Abstract
Ajuga turkestanica (Rgl.) Briq. (Lamiaceae) callus cultures were obtained from a wild plant, and the morphogenesis processes in these cultures were studied. It was possible to obtain gemmogenesis or rhizogenesis in the first six to eight growth cycles, depending on the hormonal composition of the medium; the capacity for morphogenesis was lost after 10–15 growth cycles. Suspension cell cultures were initiated from some calluses with strong growth. As a result, about 100 calluses and suspension plant cell lines of A. turkestanica were obtained; a number of them were characterized in terms of growth rate and were analyzed via HPLC-MS for the presence of phytoecdysteroids. 20-Hydroxyecdysone and turkesterone were found in most of the studied lines; the content of the first compound in the most productive lines was 30–50 times higher than in the second (2.0–2.5 mg/g versus 0.04– 0.05 mg/g dry weight, respectively). An increase in the phytoecdysteroid content in plant cells cultured in vitro was observed by the end of the growing cycle. However, phytoecdysteroids were not found in many of the obtained plant cell lines. Further research is needed to clarify the causes of the presence or absence of phytoecdysteroids in A. turkestanica plant cell cultures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Higher plants are characterized by the ability to synthesize secondary metabolites that are widely used in pharmacology, cosmetology, and the food industry. They are mainly collected from natural biocenoses, which may threaten the extinction of the used species. The development of biotechnological approaches to the obtainment of renewable materials based on the industrial growth of plant cell cultures and organs in vitro is an effective way to solve the problem of the conservation of rare and endangered species. A complex of basic studies on the growth of cultivated plant cells and the biosynthesis of secondary compounds by them is needed to obtain industrial producer strains. These studies will make it possible to study the physiological processes characteristic of a given species or plant organ in vitro, to assess its sensitivity to growth regulators of various types, and to optimize the formation of target products [1].
Ajuga turkestanica (Rgl.) Briq. (Lamiaceae) is an endemic plant of Western Tien Shan and Hissaro-Alai. The ecdysteroids synthesized by this plant have a broad spectrum of biological activity, in particular, tonic, adaptogenic, and anabolic activities [2–4]. The presence of turkesterone, a C11-hydroxylated ecdysteroid with anabolic activity, is a unique feature of the species.
Cultivated A. turkestanica callus tissue initiated from an intact plant ovary was previously obtained and studied [5]. The goal of the present work was to obtain leaf cell cultures from wild A. turkestanica plant leaves and to study the effect of growth regulators, synthetic analogues of phytohormones, on the morphogenetic, growth, and biosynthetic processes in these plant cell cultures.
EXPERIMENTAL
Materials
The original A. turkestanica plant was collected in the blooming phase in 2014 near the village of Baisun, in the foothills of the Hissar range of the Republic of Uzbekistan. Callus cultures were obtained from leaf explants according to the routine method [6]. The cultures were grown at 26°С in the dark on Murashige—Skoog nutrient medium [7] with sucrose (30 g/L), myo-inositol (100 mg/L), thiamine (0.4 mg/L), and synthetic analogues of phytohormones in various combinations. The cultivation cycle (the time interval between the transplantations) for callus cultures was 30 days. In order to optimize the nutrient medium for callus tissue cultivation, NAA and 2,4-D as auxins and BAP as a cytokinin were tested in the first cultivation cycles. The following combinations of growth regulators in the nutrient media were used, mg/L: NAA (0.1), NAA (1.0); NAA (1.0) + BAP (0.2); 2,4-D (0.1); 2,4‑D (1.0) + BAP (0.2). A medium without growth regulators was also used.
The growth and physiological characteristics of the plant cell cultures were determined in agreement with conventional methods [8]. The increase in cell biomass was calculated as the ratio of cell dry weight by the time of transplantation (30th day of cultivation) to the graft weight (100 ± 5 mg).
