Abstract
Human erythrocyte hemoglobin (Hb) has two reactive cysteines located on the surface of β-subunits. These cysteines play an important role in the adjustment of Hb functions. It is known that they are involved in the transport of intracellular nitric oxide (NO), redox signaling, and the regulation of dimeric-tetrameric Hb equilibrium. It is shown in this work that the incorporation of Cys-93β as ligands in iron-NO complexes is another way to regulate of SH group reactivity. Such complexes stabilize the SH group as a thiolate anion (R–S–), the reactivity of which is significantly higher than that of the protonated form of thiol (Cys-SH). This is why the thiols included in the complexes show increased activity in relation to electrophilic agents, such as ThioGlo1. Conversely, as part of the complexes, thiols are protected from oxidation by tert-butyl hydroperoxide. The incorporation of SH groups into complexes of iron and NO can be considered a means of thiol protection from irreversible oxidation upon oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The importance of cysteines for cell physiology is enormous, since these amino acids are subject to a wide range of posttranslational modifications, many of which are reversible. Thiol groups possess such properties as nucleophilicity, the ability to reverse oxidation-reduction, and metal binding, due to which they are extremely sensitive to acid-base, redox, and electrophilic-nucleophilic equilibrium [1–5]. Even a slight change in the concentration of oxidizing agents or electrophiles affects the state of protein SH groups. Thus, cysteines play a key role in the transmission of redox signals in living systems.
SH groups included in the active center, as well as those localized on the surface of the protein molecule, are critical for the functioning of proteins. Therefore, such cysteines are located in evolutionarily conserved domains of molecules [6]. Various compounds possessing electrophilic properties can interact with protein SH groups, e.g., active forms of oxygen and nitrogen and active carbonyl compounds, as well as metal ions (iron, zinc, copper, calcium, etc.). [7]. The most common posttranslational modifications of sulfhydryl groups are oxidation to disulfides (R–S–S–R) and cysteinesulfenic acid (R–SOH), nitrosylation (R–SNO), sulfhydrogenation (R–S–SH), the formation of Michael adducts, and hemitioacetals [4, 8–10]. The listed posttranslational modifications alter the physicochemical properties of proteins, their reactivity, and their aggregation ability.
It was shown that sulfhydryl groups play an important role in the signal transduction system inside the cell. The optimal cell response to external exposure is often realized by the modification of SH groups of enzymes, receptors, transcription factors, and other regulatory proteins that integrate various information flows [4, 9–11]. These posttranslational modifications, in contrast to phosphorylation, are usually nonenzymatic. Another modification of SH groups of cysteines is the formation of dinitrosyl iron complexes (DNICs), which are complexes containing iron ion (Fe2+) and two molecules of nitric oxide (NO). DNIC associated with protein cysteines are a stable form of NO deposition in the body [12, 13]; however, it cannot be ruled out that these complexes also function as regulators of the activity of the protein molecule.
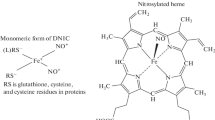
Hemoglobins (Hbs) of many vertebrates have reactive cysteine residues located on the surface of the protein molecule [14]. Human Hb contains two such residues in the tetramer β-subunits (Cys-93β). It was shown that DNICs form with the participation of these cysteines [15, 16] (Fig. 1). Another amino acid in the protein usually acts as the second ligand, which binds the iron in DNICs (denoted as L in Fig. 1). We assume that this ligand is most often a tyrosine residue.
The ability of DNICs associated with Cys-93β to protect Hb from oxidative modification caused by hydroperoxides was shown earlier [15–17]; a hypothesis describing the site-specific antioxidant effect of DNICs against proteins was formulated. In model systems with low molecular weight DNICs containing thiol ligands, it was shown that the antioxidant properties of the complexes are based on the following effects: the binding of iron and NO ions, the interception of reactive oxygen and nitrogen species, cooperative interaction with other antioxidants, and the ability to restore the oxoferryl form of heme [15, 16, 18, 19]. We hypothesized that DNICs associated with Hb may also be modulators of Cys-93β reactivity.
The goal of the study was to analyze the effect of DNIC son the reactivity of SH-groups of Hb upon exposure to tert-butyl hydroperoxide. The latter is a water-soluble analog of lipid hydroperoxides and is widely used to model peroxidation processes in living systems [19, 20].
