Abstract
Metabolism can be represented as a network of enzymatic and nonenzymatic (spontaneous) biochemical reactions. One of the most important parts of nonenzymatic metabolism are the processes involving the formation and redox transformations of highly reactive compounds: reactive forms of oxygen, nitrogen, sulfur, halogens, and reactive carbonyl compounds. All of these reactive forms have one common property—spontaneous interaction with protein amino acid residues. The reactive forms (or reactive metabolites) have long been studied in terms of their ability to have toxic effects on cells. Later, the direction of research shifted to the study of their signaling and regulatory properties. It became clear that low concentrations of these reactive compounds were necessary for living systems. They can regulate the growth and development of the organism. On the one hand, they ensure the stability of biological macromolecules and living systems; on the other hand, they promote phenotypic variability by unmasking latent variability. The review discusses the role of these compounds as regulators of metabolic plasticity and evolutionary ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The formation of the concept of metabolism took place over a long time and, as biological knowledge developed, it was enriched with new reactions and processes. At the same time, the classic textbook Biochemistry by Leninger [1] published in the 1970s included no section devoted to the biochemistry of highly reactive compounds (redox-active substances). There are also no such sections in subsequent publications or in other well-known textbooks on biochemistry. The fact is that redox-active substances are not considered biomolecules. It was believed that, if they do form in living organisms, this usually takes place by chance. This opinion prevailed until there was enough evidence of their necessity for the normal functioning of the cell and the body [2].
The reactive forms of oxygen, nitrogen, sulfur and halogens are most often called redox-active metabolites. These substances, which differ in their chemical structure, have a number of common properties by which they can be attributed to the same group of reactive biological compounds, or, simply, reactive compounds. First, many of these substances are metabolic byproducts. Second, their reactive activity depends on the redox conditions in the cell. Third, they spontaneously (without enzymatic control) react with biomolecules—proteins, carbohydrates, lipids, and nucleic acids, forming a wide range of modified substances with altered structural and functional properties. Fourth, these substances have a multidirectional effect on biological systems, depending on the concentration.
Reactive metabolites form in living organisms either purposefully, with the participation of enzymes, or spontaneously in nonenzymatic reactions (Table 1). An excess of the basal level by these substances leads to the development of a certain type of metabolic stress: oxidative (or oxidizing) stress caused by the action of reactive oxygen species (ROS), nitrosative stress induced by the action of reactive nitrogen species (RNS), carbonyl stress induced by the action of reactive carbonyl compounds (RCC), and halogenated stress caused by the action of reactive halogen compounds. All of these stresses are varieties of redox stress [3], which also includes reductive and electrophilic stress. It is known that ROS and RNS play a dual role in the cell metabolism: they act as signaling molecules at low concentrations, while they have a damaging effect on biomolecules at high concentrations [2, 4–8]. Reactive carbonyl compounds (methylglyoxal, glyoxal, malonic dialdehyde, crotonaldehyde, acrolein, 4-hydroxy-2-nonenal, 4-hydroxy-2-hexanal) also have a dual effect [9–12]. As early as the 1970s, it was shown by Szent-Györgyi that an increase in the utilization of reactive carbonyl compounds led to the cessation of cell growth, as did disturbances in the RCC detoxification system [13].
To date, there are sufficient data showing that both the lack of reactive compounds and their excess negatively affect the cellular state. It is as if the cellular metabolism is programmed for a certain balance of oxidants and reducing agents, acceptors, and donors. A shift in this balance towards oxidants or acceptors leads to the occurrence of oxidative or electrophilic stress. In contrast, a shift in the balance towards reductants or donors results in reductive stress.
THE CONCEPT OF NONENZYMATIC METABOLISM
The concept of “metabolism” is one of the fundamental concepts of biochemistry and physiology. In the modern understanding, metabolism is considered a complex system with a large number of substances and relationships between them. Over the past 20 years, it has also included reactions that do not completely fit into the traditional understanding of metabolism, if only because they are not highly integrated and not always targeted. These reactions form the so-called alternative metabolism, for which the following terms can be found in the literature: “parametabolism,” “latent metabolism,” “noncanonical metabolism,” and “paralogical metabolism.”
Bartosz was the first to draw attention to the presence of nonenzymatic reactions in metabolism and to point out their importance for living systems [14]. Then, A.G. Golubev described the main, spontaneously proceeding reactions and provided a list of products of nonenzymatic interactions [15]. Even the very title of his article, “The Wrong Side of Metabolism,” was used by him to attempt to show that the concept of metabolism is broader than traditional concepts and includes reactions that do not obey the “molecular logic of living things.” To designate this reverse side of metabolism, the terms “parametabolism” and “parametabolites” were introduced.
Parametabolites are byproducts of canonical metabolic pathways and products of noncanonical metabolic pathways. The list of substances that can be attributed to the group of parametabolites is quite extensive and includes the aforementioned ROS and RNS and reactive compounds of sulfur and halogens [12, 15, 16]. A separate group of reactive parametabolites is formed by the oxidation products of glucose and lipids—RCCs. They enter into nonenzymatic glycation reactions [9–12]. About 500 of the nonenzymatic reactions found in biological systems have been described [17]. Most of them are extremely rare, since less abundant compounds are involved in them. Among the nonenzymatic reactions, the most widely represented ones are reactions involving free radicals and nonenzymatic glycation reactions.
In 1998, the concept of “underground metabolism” was introduced into biological science [18]. Latent metabolism involves reactions that are catalyzed by native enzymes when they use endogenous metabolites as alternative substrates. In 2015, Keller et al. proposed the use of the concept of “noncanonical metabolism,” which combines nonenzymatic reactions and side enzymatic reactions [19, 20].