The suspension cell cultures were initiated from strongly growing callus lines after the 15th cultivation cycle, and the optimization of the callus culture growth was continued. Gambourg medium (B5) [9] was used in this series of experiments, the set of synthetic analogues of phytohormones was broadened (with kinetin as an additional cytokinin), and the range of their concentrations was expanded.
The experiments were carried out in triplicate. The sample size was n ≥ 25. The obtained data were statistically processed with the Microsoft Office Excel 2003 software.
Preparation of Samples for Ecdysteroid Analysis
The ground air-dry biomass (36–40 mg, a precise aliquot) was extracted three times with water (1 mL) for 30 min with sonication (ultrasonic bath, Sapphire, Russia) and then centrifuged at 10 000 rpm for 10 min (MCF microcentrifuge, Sistemy Analiza, Russia) with subsequent collection of the supernatant. The combined water extracts were loaded onto a Supelclean ENVI-18 (Supelco, United States) cartridge for solid phase extraction. The cartridge was washed with water (3 mL), and the analytes were eluted with ethanol (3 mL). The obtained solution was vacuum-evaporated at 45°С. Before the analysis, the extracts were dissolved in an ethanol–water mixture (7.5 : 2.5, vol) and passed through a nylon filter with a pore size of 0.2 μm (Acrodisc, Pall Corporation, United States).
HPLC-MS
The analysis was carried out in an Agilent 1260 Infinity device (Agilent Technologies, United States) equipped with a mass-selective detector (6100, Agilent Technologies) with a column of Poroshell 120 EC-C18 (100 × 3 mm, 2.7 μm, Agilent). The column temperature was 43°C, the flow rate of the mobile phase was 0.4 mL/min, and the injection volume was 0.4 μL. The mobile phase consisted of a solution of formic acid (0.03% vol) in water (solvent A) and acetonitrile (solvent B) used for gradient elution. The composition of mobile phase changed during the analysis in the following way (solvent B, volume %): 0–13 min—15%, 13–13.5 min—15 → 95%, 13.5–15 min—95%, 15–15.5 min—95 → 15%, 15.5–17 min—15%. A solution of formic acid (0.03% vol) in water (solvent A) or acetonitrile (solvent B) was used as the mobile phase. The analysis was carried out in negative ion mode with an m/z range of 100–1300 and a fragmenter of 70. The parameters of the ionization source were as follows: quadrupole temperature, 100°С; carrier gas (nitrogen) temperature, 250°С; nitrogen supply rate (spraying gas), 13 L/min; nitrogen pressure, 1811 Torr; and capillary voltage, 3.5 kV. The chromatograms were recorded in the selected ion mode (m/z 525 for ecdysterone and 541 for turkesterone, both of which correspond to [M–H + HCOOH]–).
The identification of ecdysteroids via HPLC-MS was performed on a Waters Acquity UPLC chromatograph (Waters, United States) equipped with a XEVO QTOF hybrid quadrupole time-of-flight mass spectrometer (Waters). A sample (1 μL) was loaded onto a column of ACQUITY UPLC BEH Phenyl (50 × 2.1 mm, 1.7 μm; Waters). The column temperature was 40°С, and the volumetric flow rate of the mobile phase was 0.4 mL/min. The composition of mobile phase changed during the analysis in the following way (solvent B, volume %): 0–1 min—15%, 1–5 min—15 → 30%, 5–15 min—30 → 38%, 15–15.5 min—38 → 45%, 15.5–23 min—45%, 23–23.5 min—45 → 95%. The analysis was carried out in positive ion mode with an m/z range 100–1200 and the subsequent ionization source parameters: ionization source temperature, 120°С; desolvation temperature, 250°С; capillary voltage, 3.0 kV; sample input cone voltage, 30 V; and nitrogen (desolvation gas) supply rate, 600 L/h. The obtained data were processed with the MassLynx (Waters) program.