EXPERIMENTAL
Reagents. The following reagents were used in the work: human methemoglobin (oxidized Hb), tert-butyl hydroperoxide (t-BOOH), methylglyoxal, PAGE, DDS-Na, Tris, glycine, NaN3, dithiothreitol (DTT), dimethyl sulfoxide (DMSO), 4-hydroxy-(2,2,6,6-tetramethylpiperidin-1-yl)oxyl (4-hydroxy-TEMPO), Coomassie brilliant blue R-250 (all from the Sigma-Aldrich, United States); 3H-naphthol [2,1-b] pyran-s-carboxylic acid (ThioGlo1) from Calbiochem (United States); and other reagents from Himmed (Russia).
The DNIC was synthesized as described in [15–17, 21]. A DNIC with glutathione ligands (GS-DNIC) was prepared by mixing FeSO4 and reduced glutathione (GSH) in a 1 : 2 molar ratio in a Thunberg vessel in a NO atmosphere. DNICs with phosphate ligands were synthesized in a similar way by passing gaseous NO in a Thunberg vessel through a solution of FeSO4 in 100 mM phosphate buffer at a pH of 6.8.
Hb-bound DNICs were prepared via the addition of phosphate DNICs (DNIC-\({\text{PO}}_{{\text{4}}}^{-}\)) to a solution of hemoglobin in 20 mM K-phosphate buffer with pH 6.8 in a molar ratio of Hb : DNIC of 1 : 3. The DNIC concentration was calculated by the integrated signal intensity of the electron paramagnetic resonance (EPR) of these complexes with the 4-hydroxy-TEMPO spin label as the external standard. The DNIC preparations were stored at –70 °C.
Obtainment of metHb-N3 and its treatment with tert-butyl hydroperoxide. NaN3 was added to a 0.15 mM Hb solution in 20 mM K-phosphate buffer with a pH of 6.8 to a final concentration of 12 mM to exclude allosteric transitions in the Hb. The solution turned a dark cherry color, indicating the formation of a low-spin form of HbIII-N3. Henceforth, the term hemoglobin refers to this complex.
To prepare the reaction mixture, t-BOOH was added to a HbIII-N3 solution in a molar ratio of 1 : 10. In experiments on the dependence of the reaction effects on the concentration of the oxidizing agent, t‑BOOH was added to final concentrations of 0.6, 1.6, 2.6, 6.8, and 11.0 mM and incubated for 5 min at room temperature prior to the measurements.
Definition of free SH groups. Sulfhydryl groups were quantified using the ThioGlo1 thiol-specific fluorescent label. The addition of ThioGlo1 protein to the solution resulted in the formation of a thiol adduct with a maximum emission of fluorescence at 500 nm at an excitation wavelength of 379 nm [22].
Samples were prepared for the analysis as follows: 5 μL of 2.5 mM ThioGlo1 in DMSO was added to 5 μL of a reaction mixture containing 0.15 mM Hb and incubated for 3 min. The resulting solution (10 μL) was added to a quartz spectrofluorimetric cuvette containing 490 μL of a 20 mM K-phosphate buffer at a pH of 6.8. To deoxygenate the Hb, a cuvette with the reaction mixture was purged with argon for 10 min.
Measurement of the content of SH groups in glutathione and glutathione DNIC samples in reaction with t-BOOH showed that the reaction mixture contained 0.08 mM GS-DNIC (in Fe2+) or an equivalent amount of GSH in 10 mM K-phosphate buffer at a pH of 7.4 and 0.2 mM t -BOOH (GSH/GS-DNIC : t-BOOH = 1 : 2.5).
Measurement of the autofluorescence of tryptophan and dithyrosine. The state of tryptophan and tyrosine residues in the Hb molecule was studied via fluorescence spectroscopy. The protein samples were prepared as described above for metHb-N3. Ten microliters of protein solution was added to a cuvette containing 490 μL of 20 mM K-phosphate buffer at a pH of 6.8. The tryptophan autofluorescence was selectively excited at a wavelength of 295 nm. The fluorescence was recorded at 330 nm. The slit width was 5 nm for the exciting light and 10 nm for the emitted light.