The appearance of reactive “random” metabolites is due to several factors: (1) excessive chemical potencies of reactive metabolites and (2) a low selectivity of enzymes—the appearance of substrate and/or catalytic multispecificity, (3) reorganization of the metabolic flow.
Keller proposed to divide the nonenzymatic reactions occurring in the cell into low-specificity reactions involving a wide range of substrates, specific reactions, and reactions that proceed simultaneously by enzymatic and nonenzymatic pathways [19]. Table 2 shows the examples of such reactions.
Living systems provide various means to control enzymatic and nonenzymatic reactions. The regulation of enzymatic reactions (speed and direction) occurs due to a change in the conformation of the enzyme molecule, while the regulation of nonenzymatic reactions takes place due to changes in the physicochemical parameters of the environment, as well as at the level of generation and/or detoxification of reactive compounds (Table 3). There is no clear boundary between these two pathways. On the one hand, reactive forms that participate in nonenzymatic reactions carrying out posttranslational modification of proteins and regulatory RNAs change their conformation and thereby affect enzymatic metabolism [21]. On the other hand, the functioning of antioxidant and antiglycating enzymes determines the stationary concentrations of reactive forms.
THE ROLE OF NONENZYMATIC REACTIONS IN BIOLOGICAL EVOLUTION
While many active metabolites play a significant role in the development of various pathologies, the expediency of their presence in the cell is increasingly being noted. Table 4 lists the main functions of nonenzymatic reactions and processes in cellular metabolism.
The appearance of reactive metabolites is of particular importance under stress conditions, when they act as mediators of phenotypic and genetic variability. The reorganization of metabolic networks that often occurs under stress conditions allows for an enhanced generation of reactive compounds (ROS and RCC) [12, 22], which can increase the likelihood of the adaptation of cells or a cellular population in different ways.
The influence of stress conditions on metabolism and metabolome is discussed in detail in the article [20]. Here we will only briefly describe the main ways to increase the evolutionary potential of biological systems with the participation of random, chemically reactive metabolites.
The protective effect of reactive compounds can manifest itself directly and indirectly. Direct protection consists of the stabilization of cell proteins, e.g., due to the formation of intraprotein disulfide bonds [23, 24] and complexes with metals [25, 27]. Mediated protection includes the following processes:
(1) Regulation of the central signaling pathways of the cell, including those that are responsible for the reaction to stress [11, 12, 28];
(2) Reprogramming of the epigenome via the direct modification of histones and indirectly via the modification of histone deacetylases and DNA methylases [11, 12];
(3) Reorganization of metabolism, the appearance of additional reactions due to the multispecificity of enzymes [29];
(4) Triggering of the mechanism of stress-induced mutagenesis [22, 30].
We described the functioning of reactive metabolites as stress sensors and regulators of metabolic memory in an earlier article [12]. Here, we will consider the role of reactive forms in the reorganization of metabolism and the triggering of stress-induced mutagenesis.
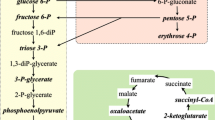
Acting as posttranslational enzyme modifiers, ROS, RNS, and RCCs can influence their selectivity towards canonical substrates or catalyzed reactions. As a result, additional reactions appear in the metabolism, some of which may be beneficial for the body under changed (stressful) conditions. This is illustrated in Fig. 1. The described mechanism is of great importance for an increase in the adaptive potential of bacterial cell populations [22].
Role of reactive metabolites in the reorganization of metabolism under stressful conditions: the shutdown of some reactions due to enzyme inactivation and the appearance of additional reactions catalyzed by modified forms of enzymes. The reaction products may include reactive compounds: ROS, RNS, and RCC.
The consolidation of a new type of metabolic pathway organization requires coding systems that are not necessarily new. They may be a byproduct of processes initially used for other goals. Such coding systems can include some posttranslational modifications that were accidental and harmful when they arose but were subsequently adapted for metabolic needs. For example, the nonenzymatic nature of nitrosylation, a widespread protein modification used in signal transduction, indicates its “accidental” metabolic past [31].
Let us also consider another potential method of cell protection under stress conditions, which consists of the triggering of the mechanism of stress-induced mutagenesis. It is believed that the evolution of organisms occurs in the direction of decreasing entropy. Control over entropy sequentially extends through the stages of information transfer from DNA to protein [20]. The most significant antientropic mechanism is a multilevel error-rate control system that operates at the level of the replication, transcription, translation, folding, and sorting of proteins. However, the global, antientropic orientation of evolution does not exclude the simultaneous existence of mechanisms that increase the error rate.
It can be assumed that the formation of redox-active molecules is metabolically programmed to maintain a certain frequency of phenotypic mutations that cannot be eliminated by antientropic mechanisms in order to provide the variability needed for evolution. Phenotypic mutations are usually understood as consequences of errors in transcription and translation. We believe that it is quite acceptable to include stable, nonenzymatic, posttranslational modifications caused by chemically reactive metabolites among phenotypic mutations. This idea is supported by the fact that the formation of reactive metabolites is strongly stimulated by various types of stress. These compounds increase phenotypic variability through the combined effect of posttranslational modifications (direct formation of variability) and manifestations of latent variability (e.g., HSP90) [12].