Quantification of Individual Ecdysteroids
The analysis was carried out via HPLC-MS; the external calibration was conducted with standard samples of ecdysterone and turkesterone (supplied by PhD I.V. Zavarzin, the Zelinsky Institute of Organic Chemistry, Russ. Acad. Sci.). The relative standard deviation of the ecdysteroid retention time under the described conditions was below 2.5%. The calibration curves for the compounds were straight-line approximated with determination coefficients of R2 > 0.99 in working concentration ranges of 5–100 μg/mL for ecdysterone and 0.5–100 μg/mL for turkesterone. The relative standard deviations of the peak area for ecdysteroids did not exceed 2.3%.
RESULTS AND DISCUSSION
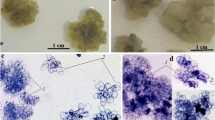
To determine the optimal phytohormone composition for culture growth, callus tissues were incubated on nutrient media containing 2,4-D, NAA, and BAP as a cytokinin (Table 1) in the first cultivation cycles. It was established that numerous aerial roots developed on the surface of the calluses in the presence of NAA (1 mg/L) or NAA (1 mg/L) in combination with BAP (0.2 mg/L) during the first four cultivation cycles, (Fig. 1), whereas stem morphogenesis was observed during the first seven cultivation cycles on the medium without growth regulators containing NAA (0.1 mg/L) (Fig. 2).
It was noted that compact calluses gave rise to loose tissue after their transfer onto a medium with 2,4-D (0.1 or 1.0 mg/L). This growth regulator, regardless of its concentration, inhibited the callus morphogenetic potential. The cultures became brown, loose, and highly watery. Therefore, 2,4-D was no longer used to grow A. turkestanica callus cultures.
As for sprouts, they were induced via the transfer of the dedifferentiated callus cultures passaged for more than a year on a medium containing BAP (0.2 mg/L). The culture acquired a globular character with pronounced foci of meristemic activity. Bud formation, followed by sprout generation, was observed 7–10 days after the transfer, and the sprout number increased on average by three times as a result of an increase in the BAP concentration in the medium to 1 mg/L.
An increase in the intensity of callus culture growth was observed during cultivation (Table 2). For instance, the mean fresh weight of calluses cultivated on the hormone-free medium was 0.6 g with a cell dry weight of 0.05 g by the end of the fourth week, while these values reached 1.7 and 0.1 g, respectively, by the tenth cycle. The relative water content in the cells varied depending on the medium composition, according to the increment indices of dry and fresh cell biomass.
Suspension cell cultures were obtained via conventional methods from strongly growing callus cultures after the 15th day of cultivation. As a result, ~100 callus and suspension lines of A. turkestanica were generated, seven of which were characterized by a high growth rate and were analyzed via HPLC-MS for the presence of the phytoecdysteroids (Fig. 3). The compounds were identified via mass-spectrometry data analysis and a comparison of the chromatographic and mass-spectrometric behavior of the analytes with that of the ecdysteroid standards (turkesterone and 20-hydroxyecdysone) (Figs. 4 and 5). The chromatograms were recorded in the selected ion mode with an m/z of 525 for 20-hydroxyecdysone and 541 for turkesterone. The selected ions corresponded to [M‒H + HCOOH]–.
HPLC-MS chromatograms of standard solutions of 20-hydroxyecdysone (0.1 mg/mL) (a), turkesterone (0.1 mg/mL) (с), and extract from A. turkestanica suspension culture (strain 4, 27th day) (b). The chromatograms were recorded in the following selected ion monitoring mode: m/z of 525 for 20-hydroxyecdysone and 541 for turkesterone (ions correspond to [M–H + HCOOH]–). Peak designations: 1, turkesterone (tR 2.2 min); and 2, 20-hydroxyecdysone (tR 7.3 min).
Both analyzed ecdysteroids were observed in two suspension and two callus lines; the content of 20-hydroxyecdysone was 40- to 50-fold higher than that in turkesterone (2.2–2.5 mg/g and 0.04–0.05 mg/g dry weight, respectively) in the most productive lines.
It was established that the phytoecdysteroid content in the biomass of the suspension cell cultures increased by the end of cultivation. At the same time, these compounds were not detected at all in two calluses and one suspension cultures.