The spectrophotometric detection of dithyrosine formation was carried out at an excitation wavelength of 325 nm and a emission of 400 nm (330–600 nm) [23]. The slit width of the exciting and emitted light was 5 nm.
Prior to measurement, the Hb samples were diluted 25 times for measurement of the tryptophan fluorescence and 12.5 times for measurement of the dithyrosine fluorescence.
In all cases, fluorescence was recorded in quartz cuvettes with an optical path length of 1 cm on an R-F‑5301 PC spectrofluorimeter (Shimadzu, Japan) at high sensitivity (“high”), a medium scanning speed (“medium”) (in accordance with the designations on the instrument).
SDS-PAGE. Electrophoresis was performed in blocks of 12% PAG with a size of 15×15×1 mL according to the Laemmli method [24] with a VE series vertical electrophoresis device (Helikon, Russia). Sample buffer was added in a 1 : 1 ratio to Hb samples prepared as described above and heated for 5 min at 95°C, and 10 μL was then loaded into the gel. The sample buffer was prepared on the basis of 0.1 M Tris-HCl buffer at a pH of 6.8 and contained 4% DDS-Na, 0.2% bromophenol blue, and 20% glycerol. A solution of 3% DTT was added to the buffer to create reducing conditions. The electrode buffer was 0.2 M Tris-glycine buffer, pH 8.3, containing 0.1% DDS-Na.
Electrophoresis was carried out at 4°C, I = 50 mA, and U = 150 V. An Elf-4 power source (NPO DNK-Technologia, Russia) provided the necessary conditions for electrophoresis. After the completion of the protein-separation process, the gel was fixed and stained with Coomassie brilliant blue R-250 solution.
The measurements were performed in at least three replicates for each Hb sample. Statistical processing of the obtained data was carried out based on the calculation of the arithmetic mean values and their errors. The differences in indicators as compared with the control were determined by the variation statistics with the Student’s t-test. The data are presented as mean ± standard deviation.
RESULTS AND DISCUSSION
As noted above, in intact human Hb, there are only two available reactive cysteines (Cys-93β) [25] located near histidine (His-92), which coordinates the iron ion. This makes them sensitive to protein-conformation transformations caused by the attachment of the ligand to the iron of the heme [26, 27]. The reactivity of SH groups depends both on the nature of the heme ligand and on the degree of saturation of the heme groups by the ligand.
To assess the reactivity of sulfhydryl groups, we used a highly specific fluorescent reagent for reduced thiols, ThioGlo1, which forms covalent adducts with reduced SH groups [22]. Since the electronic state of the heme can significantly affect the fluorescence characteristics of thiol adducts, a preliminary study of the relationship between the reactivity of SH groups and the conformational state of a protein molecule was carried out. It was shown that the fluorescence intensity of the thiol adduct with ThioGlo1 varies depends on the degree of Hb oxygenation (conformational R-T transition) (Fig. 2). This fact confirmed the presence of an allosteric interaction between heme and Cys-93β, which allowed this methodological approach to be used to study the modifications of SH groups in Hb. The experimental data are consistent with the results in [26], which showed that the addition of CO or O2 to deoxyHb led to an eightfold increase in the reaction rate of N-ethyl maleimide with SH groups. To avoid the effect of allosteria on the state of the Cys-93β, sodium azide (NaN3) was added to the metHb solution to obtain a stable, low-spin complex, HbIII-N3.
Effect of the level of Hb oxygenation on the reactivity of SH groups as measured with ThioGlo1: a, decrease in the fluorescence intensity (rel. units) of ThioGlo1 upon Hb deoxygenation (the arrow indicates aeration of the reaction mixture with argon); b, Hb absorption spectra at varying levels of oxygenation: 1, all four subunits are oxygenated (4HbO2); 2, one subunit is oxygenated (3HbIIHbO2).
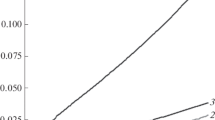
The change in the reactivity of SH groups after their inclusion in the DNIC composition was studied with respect to the concentration of ThioGlo1 (system 1) and the concentration of the oxidizing agent, t-BOOH (system 2) (Fig. 3). In the first system, the fluorescence of the thiol adduct increased linearly with an increasing concentration of ThioGlo1 in the reaction mixture (Fig. 3a). In experiments with hemoglobin DNIC (Hb-DNIC), the fluorescence intensity was higher as compared to Hb. In system 2, the fluorescence intensity of the thiol adduct Hb-DNIC also exceeded the fluorescence intensity of Hb (Fig. 3b), and this difference also increased with an increase in the concentration of the oxidizing agent. It is likely that the inclusion of SH groups in the DNIC composition prolonged the time of their presence in the form of a thiolate anion (R–S–), which is characterized by pronounced nucleophilic properties. This increased its reactivity towards to ThioGlo1.