The role of ROS in the evolutionary process and adaptation to stress has been described [32, 33]. RCCs are less studied in this context. At present, their role in metabolism as mediators of ROS signals is actively discussed [34–36]. ROS induce lipid peroxidation reactions, the products of which (unsaturated aldehydes and ketones) covalently modify proteins in nonenzymatic reactions of the formation of Schiff bases and Michael adducts.
To date, the mechanism of evolutionability, with the participation of prion-like fungi proteins, has been studied fairly well. It has been shown that prions function as an evolutionary “capacitor,” accumulating critical genetic variations and exposing them under stress conditions [37]. Prions provide a mechanism for the rapid emergence of new phenotypes under stress conditions, some of which may have adaptive value. A parallel can be drawn between the prion mechanism of evolutionability and a similar mechanism involving reactive forms. First, the formation of prions and reactive metabolites is stimulated by stress. Second, both prions and reactive metabolites primarily affect protein homeostasis. Third, these substances are involved in the transmission of information based on proteins. Prions induce autocatalytic proliferation of protein aggregates, and reactive metabolites induce the formation of protein aggregates via posttranslational modifications and also affect protein determinants of epigenetic heredity. Under normal conditions, prion proteins and reactive metabolites are harmful, since the former reduce the translation accuracy, while the latter cause uncontrolled posttranslational modifications. However, under stress conditions, these substances provide a possible mechanism for the rapid acquisition of new phenotypes.
Summarizing the above, we can conclude that biological systems are basically quasi-deterministic, i.e., they combine predetermination and randomness. Randomness combined with natural selection are the tools of evolution, not only for metabolism but for the genome [38].
THE ROLE OF REACTIVE COMPOUNDS IN THE DEVELOPMENT OF ADAPTIVE REACTIONS OF THE CELL
Nonspecific Adaptive Cell Syndrome
All living organisms need to adapt to changing environmental conditions in order to survive. The structural and functional unit of the body is the cell; therefore, changes in the body that occur during the development of adaptive reactions must affect the cellular systems. The response of cells to the action of various factors combines general (nonspecific) and specific features. Nonspecific reactions are manifested especially clearly in the case of high-intensity effects that cause damage.
The stereotypical, nonspecific character of the reaction at the cellular or tissue level to the action of various factors was described for the first time by N.E. Vvedenskii [39]. He proposed the term “parabiosis” (near life), which indicates a state close to death. The literature gives other terms with similar content—“necrobiosis” (on the verge of death and life) and “paranecrosis” (near death). All of these terms denote the state of cells that develop a complex of features corresponding to the last reversible phases of damage.
There has been a huge amount of work on the study of stress at the cellular level. Special attention should be paid to the ideas of the founder of this trend in science, D.N. Nasonov, and his followers, V.Ya. Aleksandrov and A.D. Brown. D.N. Nasonov, acting on various cells with increasing doses of factors of different natures, discovered a two-phase response of the cell to stress [40]. Subsequently, the followers of D.N. Nasonov associated this phenomenon with the unfolding of the native structure of proteins and the release of reaction centers, primarily SH groups [41]. These studies showed that proteins exhibited similar changes upon excitation and damage to the cell, which made it possible to formulate the “denaturation theory of damage and excitation” [40]. The role of protein conformational changes (labilization and stabilization) in cell adaptation was also established [41]. The existence of similar, nonspecific changes in physicochemical and metabolic parameters during the development of the adaptive response of the cell was described. This enabled the concept of nonspecific adaptation syndrome, which was previously used only in relation to the organism [43, 44], to be applied to the cell [42]. A.A. Matveev proposed to designate the nonspecific component of the cellular response by the term “universal cellular response” [45].
The development of the adaptation syndrome of the cell is accompanied by changes in the morphological, physicochemical, and biochemical parameters. Here are the most common, early, and permanent signs of cell damage:
(1) Activation of free radical oxidation of membrane phospholipids;
(2) Disruption of the functioning of membrane enzymes;
(3) Changes in membrane properties—microviscosity, phospholipid composition, permeability, etc.;
(4) Exit of various substances from cells (inorganic ions, lactic, pyruvic, ketoglutaric acids, proteins, amino acids);
(5) Uncoupling of oxidative phosphorylation, cessation of respiration;
(6) Increased aerobic glycolysis;
(7) Acidotic shift (shift in the pH of the cytoplasm from 7.32–7.45 to 6.5–5.39);
(8) Exit of potassium from the cell, entry of sodium and water.
These changes can be biologically reasonable, i.e., they are, to a certain extent, adaptive in nature and are aimed at the stabilization of metabolism under stress. Table 5 presents some of the processes that occur in the cell in the early stages of the action of stress factors before the synthesis of stress proteins.
Many of these processes are intended to increase the stability of macromolecules and reduce the sensitivity and functionality of the cell, which leads to decreased metabolic activity. This condition can be referred to as an “emergency regime.” The transition of the cell to such a regime is accompanied by the blocking of a number of reactions that can be dispensed with and the involvement of other reactions that ensure the stability of the cellular system. The metabolic activity is narrowed to preserve the reserves of the cell for subsequent recovery.
One of the factors that stabilize protein molecules and reduce their reactivity are reactive metabolites (ROS and RCCs), the level of which increases at the initial stages of damage (development of a stress reaction) [46]. ROS are produced as a result of metabolic disturbances or as part of a network of perception and transmission of stress signals. Metabolic ROS directly alter the redox status of enzymes, thereby rebuilding metabolic flows. In addition, they can influence transcription and translation by altering the activity of key regulatory proteins. Signaling ROS are generated in response to stress by stress sensors, e.g., NADPH oxidase.
