mutase, phosphoglucomutase, creatine kinase, and, in addition, triose phosphate isomerase. In the breast muscle of poultry, glycolysis is one of the main pathways for obtaining energy for muscle contraction and for the energy requirements during growth. In order to maintain muscle weight as well as meet the needs of the contractile muscles, poultry need a significant amount of energy. Therefore, it is not surprising that glycolysis enzymes predominated in the fraction of soluble proteins. For all samples in this category, there was also a “minimum” conserved set of proteins that did not change their production. The main differences between the samples were recorded in the proteome of the leg muscles of poultry from group 3, where additional isoforms of α‑enolase (XP_015152319.2), glyceraldehyde-3-phosphate dehydrogenase (AZN23181.1), malate dehydrogenase (NP_001006395.1), and creatine kinase (NP_990838. 1) were revealed (Fig. 2c). It is interesting to note that in this experimental group of broilers the most intensive increase in muscle weight was noted, compared with other groups.
In the leg muscles of poultry from group 3, an increased level of the M-type creatine kinase protein (NP_990838.1), one of the four forms of CK, was found (Fig. 2c). It is known that CK is a potential biomarker of meat WHC [8], indeed, the WHC value of the leg muscles of poultry from group 3 was the highest (Table 3). It was previously shown [35] that the production of M-type creatine kinase and serum albumin was lower in samples with low WHC [8].
In addition, proteins such as phosphatidylethanolamine-binding protein 1, carbonic anhydrase II, and serum albumin (except group 4) were identified in muscle tissue samples from all groups of poultry (Table 4).
It is known that the discovered enzyme carbonic anhydrase II catalyzes the reversible reaction of carbon dioxide hydration and carbonic acid dehydration, and is involved in maintaining the pH balance in muscle tissues. Phosphatidylethanolamine-binding protein 1, together with vimentin, is a proteomic marker of rheumatic cartilage diseases [36] and its homologue is able to interact with MAP kinases in humans [37].
For sample no. 3 (FFA diet), the presence of apolipoprotein A1 (ApoA-I) in the tissues of the leg muscles of broilers was shown (Table 4, Fig. 2). ApoA-I is involved in lipid metabolism and, as a major component of high-density lipoprotein (HDL), plays an important role in the regulation of cholesterol levels in peripheral tissues through its reverse transport to the liver. ApoA-I deficiency is associated with excessive accumulation of intracellular cholesterol in humans and poultry. It has been shown that the expression level of ApoA-I decreased in hemorrhagic fatty liver syndrome in chickens [38], and a decrease in the expression level of ApoA-I in broiler tissues with a high content of abdominal fat has also been demonstrated [39]. Indeed, the presence of the ApoA-I fraction in leg muscle tissues of group 3 broilers correlated with the lowest fat content in femoralis muscle tissues among all groups, 5.9 ± 0.9% (Table 3).
There have been attempts in the literature to associate the expression (and activity) of mitochondrial proteins with feed efficiency (FE) of poultry [40]. It has been shown that the activity of complexes I and II of the electron transport chain is higher in the mitochondria of white and red meat samples obtained at high FE. Expression of mitochondrial proteins at low FE is associated with a low ability to transfer electrons in the electron transport chain during oxidative phosphorylation.
The appearance in the samples of group 3 of additional isoforms of various complexes of the electron transport chain indicates changes in metabolism.
It should be noted that there were no differences in the production of the chaperone complex in the proteome of poultry of different groups: no changes in the composition of isoforms were revealed for the fraction of HSP70 and HSP71 proteins. It was assumed that these proteins are involved in the regulation of the assembly and maintenance of the structure of muscle tissue, in the protection of structural proteins, including desmin, actin, and titin under stress [41], and in the regulation of glycolysis [42]. At the same time, a decrease in their production was associated with the development of the PSE syndrome, the characteristic features of which are exudative pale, soft, watery meat with a soft, friable texture and the release of meat juice due to reduced WHC [42, 43].