In the next step, the time variation of the fluorescence of thiol adducts with ThioGlo1 was studied after the addition of t-BOOH to the Hb solution in a 10 : 1 molar ratio. t-BOOH in both variants, Hb and Hb-DNIC, initially caused a slight (~7%) increase in fluorescence intensity and then a decrease that indicated the oxidation of SH groups (Fig. 4). In 80 min, the fluorescence intensity in both cases decreased by ~15%. The initially observed sharp increase in fluorescence was apparently associated with the formation of activated forms of thiol: thiolate anion (R–S–) and sulfenic acid (R–SOH). In these forms, the binding constant of ThioGlo1 differed from R–SH. It is known that activated thiols occur upon the mild oxidation of cysteine residues [4, 9], which corresponds to the experimental conditions. It should be noted that the residence time of thiols in the activated state was longer for Hb-DNIC than for Hb.
To check whether the observed increase in the fluorescence of thiol adducts is really related to the inclusion of SH groups in the complexes, we studied the interaction of t-BOOH with a low molecular weight thiol, reduced glutathione (GSH). The addition of t-BOOH to a solution of glutathione in a molar ratio of 1.0 : 2.5 showed that the GSH thiol groups were oxidized, and they were present at only half of the initial level by the end of the measurements (20 min) (Fig. 5, curve 1).
It should be noted that, both in the case of low molecular weight glutathione DNIC (GS-DNIC) and protein (Hb-S-DNIC), the fluorescence increased at the beginning and only then decreased (Fig. 5, curve 2). Moreover, only 30% of thiols were oxidized in 20 min. Earlier, we obtained similar results when GSH and GS-DNIC were oxidized with hypochlorous acid [19].
Protein-bound glutathione DNICs react poorly with organic hydroperoxides. However, the destruction of DNIC can be triggered by the oxoferryl form of Hb (Hb–FeIV=O), which forms in the reaction of metHb with t-BOOH [16]. A possible molecular mechanism of the antioxidant action of DNICs in this case is associated with the reduction of Hb–FeIV=O radical [17, 28, 29]. The antioxidant effect may also be due to the restoration of the oxoferryl form of the heme and nitrosylation of the heme group with the release of nitric oxide during DNIC decomposition [28, 29]. The oxoferryl form of heme is a very strong oxidizing agent that modifies the protein chain of hemoproteins [30, 31]. In this case, phenoxyl radicals form as a result of the one-electron oxidation of tyrosine residues. These phenoxyl radicals can oxidize cysteine residues with the formation of thiyl radicals [32].
The residues Tyr42 and Tyr24 are known to be oxidized in Hb; thus, the state of tyrosine residues was determined along with spectrofluorimetric measurements of the state of thiols. Their fluorescence increased during dithyrosine formation as a result of the interaction of two tyrosine radicals. In experiments with Hb, there was first a decrease in fluorescence and then an increase; it was 15% of the initial level at the end of the measurement (Fig. 6). The increase in tyrosine fluorescence coincided in time with a decrease in the fluorescence of thiol adducts with ThioGlo1 (Fig. 4), which indicated the simultaneous oxidation of thiols and tyrosines in Hb. The results are consistent with [32], which showed that the appearance of tyrosine radicals preceded the oxidation of thiols and the formation of a thiyl radical. In the experiment with Hb-DNIC, fluorescence increased by 6% in the first minutes and then did not change further (Fig. 6). Thus, the presence of DNA in the protein composition protected tyrosine residues from oxidation. This fact can be explained by the formation of 3-nitrotyrosine in the reaction of NO (the source of which was DNIC) with tyrosine radicals [33].