The adaptation of the cell to stressful environmental conditions can be short-term or long-term. In the second case, changes in the transcription apparatus are turned on, which leads to the synthesis of stress proteins (heat shock proteins, chaperones, antioxidant enzymes, antifreeze proteins, etc.). In addition, a prolonged and systematic repetition of exposure that does not go beyond the boundaries of physiological stress can induce rearrangements in the epigenome [47]. Since it takes time to implement a response that affects gene expression, the cell provides fast response mechanisms that form a short-term adaptation. An example of the latter is a change in the conformation and biological activity of an enzyme upon the binding of a reactive metabolite to its regulatory center. Regulatory centers can be both structures that are complementary to the structure of the bioregulator and reactive amino acid residues (e.g., those of cysteine or histidine) in a specific region of the polypeptide chain. The first type of regulation is allosteric, and the second is site-specific [12].
Reactive Metabolites As Inducers of Metabolic Plasticity
Various experimental models have shown that the adaptation mechanisms of living systems obey the hormesis rule. Hormesis is the name given to the process in which low doses of toxic substances or stress factors make a cell or the entire body more resistant to higher toxic doses of a stressor by activating stress resistance mechanisms. Hormesis is an evolutionarily conservative, universal adaptive response that provides metabolic plasticity of living systems [48–50]. It is graphically expressed as a two-phase, U-shaped or inverted U-shaped curve in dose-response coordinates (Fig. 2). This curve describes the change in the level of reactivity of a living system with respect to the strength of the influence of any factor. Two phases on the hormesis curve (the activation phase and the phase of suppressed function) are not considered to be different processes but different stages of the same process of adaptive response development [49]. The hormesis curve describes the limits to which the functions of a cell (proliferation, growth, migration, etc.) or an organism (complex behavior, learning, memory, etc.) can change under the influence of chemical or physical factors [50].
The history of the study of biphasic reactions dates back to the 1880s, when Schultz tested various disinfectants on yeast cells [51]. The term “hormesis” was first mentioned in 1943 in the work of Southam and Ehrlich, who studied the effect of red cedar extracts on the metabolism of fungi [52]. Currently, the concept of hormesis is being actively developed by the Calabrese group [49, 50, 53].
The hormesis response is inherent to the different levels of the organization of living matter, which vary from proteins to the whole organism [50]. This is the way that living organisms adapt to short-term and long-term stresses [50, 54]. However, there is still no complete understanding of the molecular mechanisms that are responsible for hormesis. We can only say that each level of the structural organization is characterized by its own mechanisms. The cellular response usually includes a complex adaptive program to increase antioxidant and electrophilic protection. This is due to the dual action of reactive metabolites (ROS, RNS, and RCCs), which, in low doses, activate the signaling pathways of response to stress through nonenzymatic PTMs of metabolic and signaling enzymes, cytoskeletal proteins, and transcription factors (NF-kB, FoxOs, Nrf2 and other redox adjustable factors) [11]. Figure 3 shows the role of reactive metabolites as inducers of hormesis. Reactive metabolites mediate the adaptive hormesis response of a cell or organism to stress through nonenzymatic posttranslational modifications (PTMs) of proteins: oxidation, nitration, nitrosylation, chlorination, lipoxidation, and glycation.
Mediation of the adaptive hormesis response of a cell or organism to stress via nonenzymatic, posttranscriptional protein modifications. HNE is 4-hydroxy-2-nonenal, MDA is malondialdehyde, MG is methylglyoxal, TPI is triose phosphate isomerase, GAPDH is glyceraldehyde-3-phosphate dehydrogenase, OXPHOS is oxidative phosphorylation.
ROS play an essential role in providing hormesis [55, 57]. It is believed that their production at low and moderate stress helps the cell or body to adapt to stronger influences. Therefore, regular, moderate physical activity, during which ROS are formed, is recommended in order to improve the health and overall resistance of the organism [56]. The ROS sources during exercise include not only mitochondria but also enzymatic reactions catalyzed by NADPH oxidase, xanthine oxidase, and monoamine oxidase. The production of ROS can be caused by short-term hypoxia due to intensive oxygen consumption by skeletal muscles [58]. The activation of antioxidant systems also occurs in other cases, e.g., in the case of moderate alcohol consumption, which causes ROS production [59].
In addition to physical activity, reduced caloric intake or a low-carbohydrate diet can improve health and life expectancy [60–62]. This effect is associated with a decrease in glycolytic flow. This, in turn, is associated with a decrease in the production of methylglyoxal (MG), which contributes to cell aging due to nonenzymatic glycation reactions. Conversely, insignificant amounts of MG are required for the cell to maintain the activity of the chaperone and the antiapoptotic protein Hsp27 [62]. Therefore, consideration is given to the beneficial effect of intermittent fasting, which makes it is possible to maintain the production of MG and ROS at a level that is sufficient to activate signaling protection pathways but insufficient for the manifestation of toxic effects [61, 62].
The fact that ROS and RCCs are necessary for the body to maintain the activity of defense systems casts doubt on the advisability of the consumption of large amounts of antioxidants and the exclusion of carbohydrates from the diet.
PROTEIN CYSTEINES ARE SENSORS OF REACTIVE METABOLITES
The manifestation of a nonspecific component in the adaptive response of a cell is largely a consequence of the interaction of reactive metabolites with reactive cysteine residues of various proteins, including enzymes, transcription factors, membrane channels, receptors, and cytoskeletal proteins [63–65]. Redox modifications of cysteines affect the catalytic activity, metabolic rate, subcellular localization of proteins, and protein–protein [66] and protein–membrane interactions [67]. They are often combined with phosphorylation/dephosphorylation and calcium-dependent signal transduction pathways [68]. This is how random reactions are integrated with enzymatic metabolism.