Antioxidant capacity of the breast and muscle tissue of poultry. It is known from the literature that the AOC of muscle tissue affects the quality of meat. The studies have shown that the oxidation of muscle proteins caused by oxidative stress resulted in the loss of essential amino acids (e.g., tryptophan) and affected the WHC of meat proteins, the color and texture of the resulting meat products, as well as the digestibility of meat and led to a decrease in its nutritional value [44, 45]. Oxidation of porcine myofibrillar proteins also reduced their ability to jellify, which is important for the textural and structural characteristics of meat products [46]. As can be seen from the results shown in Table 5, the highest AOC values of the muscle tissue of the breast and leg muscles were obtained for broilers from group 3, which received FDA in addition to the regular diet.
Thus, with the introduction of a fermented protein feed additive into the diet of broiler chickens of the Smena 9 cross, in the proteomes of the breast and leg muscles of the bird, fragments of proteins that are biomarkers of tenderness (actin, troponin, heavy and light chains of myosin) and WHC of meat (creatine type M kinase), as well as the appearance of additional isoforms of various complexes of the electron transport chain (cytochrome bc1 complex, or complex III of the respiratory electron transport chain), characterizing the effectiveness of this feeding ration, were noted. The introduction of FFA into the diet of poultry also led to an increase in the antioxidant capacity of the tissues of the breast and leg muscles of broilers, and a decrease in the fat content in the tissues of the leg muscles, which correlated with the presence of apolipoprotein A1 (ApoA-I) in the proteomes of these tissues.
REFERENCES
Eremeev, N.L., Nikolaev, I.V., Keruchen’ko, I.D., Stepanova, E.V., Satrutdinov, A.D., Zinov’ev, S.V., et al., Appl. Biochem. Microbiol., 2009, vol. 45, no. 6, pp. 648–655. https://doi.org/10.1134/S0003683809060131
Mamelona, J., Saint-Louis, R., and Pelletier, E., Int. J. Food Sci. Technol., 2010, vol. 45, pp. 147–154. https://doi.org/10.1111/j.1365-2621.2009.02114.x
Khiari, Z., Poult. Sci., 2014, vol. 93, pp. 2347–2362. https://doi.org/10.3382/ps.2014-03953
Fisinin, V.I., Ismailova, D.Yu., Volik, V.G., Lukashenko, V.S., and Saleeva, I.P., S-kh. Biol., 2017, vol. 52, no. 6, pp. 1105–1115. https://doi.org/10.15389/agrobiology.2017.6.1105rus
Fisinin, V.I., Lukashenko, V.S., Saleeva, I.P., Ovseichik, E.A., Zhuravchuk, E.V., Volik, V.G., and Ismailova, D.Yu., Ptitsevodstvo, 2018, vol. 11, no. 12, pp. 20–22.
Silkina, V.A., Genet. Razvedenie Zhivotn., 2015, no. 1, pp. 26–29.
Piras, C., Roncada, P., Rodrigues, P.M., Bonizzi, L., and Soggiu, A., Proteomics, 2016, vol. 16, pp. 799–815. https://doi.org/10.1002/pmic.v16.5
Vlachos, A., Arvanitoyannis, I.S., and Tserkezou, P., Crit. Rev. Food Sci. Nutr., 2013, vol. 56, pp. 1061–1096. https://doi.org/10.1080/10408398.2012.691573
Wu, W., Fu, Y., Therkildsen, M., Li, X.M., and Dai, R.T., Food Rev. Int., 2015, vol. 31, pp. 13–28. https://doi.org/10.1080/87559129.2014.961073
Paredi, G., Raboni, S., Bendixen, E., de Almeida, A.M., and Mozzarelli, A., J. Proteomics, 2012, vol. 75, no. 14, pp. 4275–4289. https://doi.org/10.1016/j.jprot.2012.04.01
Corzo, A., Kidd, M.T., Dozier, W.A., Shack, L.A., and Burgess, S.C., Br. J. Nutr., 2006, vol. 95, no. 4, pp. 703–708. https://doi.org/10.1079/bjn20051716
Molette, C., Remignon, H., and Babile, R., Poult. Sci., 2005, vol. 84, no. 1, pp. 119–127. https://doi.org/10.1093/ps/84.1.119
Mekchay, S., Teltathum, T., Nakasathien, S., and Pongpaichan, P., J. Poult. Sci., 2010, vol. 47, no. 1, pp. 8–12.