Since the observed differences in thiol properties can be caused by protein-conformation changes under the influence of DNIC, the intrinsic fluorescence of Hb due to the luminescence of tryptophan residues was studied. This method is widely used to assess the conformational state of proteins. Figure 7a shows the obtained fluorescence emission spectra of Hb and Hb-DNIC. Analysis of the spectra showed that Hb‑DNIC formation did not lead to a shift in the maximum of the tryptophan-fluorescence spectrum, the position of which depends on the rearrangement of the immediate environment of this amino acid. Thus, the DNIC has no effect of on the conformational state of the Hb molecule. Therefore, the observed differences in the reaction properties of SH groups in both cases are most likely due to the different states of thiols and not to conformational changes in the protein.
Tryptophan is also a target for attack by hydroperoxides in Hb, in addition to cysteine [34, 35]; therefore, the dependence of the tryptophan-fluorescence emission intensity at 327 nm on the t-BOOH concentration was studied (Fig. 7b). This dependence was wavelike. The curves obtained for Hb contain two extrema at oxidizer concentrations of 0.6 and 6.8 mM; only the first extremum is clearly expressed in the case of Hb-DNIC. For both protein variants, the t-BOOH concentrations at which the fluorescence practically does not differ from the initial one can be determined: without t-BOOH, it is 2.6 and 11 mM. The appearance of tryptophan-fluorescence extrema can be explained by local conformational rearrangements of the Hb molecule due to oxidative modification. Conformational rearrangements are likely of a non-denatured origin, and they were much less pronounced in the case of Hb-DNIC. The fluorescence intensity in the entire range of t-BOOH concentrations did not fall below the initial level, which indicated the absence of tryptophan oxidation.
The aggregation of Hb molecules was studied via SDS-PAGE with dithiothreitol-reducing disulfide bonds. Analysis of the data presented on the electrophoregram shows that, in the presence of t-BOOH, covalent cross-linked dimers formed in both protein variants (Fig. 8), but the number of high molecular weight forms in Hb was higher than in Hb-DNIC. Since the protein samples contained DTT, it can be concluded that the Hb dimerization was due to non-disulfide bonds. Most likely, tyrosine radicals were involved in the formation of interprotein cross-links. Since the tyrosine oxidation in the Hb-DNIC sample was less pronounced than that in Hb (Fig. 6), the formation of cross-linked proteins was less intense. The obtained data are consistent with previously obtained results demonstrating the ability of DNICs to prevent oxidative modification of the protein associated with them [15–17]. This ability of DNICs is explained by the fact that they act simultaneously as a chelator and antioxidant. However, large protein aggregates appeared at Hb-DNIC at t-BOOH concentrations of 6.8 and 11 mM but did not enter the separating gel. It seems that dinitrosyl complexes are destroyed at a high concentration of an oxidizing agent, and they release Fe2+ ions, which react with peroxides to form free radicals that induce further protein oxidation [36]. The dual role of DNICs as an antioxidant at low concentrations and an oxidizing agent and a prooxidant at high concentrations has been repeatedly emphasized in [15–18, 21, 37, 38].
An additional explanation of the observed facts can be given. Thiols located on the surface of the protein are readily accessible targets for reactive oxygen species (ROS). Reacting with an oxidizing agent, they take the blow and thereby protect other amino acids from irreversible oxidative modification. If thiols are blocked and cannot react with ROS, tyrosine and tryptophan fall under attack, which leads to multiple intermolecular crosslinking and the accumulation of large protein aggregates. The role of Cys-93β in protecting Hb from denaturation and polymerization in the reaction with H2O2 was shown in [39, 40].
The role of Cys-93β in the functioning of Hb and erythrocytes is still not fully understood. The important biological importance of these thiols is indicated by the high conservatism of the surface cysteine residue in Hb vertebrates [14], the allosteric regulation of its reactivity [26, 27], and the high intracellular concentration (~10 mM, based on the concentration of Hb in human erythrocytes at ~40% hematocrit). The hypothesis of the participation of Cys-93β in NO metabolism and in its export is actively discussed [10, 41–45]. Since the reactivity of Cys-93β depends on the degree of Hb saturation with oxygen, these thiols can participate in intracellular redox signaling, which occurs via the interaction of Hb with components of the erythrocyte membrane [45]. Thiol groups can participate in the regulation of the equilibrium of the dimeric and tetrameric forms of Hb, which determines the formation of \({\text{O}}_{{\text{2}}}^{{ \bullet -}}\) and, accordingly, H2O2 inside the cell [46]. There is evidence of the involvement of Cys93β in the regulation of Hb oxidation. Thus, this thiol can react with the superoxide that forms in the heme pocket of the β-chain during autooxidation with the formation of a thiyl radical [47]. It was shown that Cys93β almost halves the H2O2-dependent oxidation of oxygenated Hb [48]. It was hypothesized that the Cys-93β in erythrocytes is an allosterically regulated antioxidant [48]. The role of Cys93β in providing a safe path for the transfer of electrons generated in the heme pocket is discussed in [39]. The cysteine radical that forms in this process can be reduced by glutathione, the content of which in erythrocytes is high.