CONCLUSIONS
The growth, morphogenetic, and biosynthetic characteristics of Ajuga turkestanica cell cultures were studied. It was shown that the addition of auxins and cytokinins in certain concentrations ensures morphogenesis regulation, and some of the obtained calluses and suspension cultures (although not all) retain the ability to produce ecdysteroids (20-hydroxyecdysone and turkesterone).
Based on the results, it can be concluded that the plant cell cultures are unstable in terms of phytoecdysteroid biosynthesis. Even if compounds are produced, the level of their accumulation in plant cell cultures is lower than that in leaves of intact A. turkestanica plants. It is two to three times lower for 20-hydroxyecdysone and 20–30 times lower for turkesterone.
Thus, the growth, morphogenetic, and biosynthetic characteristics of Ajuga turkestanica cell cultures were studied. It was found that the addition of auxins and cytokinins to the medium at certain ratios provides the regulation of morphogenesis. It was shown that some of the obtained callus and suspension cultures retain the ability to form phytoecdysteroids (20-hydroxyecdysone and turkesterone), though in smaller amounts than in the leaves of intact plants. Phytoecdysteroids were not found in a number of obtained cell cultures. Additional studies are required to clarify the causes for the synthesis of these compounds in cell cultures in vitro.
REFERENCES
Nosov, A.M., Plant cell culture: Unique system, model, and tool, Russ. J. Plant Physiol., 1999, vol. 46, no. 6, pp. 837–844.
Kutepova, T.A., Syrov, V.N., Khushbaktova, Z.A., and Saatov, Z., Hypoglycemic activity of the total ecdysteroid extract from Ajuga turkestanica,Pharm. Chem. J., 2001, vol. 35, pp. 608–609.
Syrov, V.N., Yuldasheva, N.Kh., Egamova, F.R., et al., Assessment of hypoglycemic action of phytoecdysteroids, Exp. Clin. Pharmacol., 2012, vol. 75, no. 5, pp. 28–31.
Syrov, V.N., Islamova, Zh.I., Egamova, F.R., et al., Stress-protective properties of phytoecdysteroids, Exp. Clin. Pharmacol., 2014, vol. 77, no. 7, pp. 35–38.
Zakirova, R.P., Abdukadyrov, I.T., and Yakubova, M.R., A possibility of increasing in ecdysteroid content in callus and suspension cultures of Ajuga turkestanica by induced morphogenesis, Biotekhnologiya, 2012, no. 3, pp. 44–47.
Butenko, R.G., Plant Tissue Culture and Plant Morphogenesis, Jerusalem: Israel Program for Scientific Translation Ltd., 1968.
Murashige, T. and Skoog, F.A., A revised medium for rapid growth and bioassays with tobacco tissue culture, Physiol. Plant., 1962, vol. 15, no. 13, pp. 473–497.
Nosov, A M., Assessment methods and growth characteristics of plant cell cultures, in Molecular Genetics and Biochemical Methods in Modern Plant Biology, Moscow: BINOM, pp. 386–403.
Gambourg, O.L. and Elevegh, D., Culture methods and detection of glucanases in suspension cultures of wheat and parleys, Can. J. Biochem., 1968, vol. 46, pp. 417–421.
Funding
The work was financially supported by the Russian Science Foundation (Project no. 16-14-00126).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Gordon
Abbreviations: BAP—6-benzylaminopurine; 2,4-D—2,4-dichlorophenoxyacetic acid; HPLC-MS—high-performance liquid chromatography with mass-spectrometric detection; NAA—α‑naphtylacetic acid.
Rights and permissions
About this article
Cite this article
Zakirova, R.P., Sobolkova, G.I., Kharitonov, T.D. et al. Obtainment and Characteristics of Phytoecdysteroid-Producing Аjuga turkestanica (Rgl.) Plant Cell Cultures. Appl Biochem Microbiol 56, 809–814 (2020). https://doi.org/10.1134/S0003683820070078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820070078