It is no coincidence that cysteine was evolutionarily chosen to be a sensor of the redox state of the cell and reactive metabolites. First, this amino acid interacts with many ROS and RCCs [36, 63, 64], and, second, most of the cysteine modifications are reversible. The reactions between reactive forms and protein cysteines are usually site-specific. They depend on the microenvironment in the protein molecule, which determines the degree of ionization and the availability of reactive metabolites for impact [12, 64, 69]. The proteins containing reactive SH groups are called redox switches [36, 68]. Figure 4 shows the main products of the reaction of cysteine with reactive metabolites. The set of posttranslational modifications of protein cysteines forms the so-called cysteine proteome of the cell.
Due to the high sensitivity to redox-active metabolites, cysteines function as sensors for the redox environment of the cell and reactive metabolites (oxidants and electrophiles). Oxidized or reduced thiols are a kind of switches for signal transduction pathways that are sensitive to changes in the redox state under stress conditions [68]. Cysteines are most often attacked by ROS, namely, H2O2 and organic peroxides. The reaction involves only deprotonated cysteines in the form of thiolate (–S–). The oxidation of thiolate with peroxides leads to the formation of sulfenic acid (–SOH), which is a key player in redox signaling [64, 70]. Cysteine sulfenic acids are unstable and can be additionally oxidized to irreversible forms: sulfinic (–SO2H) and sulfonic (–SO3H) acids. Other modifications are possible: the formation of disulfide bonds (–SS–), S‑glutathionylation (–SSG) and the formation of sulfenamide (–SN=).
The multidirectional action of reactive metabolites is partly due to their ability to activate or block sulfhydryl groups of redox switch proteins depending on concentration. Thus, the sensitivity and resistance of the cell to these redox conditions is adjusted. These substances can release the reaction potential of SH groups in a certain concentration range. The activation mechanisms can be different. The mechanism of greatest interest is an increase in the reactivity of SH groups via decomposition of the labile complex that includes them [23, 26, 69]. These may also include complexes with metals (Fe, Zn, Cu, Ca).
The binding of metals by proteins can serve as a mechanism for the temporary “shutdown” of a protein from metabolic transformations under the conditions of a strong violation of redox conditions (oxidative, nitrosative, and carbonyl stress).
As the spatial structure is disrupted in proteins, additional metal binding centers appear. As a result, stable protein aggregates with reduced functional activity form in the cell [71]. The progressive formation of metal–protein complexes is observed during aging and as a result of the effect on the protein by damaging agents, which accumulate during the development of oxidative stress and/or disruption of metabolic processes in the body [72, 73].
One way to stabilize proteins and reduce their reactivity can be the inclusion of protein SH groups in complexes with NO (R–S–Fe2+(NO)2) [69] (Fig. 4). The formation of such complexes blocks cysteine residues under normal conditions and sensitizes them under conditions of electrophilic/oxidative stress, since the thiols in the structure of the complex are in an active ionized form (in the form of a thiolate anion) and do not require preliminary deprotonation.
REACTIVE METABOLITES ARE REGULATORS OF CANCER CELL METABOLISM
A very important process that can be discussed in this context is the role of ROS in the regulation of cancer cell metabolism. Such regulation is also subject to the hormesis mechanism. The antioxidant potential of tumor cells maintains a certain level of ROS that activate prooncogenic signals that increase cell survival, proliferation, and resistance to apoptosis but do not have a damaging effect [74].
It is known that the pronounced features of cancer cell metabolism include enhanced glycolysis, even in the presence of a high oxygen concentration (the Warburg effect or aerobic glycolysis), and constant high levels of ROS [5, 74, 75]. First, cancer cells activate glycolysis in response to ROS, and then ROS inhibit glycolysis enzymes—glyceraldehyde-3-phosphate dehydrogenase, pyruvate kinase M2, and phosphofructokinase [76]. Due to this, the metabolic flow of glucose is redirected to the alternative pentose phosphate pathway [75], in which NADPH, a reducing equivalent for antioxidant enzymes, including the system of glutathione and thioredoxins, is formed [77]. However, the inactivation or inhibition of glyceraldehyde 3-phosphate dehydrogenase promotes the entry of triose phosphate intermediates of glycolysis into the glyoxalase pathway, in which an active carbonyl compound methylglyoxal is formed [78]; an excessive accumulation of it can lead to cell death. Due to this, glyceraldehyde-3-phosphate dehydrogenase is an important therapeutic target in cancer treatment.
REACTIVE COMPOUNDS AS PROTOBIOREGULATORS
Biological systems have a special type of signal transduction that is based on the protein modification by reactive compounds (oxidants and electrophiles). The negative and positive aspects of such signaling are dictated by the history of the emergence of living systems, the roots of which go deep into the evolutionary past of the cell, possibly even into the precellular era, when the first protein catalysts appeared.
The perception and transmission of signals in modern biological systems are carried out by proteins, which change their structure and properties under the action of certain substances. Modern metabolism is based on the selective interaction of proteins with substances; however, selectivity in protometabolic systems was the exception rather than the rule. Proteins most likely reacted to various agents in a complex manner with all of their available reaction centers, perceiving them as signals (stimuli). The ability to react nonspecifically has also been preserved in modern proteins. It is found under the conditions of high doses of stimuli. While nonspecific reactions are generally undesirable in the modern metabolism, this was the usual way of receiving signals in the protometabolism; it allowed the system to adapt to the environment. The consequences of nonspecific interactions could be both beneficial and harmful to the system.