The results on Hb show that DNICs can be regulators of the reactivity of protein SH groups, while DNIC stability is determined by the redox conditions inside the cell. Under normal conditions, the inclusion of SH groups in DNICs is a reversible, posttranslational modification, which reduces the reactivity of thiols but does not exclude participation in redox transformations. Under oxidative stress, the complexes decompose, releasing the thiolate anion, which is in a state of increased readiness for reaction with ROS and electrophilic compounds, since it does not need preliminary deprotonation.
The formation of adducts with electrophilic compounds leads to stable modification of the protein. In other words, DNICs are a kind of label for reactive cysteines; they predispose them to stable modifications, thereby ensuring the selectivity of this modification. The regulatory effect of DNICs is also associated with their effect on the balance between thiol and thiolate in the protein, shifting it towards thiolate. These complexes increase the energy barrier of the reaction with peroxides by stabilizing thiolate anions. Thus, DNICs adjust the reactivity of cysteine towards the action of peroxides and electrophiles, as illustrated by the scheme in Fig. 9.
CONCLUSIONS
The adjustment of cysteine reactivity by DNICs can be attributed to the mechanisms of the regulation of the protein structure and function via the binding of metal ions or their complexes [1, 7]. The thiolate group is known to be a good ligand for iron, copper, and zinc ions [1, 7, 49, 50]. Moreover, the binding of metals by cysteine ligands not only changes the protein structure but also has a complex protective effect due to the simultaneous decrease in the reactivity of thiol and the metal. This makes it possible to delay the formation of stable modifications of protein thiols under conditions of insignificant oxidative stress and, conversely, accelerate them at high stress levels.
REFERENCES
Giles, N.M., Watts, A.B., Giles, G.I., Fry, F.H., Littlechild, J.A., and Jacob, C., Chem. Biol., 2003, vol. 10, no. 8, pp. 677–693.
Paulsen, C.E. and Carroll, K.S., ACS Chem. Biol., 2010, vol. 5, no. 1, pp. 47–62.
Paulsen, C.E. and Carroll, K.S., Chem. Rev., 2013, vol. 113, no. 7, pp. 4633–4679.
Go, Y.M., Chandler, J.D., and Jones, D.P., Free Radic. Biol. Med., 2015, vol. 84, pp. 227–245.
Klomsiri, C., Karplus, P.A., and Poole, L.B., Antioxid. Redox Signal., 2010, vol. 14, no. 6, pp. 1065–1077.
Gupta, V. and Carroll, K.S., Biochim. Biophys. Acta, 2013, vol. 1840, no. 2, pp. 847–875.
Novikova, N.N., Kovalchuk, M.V., Yurieva, E.A., Konovalov, O.V., Stepina, N.D., Rogachev, A.V., Yalovega, G.E., Kosmachevskaya, O.V., Topunov, A.F., and Yakunin, S.N., J. Phys. Chem. B, 2019, vol. 123, no. 40, pp. 8370–8377.
Lo, ConteM. and Carroll, K.S., J. Biol. Chem., 2013, vol. 288, no. 37, pp. 26480–26488.
Wible, R.S. and Sutter, T.R., Chem. Res. Toxicol., 2017, vol. 30, no. 3, pp. 729–762.
Kosmachevskaya, O.V., Shumaev, K.B., and Topunov, A.F., Biochemistry (Moscow), 2019, vol. 84, suppl. 1, pp. S206–S224.
Foyer, C.H., Wilson, M.H., and Wright, M.H., Free Radic. Biol. Med., 2018, vol. 122, pp. 137–149.