Surowiec, I., Koistinen, K.M., Fraser, P.D., and Bramley, P.M., Meat Sci., 2011, vol. 89, no. 2, pp. 233–237.
Zapata, I., Reddish, J.M., Miller, M.A., Lilburn, M.S., and Wick, M., Poult. Sci., 2012, vol. 91, no. 7, pp. 1654–1659. https://doi.org/10.3382/ps.2011-02029
Phongpa-Ngan, P., Grider, A., Mulligan, J.H., Aggrey, S.E., and Wicker, L., J. Agricult. Food Chem., 2011, vol. 59, no. 24, pp. 13181–13187.
Laville, E., Sayd, T., Terlouw, C., Blinet, S., Pinguet, J., Fillaut, M., Glannison, J., and Chérel, P., J. Agric. Food Chem., 2009, vol. 57, no. 11, pp. 4913–4923. https://doi.org/10.1021/jf900286x
Marcos, B. and Mullen, A.M., Meat Sci., 2014, vol. 97, no. 1, pp. 11–20. https://doi.org/10.1016/j.meatsci.2013.12.008
Sayd, T., Morzel, M., Chambon, C., Franck, M., Figwer, P., Larzul, C., et al., J. Agric Food Chem., 2006, vol. 54, pp. 2732–2737. https://doi.org/10.1021/jf052569v
Bouley, J., Meunier, B., Chambon, C., De, S.S., Hocquette, J.F., and Picard, B., Proteomics, 2005, vol. 5, pp. 490–500. https://doi.org/10.1002/()1615-9861
Hamelin, M., Sayd, T., Chambon, C., Bouix, J., Laville, E., Milenkovic, D., et al., J. Anim. Sci., 2007, vol. 84, pp. 3266–3276. https://doi.org/10.2527/jas.2006-162
Volik, V.G., Ismailova, D.Yu., Zinov’ev, S.V., and Erokhina, O.N., Ptitsa Ptitseprod., 2017, no. 2, pp. 40–42.
Volik, V.G., Ismailova, D.Yu., Lukashenko, V.S., Saleeva, I.P., Fedorova, T.V., Ovseichik, E.A., Zhuravchuk, E.V., and Zinov’ev, S.V., Uch. Zap. Kazan. Univ., Ser. Estestv. Nauki, 2019, vol. 161, no. 3, pp. 422–439. https://doi.org/10.26907/2542-064X.2019.3.422-439
Lukashenko, V.S., Lysenko, M.A., and Stolyar, T.A., Metodika provedeniya anatomicheskoi razdelki tushek, organolepticheskoi otsenki kachestva myasa i yaits sel’skokhozyaistvennoi ptitsy i morfologii yaits (Methodology for Anatomical Cutting of Carcasses and Organoleptic Assessment of the Quality of Poultry Meat and Eggs and Egg Morphology), Lukashenko, V.S., Ed., Sergiev Posad: VNITIP, 2013.
Zhuravskaya, N.K., Alekhina, L.T., and Otryashenkova, L.M., Issledovanie i kontrol’ kachestva myasa i myasoproduktov (Research and Quality Control of Meat and Meat Products), Moscow: Agropromizdat, 1985.
Vasina, D.V., Pavlov, A.R., and Koroleva, O.V., BMC Microbiol., 2016, vol. 16, article ID 106. https://doi.org/10.1186/s12866-016-0729-0
Nikolaev, I.V., Sforza, S., Lambertini, F., Ismailova, D.Yu., Khotchenkov, V.P., Volik, V.G., et al., Food Chem. A, 2016, vol. 197, pp. 611–621.
Picard, B., Lebret, B., Cassar-Malek, I., Liaubet, L., Berri, C., Le Bihan-Duval, E., and Renand, G., Meat Sci., 2015, vol. 109, pp. 18–26. https://doi.org/10.1016/j.meatsci.2015.05.003
Huang, H. and Lametsch, R., in Proteomics in Foods: Principles and Applications, Toldrá, F. and Nollet, L.M.L., Eds., New York: Springer, 2013, pp. 103–109.
Alessandro, A., Marrocco, C., Rinalducci, S., Mirasole, C., Failla, S., and Zolla, L., J. Proteom., 2012, vol. 75, no. 14, p. 4381.