The ability of reactive compounds to interact reversibly with reaction residues of cysteines in proteins forms the basis of intracellular redox signaling. It is the spontaneous nature of these reactions and the absence of strict selectivity that lead to the duality of their effects. At low concentrations, redox-active molecules predominantly modify regulatory proteins; at higher concentrations, they also modify other cellular proteins. In the first case, they act as adequate stimuli (signaling molecules); in the second case, they act as inadequate stimuli, inducing a complex of nonspecific adaptive changes that can turn into pathological ones.
Reactive oxygen species, RNS, and RCCs can be classified as universal agents that act as nonspecific allosteric effectors inherited by the modern metabolism from its evolutionary past, and nonspecific reception can be considered a steps towards the emergence of specific reception. Presumably, redox signaling arose due to the stabilization of adaptively valuable posttranslational changes in proteins, which occurred in violation of the homeostatic parameters of the intracellular environment as early as the stage of primary cells. This scenario of the origin of receptors from a pool of proteins that were initially subject to modification by redox-active compounds or participated in their metabolism seems to be quite plausible.
It should also be noted that many ROS and RCC are byproducts of the pathways of energy supply to the cell: electron transport chains of mitochondria and glycolysis. This means that their intracellular concentration carries information about the state of catabolic pathways and the lack of food substrates, or, conversely, their excess. For example, methylglyoxal, a glycolysis byproduct, signals the inability of enzymatic pathways to utilize glucose. In addition, under the conditions of stressful factors, the energy systems of the cell suffer the most. In this case, ROS and RCCs act as stress mediators.
CONCLUSIONS
To date, the list of physiological processes has been supplemented by phenomena such as oxidative mitogenesis, epigenetic inheritance, phenotypic plasticity, and hormesis. The views of enzymatic metabolic pathways proved to be insufficient to explain the mechanisms underlying these phenomena. Therefore, it is necessary to identify an area in the general metabolism of the cell that is only partly associated with the functioning of enzyme systems and is, in fact, a network of nonenzymatic redox reactions that cause posttranslational modifications of proteins: oxidation, nitrosylation, nitration, lipoxidation, chlorination, glycation, acylation, thiolation, formation of Michael’s products, etc.
The emergence of specificity based on the complementarity of the structures of interacting molecules has become a prerequisite for the channeling of metabolism. It is known that enzymes catalyze reactions that can occur even in their absence, since they are thermodynamically possible. Due to the increase in the speed of desired reactions, the likelihood of side reactions is minimized. While most metabolic processes were taken under enzymatic control, nonenzymatic reactions are present in the metabolism of modern organisms.
Reactive metabolites play an important role in the regulation of metabolic plasticity and the evolution of living systems. On the one hand, they ensure the stability of biological macromolecules and systems; on the other hand, they contribute to phenotypic variability by unmasking latent variability. The complex organized system of redox reactions involving reactive metabolites and the cysteine proteome, as well as the widespread occurrence of these reactions in all living organisms, which vary from bacteria to humans, leave no doubt that the formation of these “random” reactive compounds is not at all accidental and is one of the mechanisms metabolic regulation.
REFERENCES
Lehninger, A.L., Biochemistry: The Molecular Basis of Cells Structure and Function, New York: Worth Publ., 1970.
Zarkovic, N., Cells, 2020, vol. 9, no. 3. e767. https://doi.org/10.3390/cells9030767
Gutteridge, J.M.C. and Halliwell, B., Biochem. Biophys. Res. Commun., 2018, vol. 502, no. 2, pp. 183–186.
Beckman, J.S. and Koppenol, W.H., Am. J. Physiol. Cell. Physiol., 1996, vol. 271, pp. 1424–1437.
Moloney, J.N. and Cotter, T.G., Semin. Cell Dev. Biol., 2017, vol. 80, pp. 50–64.
Abalenikhina, Yu.V., Kosmachevskaya, O.V., and Topunov, A.F., Appl. Biochem. Microbiol., 2020, vol. 56, no. 6, pp. 611–623.
Moldogazieva, N.T., Mokhosoev, I.M., Mel’nikova, T.I., Zavadskiy, S.P., Kuz’menko, A.N., and Terentiev, A.A., Biochemistry (Moscow), 2020, vol. 85, suppl. 1, pp. S56–S78.
Sies, H. and Jones, D.P., Nat. Rev. Mol. Cell. Biol., 2020, vol. 21, no. 7, pp. 363–383.
Ayala, A., Munoz, M.F., and Arguelles, S., Oxid. Med. Cell. Longev., 2014, vol. 2014, p. 360438.
Semchyshyn, H.M., Sci. World J., 2014, p. 417842.
Kosmachevskaya, O.V., Shumaev, K.B., and Topunov, A.F., Appl. Biochem. Microbiol., 2017, vol. 53, no. 3, pp. 273–289.
Kosmachevskaya, O.V., Shumaev, K.B., and Topunov, A.F., Biochemistry (Moscow), 2019, vol. 84, S-uppl. 1, pp. S206–S224.
Szent-Gyorgyi, A., Bioelectronics. A Study in Cellular Regulations, Defense, and Cancer, New York: Academic Press, 1968.
Bartosz, G., J. Theor. Biol., 1981, vol. 91, pp. 233–235.
Golubev, A.G., Biokhimiya, 1996, vol. 61, no. 11, pp. 2018–2039.