Vanin, A.F., Nitric Oxide, 2016, vol. 54, pp. 15–29. https://doi.org/10.1016/j.niox.2016.01.006
Vanin, A.F., Dinitrosyl Iron Complexes as a “Working Form” of Nitric Oxide in Living Organisms, Cambridge: Scholars Publishing, 2019.
Reischl, E., Dafre, A.L., Franco, J.L., and Wilhelm Filho, D., Comp. Biochem. Physiol. C Toxicol. Pharmacol., 2007, vol. 146, nos. 1–2, pp. 22–53.
Shumaev, K.B., Gubkin, A.A., Serezhenkov, V.A., Lobysheva, I.I., Kosmachevskaya, O.V., Ruuge, E.K., Lankin, V.Z., Topunov, A.F., and Vanin, A.F., Nitric Oxide, 2008, vol. 18, no. 1, pp. 37–46.
Shumaev, K.B., Kosmachevskaya, O.V., Timoshin, A.A., Vanin, A.F., and Topunov, A.F., Methods Enzymol., 2008, vol. 436, pp. 445–461.
Shumaev, K.B., Petrova, N.E., Zabbarova, I.V., Vanin, A.F., Topunov, A.F., Lankin, V.Z., and Ruuge, E.K., Biochemistry (Moscow), 2004, vol. 69, no. 5, pp. 569–574.
Shumaev, K.B., Dudylina, A.L., Ivanova, M.V., Pugachenko, I.S., and Ruuge, E.K., Biofactors, 2018, vol. 44, no. 3, pp. 237–244.
Shumaev, K.B., Gorudko, I.V., Kosmachevskaya, O.V., Grigoryeva, D.V., Panasenkoe, O.M., Vanin, A.F., Topunov, A.F., Terekhova, M.S., Sokolov, A.V., Cherenkevich, S.N., and Ruuge, E.K., Oxid. Med. Cell. Longev., 2019, vol. 2019, article ID 2798154. https://doi.org/10.1155/2019/2798154
Domanski, A.V., Lapshina, E.A., and Zavodnik, I.B., Biochemistry (Moscow), 2005, vol. 70, no. 7, pp. 761–769.
Shumaev, K.B., Gubkin, A.A., Gubkina, S.A., Gudkov, L.L., Lakomkin, V.L., Topunov, A.F., Vanin, A.F., and Ruuge, E.K., Biophysics (Moscow), 2007, vol. 52, no. 3, pp. 336–339.
Hoff, S., Larsen, F.H., Andersen, M.L., and Lund, M.N., Analyst, 2013, vol. 138, no. 7, pp. 2096–2103.
Davies, K.J. and Delsignore, M.E., J. Biol. Chem., 1987, vol. 262, no. 20, pp. 9908–9913.
Laemmli, U.K., Nature, 1970, vol. 227, no. 5259, pp. 680–685.
Blacken, G.R., Wang, Y., Lopez, J.A., and Fu, X., Blood, 2009, vol. 114, no. 22, pp. 4040–4040.
Riggs, A., J. Biol. Chem., 1961, vol. 236, no. 7, pp. 1948–1954.
Benesch, R.E. and Benesch, R., Biochemistry, 1962, vol. 1, no. 5, pp. 735–738.
Gorbunov, N.V., Osipov, A.N., Day, B.W., Zayas-Rivera, B., Kagan, V.E., and Elsayed, N.M., Biochemistry, 1995, vol. 34, no. 20, pp. 6689–6699.
Gorbunov, N.V., Yalowich, J.C., Gaddam, A., Thampatty, P., Ritov, V.B., Kisin, E.R., Elsayed, N.M., and Kagan, V.E., J. Biol. Chem., 1997, vol. 272, no. 19, pp. 12328–12341.
Reeder, B.J., Grey, M., Silaghi-Dumitrescu, R.-L., Svistunenko, D.A., Bulow, L., Cooper, C.E., and Wilson, M.T., J. Biol. Chem., 2008, vol. 283, no. 45, pp. 30780–30787.
Vlasova, I.I., Molecules, 2018, vol. 23, no. 10. e2561. https://doi.org/10.3390/molecules23102561
Bhattacharjee, S., Deterding, L.J., Jiang, J., Bonini, M.G., Tomer, K.B., Ramirez, D.C., and Mason, R.P., J. Am. Chem. Soc., 2007, vol. 129, no. 44, pp. 13493–13501.