Luccia, A.D., Picariello, G., Cacace, G., Scaloni, A., Faccia, M., Liuzzi, V., Alviti, G., and Musso, S.S., Meat Sci., 2005, vol. 69, pp. 479–491. https://doi.org/10.1016/j.meatsci.2004.10.004
Beldarrain, L.R., Aldai, N., Picard, B., Sentandreu, E., Navarro, J.L., and Sentandreu, M.A., J. Proteomics, 2018, vol. 183, pp. 25–33. https://doi.org/10.1016/j.jprot.2018.05.005
Gagaoua, M., Monteils, V., and Picard, B., J. Agric. Food Chem., 2018, vol. 66, no. 51, pp. 13552–13563. https://doi.org/10.1021/acs.jafc.8b05744
Soglia, F., Mazzoni, M., Zappaterra, M., Di Nunzio, M., Babini, E., Bordini, M., et al., Front. Physiol., 2020. https://doi.org/10.3389/fphys.2019.01581
Bottje, W., Iqbal, M., Tang, Z.X., Cawthon, D., Okimoto, R., Wing, T., and Cooper, M., Poult. Sci., 2002, vol. 81, no. 4, pp. 546–555.
Yeung, K., Janosch, P., McFerran, B., Rose, D.W., Mischak, H., Sedivy, J.M., and Kolch, W., Mol. Cell. Biol., 2000, vol. 20, no. 9, pp. 3079–3085. https://doi.org/10.1128/mcb.20.9.3079-3085.2000
Ghosh, J.G., Houck, S.A., and Clark, J.I., Int. J. Biochem. Cell. Biol., 2007, vol. 39, no. 10, pp. 1804–1815.
Song, Y., Ruan, J., Luo, J., Wang, T., Yang, F., Cao, H., Huang, J., and Hu, G., Poult. Sci., 2017, vol. 96, no. 10, pp. 3559–3563. https://doi.org/10.3382/ps/pex163
Wang, D., Wang, N., Li, N., and Li, H., Poult. Sci., 2009, vol. 88, no. 11, pp. 2285–2292. https://doi.org/10.3382/ps.2009-00190
Iqbal, M., Pumford, N.R., Tang, Z.X., Lassiter, K., Wing, T., and Cooper, M., Poult. Sci., 2004, vol. 83, no. 3, pp. 474–484. https://doi.org/10.1093/ps/83.3.474
Laville, E., Sayd, T., Santé-Lhoutellier, V., Morzel, M., Labas, R., Franck, M., Chambon, C., and Monin, G., Meat Sci., 2005, vol. 70, pp. 167–172.
Xing, T., Wang, P., Zhao, L., Liu, R., Zhao, X., Xu, X., and Zhou, G., Poult. Sci., 2016, vol. 95, no. 10, pp. 2391–2396. https://doi.org/10.3382/ps/pew181
Xing, T., Zhao, X., Zhou, G., and Xu, X., J. Agric. Food Chem., 2017, vol. 65, no. 13, pp. 2913–2922. https://doi.org/10.1021/acs.jafc.6b05835
Lund, M.N., Heinonen, M., Baron, C.P., and Estévez, M., Mol. Nutr. Food Res., 2011, vol. 55, pp. 83–95.
Zhou, F., Zhao, M., Zhao, H., Sun, W., and Cui, C., Meat Sci., 2014, vol. 96, no. 4, p. 1432.
Carvalho, M.E., Gasparin, G., Poleti, M.D., Rosa, A.F., Balieiro, J.C., Labate, C.A., and Coutinho, L.L., Meat Sci., 2014, vol. 96, no. 3, pp. 1318–1324.
Funding
The study was supported by the Russian Science Foundation (Project no. 17-16-01028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they do not have conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by V. Mittova
Rights and permissions
About this article
Cite this article
Ismailova, D.Y., Savinova, O.S., Fedorova, T.V. et al. Changes in the Proteome of Poultry Muscle Tissue when Including Various Protein Supplements into Their Diet. Appl Biochem Microbiol 58, 478–489 (2022). https://doi.org/10.1134/S0003683822040068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822040068