Golubev, A.G., St. Petersburg: L-N, 2015.
Danchin, A., Microb. Biotechnol., 2017, vol. 10, pp. 57–72.
D’Ari, R. and Casadesus, J., BioEssays, 1998, vol. 20, pp. 181–186.
Keller, M.A., Piedrafita, G., and Ralser, M., Curr. Opin. Biotechnol., 2015, vol. 34, pp. 153–161.
Piedrafita, G., Keller, M.A., and Ralser, M., Biomolecules, 2015, vol. 5, pp. 2101–2122.
Kozlov, V.A., Sapozhnikov, S.P., Sheptukhina, A.I., and Golenkov, A.V., Vestn. RAMN, 2015, vol. 70, no. 4, pp. 397–402.
Kosmachevskaya, O.V., Shumaev, K.B., and Topunov, A.F., Biochemistry (Moscow), 2015, vol. 80, no. 13, pp. 1655–1671.
Giles, N.M., Watts, A.B., Giles, G.I., Fry, F.H., Littlechild, J.A., and Jacob, C., Cell Chem. Biol., 2003, vol. 10, no. 8, pp. 677–693.
Karimi, M., Ignasiak, M.T., Chan, B., Croft, A.K., Radom, L., Schiesser, C.H., Pattison, D.I., and Davies, M.J., Sci. Rep., 2016, vol. 6, no. 1, p. 38572.
Desmet, J., Joniau, M., and Van Dael, H., Stud. Org. Chem., 1993, vol. 47, pp. 299–307.
Arnold, F.H. and Zhang, J.H., Trends Biotechnol., 1994, vol. 12, no. 5, pp. 189–192.
Sayre, L.M., Moreira, P.I., Smith, M.A., and Perry, G., Ann. Ist. Super Sanita, 2005, vol. 41, no. 2, pp. 143–164.
Evans, J.L., Goldfine, I.D., Maddux, B.A., and Grodsky, G.M., Endocrine Rev., 2002, vol. 23, no. 5, pp. 599–622.
Carbonell, P., Lecointre, G., and Faulon, J.-L., J. Biol. Chem., 2011, vol. 286, no. 51, pp. 43994–44004.
Tenaillon, O., Denamur, E., and Matic, I., Trends Microbiol., 2004, vol. 12, no. 6, pp. 264–270.
Moldogazieva, N.T., Mokhosoev, I.M., Feldman, N.B., and Lutsenko, S.V., Free Radic. Res., 2018, vol. 52, no. 5, pp. 507–543.
Forman, H.J., Ann. N.Y. Acad. Sci., 2010, vol. 1203, pp. 35–44.
Tauffenberger, A. and Magistretti, P.J., Neurochem. Res., 2021, vol. 46, no. 1, pp. 77–87.
Domingues, R.M., Domingues, P., Melo, T., Pérez-Sala, D., Reis, A., and Spickett, C.M., J. Proteomics, 2013, vol. 92, pp. 110–131.
Mano, J., Biswas, M.S., and Sugimoto, K., Plants, 2019, vol. 8, no. 10. e391. https://doi.org/10.3390/plants8100391
Viedma-Poyatos, Á., González-Jiménez, P., Langlois, O., Company-Marín, I., Spickett, C.M., and Pérez-Sala, D., Antioxidants, 2021, vol. 10, no. 2. e295. https://doi.org/10.3390/antiox10020295
Tyedmers, J., Madariaga, M.L., and Lindquist, S., PLoS Biol., 2008, vol. 6, no. 11. e294. https://doi.org/10.1371/journal.pbio.0060294
Koonin, E.V., The Nature and Origin of Biological Evolution. Upper Saddle River, NJ, USA: FT Press, 2012.
Vvedenskii, N.E., Izbrannye proizvedeniya (Selected Works), Moscow: Akad. Nauk SSSR, 1951, vol. 2.
Nasonov, D.N., Mestnaya reaktsiya protoplazmy i rasprostranyayushcheesya vozbuzhdenie (Local Protoplasmic Response and Spreading Excitation), Moscow: Akad. Nauk SSSR, 1962.
Aleksandrov, V.Ya., Reaktivnost’ kletok i belki (Cell Reactivity and Proteins), Leningrad: Nauka, 1985.
Braun, A.D. and Mozhenok, T.P., Nespetsificheskii adaptatsionnyi sindrom kletochnoi sistemy (Nonspecific Adaptation Syndrome of the Cellular System), Leningrad: Nauka, 1987.
Selye, H., The Case for Supramolecular Biology, New York: Liveright, 1967.
Garkavi, L.Kh., Kvakina, E.B., and Ukolova, M.A., Adaptatsionnye reaktsii i rezistentnost' organizma (Adaptive Responses and Body Resistance), Rostov-on-Don: Rostov. Univ., 1979.
Matveev, V.V., Cell Mol. Biol., 2005, vol. 51, no. 8, pp. 715–723.
Choudhury, F.K., Rivero, R.M., Blumwald, E., and Mittler, R., Plant Abiotic Stress, 2017, vol. 90, no. 5, pp. 856–867.
Vaiserman, A.M., Ageing Res. Rev., 2011, vol. 10, pp. 413–421.
Agutter, P.S., BioEssays, 2007, vol. 29, no. 4, pp. 324–333.
Calabrese, E.J., Environ. Pollut., 2013, vol. 182, pp. 452–460.
Calabrese, E.J., Microb. Cell, 2014, vol. 1, no. 5, pp. 145–149.
Schulz, H., Virch. Archiv Pathol. Anat. Phys. Klin. Med., 1887, no. 108, pp. 423–445.