Gunther, M.R., Sturgeon, B.E., and Mason, R.P., Toxicology, 2002, vol. 177, no. 1, pp. 1–9.
Steffek, R.P. and Thomas, M.J., Free Radic. Res. Commun., 1991, vol. 12-13, no. 2, pp. 489–497.
Jia, Y., Buehler, P.W., Boykins, R.A., Venable, R.M., and Alayash, A.I., J. Biol. Chem., 2007, vol. 282, no. 7, pp. 4894–4907.
Laguerre, M., Bily, A., Roller, M., and Birtic, S., Annu. Rev. Food Sci. Technol., 2017, vol. 8, pp. 391–411.
Shumaev, K.B., Gubkin, A.A., Gubkina, S.A., Gudkov, L.L., Sviryaeva, I.V., Timoshin, A.A., Topunov, A.F., Vanin, A.F., and Ruuge, E.K., Biophysics (Moscow), 2006, vol. 51, no. 3, pp. 423–428.
Shumaev, K.B., Gubkina, S.A., Vanin, A.F., Burbaev, D.Sh., Mokh, V.P., Topunov, A.F., and Ruuge, E.K., Biophysics (Moscow), 2013, vol. 58, no. 2, pp. 172–177.
Winterbourn, C.C. and Carrell, R.W., Biochem. J., 1977, vol. 165, no. 1, pp. 141–148.
Pimenova, T., Pereira, C.P., Gehrig, P., Buehler, P.W., Schaer, D.J., and Zenobi, R., J. Proteome Res., 2010, vol. 9, no. 8, pp. 4061–4070.
Stamler, J.S., Singel, D.J., and Piantadosi, C.A., Nat. Med., 2008, vol. 14, no. 10, pp. 1008–1009.
Jensen, F.B., J. Exp. Biol., 2009, vol. 212, no. 21, pp. 3387–3393.
Gaston, B., May, W.J., Sullivan, S., Yemen, S., Marozkina, N.V., Palmer, L.A., Bates, J.N., and Lewis, S.J., J. Appl. Physiol., 2014, vol. 116, no. 10, pp. 1290–1299.
Zhao, Y., Wang, X., Noviana, M., and Hou, M., Acta Biochim. Biophys. Sin., 2018, vol. 50, no. 7, pp. 621–634.
Kosmachevskaya, O.V., Nasybullina, E.I., Blindar, V.N., and Topunov, A.F., Appl. Biochem. Microbiol., 2019, vol. 55, no. 2, pp. 83–98.
O'Neill, J.S. and Reddy, A.B., Nature, 2011, vol. 469, no. 7331, pp. 498–503.
Balagopalakrishna, C., Abugo, O.O., Horsky, J., Manoharan, P.T., Nagababu, E., and Rifkind, J.M., Biochemistry, 1998, vol. 37, no. 38, pp. 13194–13202.
Vitturi, D.A., Sun, C.W., Harper, V.M., Thrash-Williams, B., Cantu-Medellin, N., Chacko, B.K., Peng, N., Dai, Y., Wyss, J.M., Townes, T., and Patel, R.P., Free Radic. Biol. Med., 2013, vol. 55, pp. 119–129.
Jakob, U., Eser, M., and Bardwell, J.C.A., J. Biol. Chem., 2001, vol. 275, no. 49, pp. 38302–38310.
Kaim, W. and Schwederski, B., in Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life, Meyer, G.N.A., Ed., Chichester, UK: Wiley, 1991, pp. 330–350.
ACKNOWLEDGMENTS
The equipment of the Industrial Biotechnologies Center for Collective Use of the Research Center of Biotechnology, Russian Academy of Sciences, was used for the research.
This work was supported by the Russian Foundation for Basic Research (project no. 19-29-12052) and the Ministry of Science and Higher Education of the Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by I. Shipounova
Rights and permissions
About this article
Cite this article
Kosmachevskaya, O.V., Nasybullina, E.I., Shumaev, K.B. et al. Effect of Iron–Nitric Oxide Complexes on the Reactivity of Hemoglobin Cysteines. Appl Biochem Microbiol 56, 512–520 (2020). https://doi.org/10.1134/S0003683820050099
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820050099