Southam, C.M. and Ehrlich, J., Phytopathology, 1943, vol. 33, pp. 517–524.
Calabrese, E.J., Dhawan, G., Kapoor, R., Iavicoli, I., and Calabrese, V., Gerontology, 2016, no. 62, pp. 530–535.
Zimmermann, A., Bauer, M.A., Kroemer, G., Madeo, F., and Carmona-Gutierrez, D., Microbial Cell, 2014, vol. 1, no. 5, pp. 150–153.
Radák, Z., Chung, H.Y., Koltai, E., Taylor, A.W., and Goto, S., Ageing Res. Rev., 2008, pp. 34–42.
Goto, S. and Radák, Z., Dose Response, 2010, vol. 8, no. 1, pp. 68–72.
Oliveira, M.F., Geihs, M.A., França, T.F.A., Moreira, D.C., and Hermes-Lima, M., Front. Physiol., 2018, vol. 9, p. 945.
Clanton, T.L., J. Appl. Physiol., 2007, vol. 102, pp. 2379–2388.
Wang, Q., Sun, A.Y., Simonyi, A., Kalogeris, T.J., Miller, D.K., Sun, G.Y., and Korthuis, R.J., Free Radic. Biol. Med., 2007, vol. 43, pp. 1048–1060.
Yu, B.P. and Chung, H.Y., Ann. N.Y. Acad. Sci., 2006, vol. 928, pp. 39–47.
Hipkiss, A.R., Ann. N.Y. Acad. Sci., 2006, vol. 1067, pp. 361–368.
Hipkiss, A.R., Biogerontology, 2007, vol. 8, no. 2, pp. 221–224.
Winterbourn, C.C. and Hampton, M.B., Free Radic. Biol. Med., 2008, vol. 45, no. 5, pp. 549–561.
Trost, P., Fermani, S., Calvaresi, M., and Zaffagnini, M., Plant, Cell Environ., 2017, vol. 40, no. 4, pp. 483–490.
Day, N.J., Gaffrey, M.J., and Qian, W.-J., Antioxidants, 2021, vol. 10, no. 3, e499. https://doi.org/10.3390/antiox10030499
Liebthal, M., Schuetze, J., Dreyer, A., Mock, H.-P., and Dietz, K.-J., Antioxidants, 2020, vol. 9, no. 6, e515. https://doi.org/10.3390/antiox9060515
Kosmachevskaya, O.V., Nasybullina, E.I., Blindar, V.N., and Topunov, A.F., Appl. Biochem. Microbiol., 2019, vol. 55, no. 2, pp. 83–98.
Klomsiri, C., Karplus, P.A., and Poole, L.B., Antioxid. Redox Signal., 2011, vol. 14, no. 6, pp. 1065–1077.
Kosmachevskaya, O.V., Nasybullina, E.I., Shumaev, K.B., Novikova, N.N., and Topunov, A.F., Appl. Biochem. Microbiol., 2020, vol. 56, no. 5, pp. 512–520.
Claiborne, A., Mallett, T.C., Yeh, J.I., Luba, J., and Parsonage, D., Adv. Protein Chem., 2001, vol. 58, pp. 215–276.
Reeg, S. and Grune, T., Antioxid. Redox Signal., 2015, vol. 23, no. 3, pp. 239–255.
Novikova, N.N., Kovalchuk, M.V., Yurieva, E.A., Konovalov, O.V., Stepina, N.D., Rogachev, A.V., Yalovega, G.E., Kosmachevskaya, O.V., Topunov, A.F., and Yakunin, S.N., J. Phys. Chem. B, 2019, vol. 123, no. 40, pp. 8370–8377.
Konovalov, O.V., Novikova, N.N., Kovalchuk, M.V., Yalovega, G.E., Topunov, A.F., Kosmachevskaya, O.V., Yurieva, E.A., Rogachev, A.V., Trigub, A.L., Kremennaya, M.A., Borshchevskiy, V.I., Vakhrameev, D.D., and Yakunin, S.N., Materials, 2020, vol. 13, no. 20. e4635. https://doi.org/10.3390/ma13204635
Moloney, J.N. and Cotter, T.G., Semin. Cell Dev. Biol., 2017, vol. 80, pp. 50–64.
Movahed, Z.G., Rastegari-Pouyani, M., Mohammadi, M.H., and Mansouri, K., Biomed. Pharmacother., 2019, vol. 112. e108690. https://doi.org/10.1016/j.biopha.2019.108690
Mullarky, E. and Cantley, L.C., Innovative Medicine: Basic Research and Development, Tokyo: Springer, 2015.
Stincone, A., Prigione, A., Cramer, T., Wamelink, M., Campbell, K., Cheung, E., Olin-Sandoval, V., Gruning, N.M., Kruger, A., Tauqeer, AlamM., Keller, M.A., Breitenbach, M., Brindle, K.M., Rabinowitz, J.D., and Ralser, M., Biol. Rev., 2015, vol. 90, pp. 927–963.
Kosmachevskaya, O.V., Novikova, N.N., and Topunov, A.F., Antioxidants, 2021, vol. 10, no. 2. e253. https://doi.org/10.3390/antiox10020253
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by L. Solovyova
Rights and permissions
About this article
Cite this article
Kosmachevskaya, O.V., Topunov, A.F. Nonenzymatic Reactions in Metabolism: Their Role in Evolution and Adaptation. Appl Biochem Microbiol 57, 543–555 (2021). https://doi.org/10.1134/S0003683821050100
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683821050100