Abstract
A method for preparing antibodies that specifically recognize antibiotics of the fluoroquinolone group with the same radical in the first position of the quinolone nucleus is proposed. The specificity of rabbit antisera prepared at different cycles of immunization by changing the structure of the hapten in the composition of immunogens was characterized. The selected antibodies provided a group-specific analysis of 16 representatives of fluoroquinolones, including a combination of the following compounds that are controlled in animal products: ciprofloxacin, norfloxacin, pefloxacin, ofloxacin, and enrofloxacin. Using these antibodies, an indirect competitive enzyme-linked immunosorbent assay was developed that was characterized by a detection limit of ciprofloxacin of 0.2 ng/mL and a duration of 2 h. The assay was approbated for the detection of fluoroquinolones in milk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
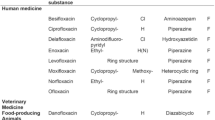
Fluoroquinolones belong to a class of structurally similar synthetic antimicrobial compounds (Fig. 1) that currently account for more than 40 substances used in medical practice, the list of which is regularly updated [1]. By the mechanism of bactericidal action, all fluoroquinolones are inhibitors of bacterial enzymes, DNA gyrase and topoisomerase II [2]. Fluoroquinolones are used to treat infections caused by gram-negative microorganisms and multi-resistant pathogens. In veterinary medicine, medicinal preparations based on enrofloxacin, flumequine, lomefloxacin, ofloxacin, ciprofloxacin, orbifloxacin, difloxacin and marbofloxacin are used [3, 4]. The toxic effect of fluoroquinolones and the risks of their non-medical ingestion into the human body with animal products led to the introduction of maximum permissible levels of their content in food products [5]. For instance, in EU countries, the maximum permissible level (MPL) of the total content of enrofloxacin and ciprofloxacin in milk is set at 100 ng/mL [6], while the same restrictions apply in Russia to the total content of ciprofloxacin, enrofloxacin, pefloxacin, ofloxacin and norfloxacin (Fig. 1) [7]. The chromatographic methods of fluoroquinolone detection that are prevalent in modern practice [8, 9] involve the use of complex, expensive equipment and are therefore not suitable for mass screening [10]. Therefore, sensitive, efficacious, rapid, easy-to-use, and inexpensive methods of controlling the total content of fluoroquinolones in milk and other food products are needed. Immunochemical methods, in particular, enzyme-linked immunosorbent assays (ELISAs), have been actively developed in recent years to control the content of antibiotics, and ELISA has become the most effective solution to this problem [11–14].
Several methodological approaches to the development of group-specific ELISAs are available based on the use of either antibodies with broad specificity or a mixture of highly selective antibodies [15–22]. The predominant approach involves the use of conjugates obtained by modifying the secondary nitrogen atom of the piperazine radical in the seventh position of the quinolone nucleus [15–18]. However, the existing test systems allow detecting only a few (no more than 11–12) of the antibiotics of the fluoroquinolone group, which necessitates the development of new assay systems for the simultaneous control of a wide range of fluoroquinolones in accordance with regulations.
The aim of this work is to prepare antibodies to control the total content of fluoroquinolones and to develop ELISA methods based on their use.
METHODS
Chemical substances. We used danofloxacin (DAN), clinafloxacin (CLI), moxifloxacin (MOX), ofloxacin (OFL), garenoxacin (GAR), pefloxacin (PEF), gatifloxacin (GAT), sarafloxacin (SAR), lomefloxacin (LOM), tozufloxacin (TOZ), sparfloxacin (SPA), difloxacin (DIF) pazufloxacin (PAZ), marbofloxacin (MAR), rufloxacin (RUF), norfloxacin (NOR), ciprofloxacin (CIP), enrofloxacin (ENR), pipemidic acid (PIP), nalidixic acid (NAL), oxinic acid (OXI), orbifloxacin (ORB), enoxacin (ENO), nadifloxacin (NAD), flumequine (FLU), bovine serum albumin (BSA), ovalbumin (OVA), casein, N-hydroxysuccinimide (HSI), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), 3,3',5,5'-tetramethylbenzidine (TMB), and methanol (Sigma-Aldrich, USA), as well as peroxidase-labeled antibodies against rabbit IgG (H+L) (Medgamal, Russia). All auxiliary reagents (salts, acids, alkalis, and organic solvents) were of analytical or chemical purity.
Synthesis of cationized BSA. The carboxyl groups of BSA were modified with ethylenediamine (according to [23, 24] with modifications); 60 mg (0.88 μmol) of BSA were dissolved in 5 mL of distilled water, and 0.5 mL of a solution containing 16.8 mg (88 μmol) of EDC and 10.2 mg (88 μmol) of HSI were added with vigorous stirring and incubated for 15 min. A solution of 13.0 mg (88 μmol) of ethylene diamine hydrochloride in 10 mL of 0.05 M carbonate buffer, pH 9.5, containing 150 μL of triethylamine was also prepared. The resulting solution was added to the activated protein and incubated for 5 h with vigorous stirring. The product was dialyzed against a 0.05 M carbonate buffer pH 9.5 and stored at –20°C.
Synthesis of immunoreagents. Fluoroquinolones were conjugated to proteins by the carbodiimide method (according to [25] with modifications); 14.7 μmol of fluoroquinolone, 5.7 mg (30 μmol) of EDC, and 3.5 mg (30 μmol) of HSI were dissolved in 1.0 mL of N,N-dimethylformamide. The mixture was incubated for 2 h with stirring. A solution of 10 mg of protein in 8 mL of 0.05 M carbonate buffer, pH 9.5, containing 50 μL of trimethylamine was prepared and incubated for 1 h at 4°C. To the protein solution, a solution of activated fluoroquinolone was added dropwise with constant stirring and incubated for 5 h at 25°C in the dark with stirring. The synthesized conjugate was purified from low molecular weight impurities by dialysis against a 0.01 M K-phosphate buffer (pH 7.4) containing 0.1 M NaCl (PBS) and stored at ‒20°C.
The conjugates CLI-NH-C5-NH-cBSA and CLI-NH-C5-NH-OVA were synthesized according to [17, 18]; 5 mg (0.11 μmol) of OVA and 2.2 mg (5.6 μmol) of CLI were dissolved in 8 mL of distilled water, and 20 μL of triethylamine were added. With vigorous stirring, 230 μL (5.6 μmol) of 0.25% glutaraldehyde were added. The solution was incubated with constant, vigorous stirring at room temperature for 1 h, and then 500 μL (30 μmol) of 0.22% sodium borohydride solution were added and incubated for another 30 min. The synthesized conjugate was separated from the low molecular weight impurities by dialysis against PBS and stored at –20°C.
Antibody production. To obtain polyclonal antibodies, 3–5 month old male rabbits of the California rabbit breed were used. Animals were immunized every 2 weeks with freshly prepared emulsion of conjugate (0.5–1.0 mg in 1.0 mL PBS) in a 1 : 1 mixture with complete Freund’s adjuvant for the first injection and with incomplete Freund’s adjuvant for subsequent injections. The emulsion was injected subcutaneously in 5–10 places in the region of the spine. Blood was collected from the marginal ear vein into vacuum tubes with gel and clotting activator. The serum was separated by centrifugation, and the IgG fraction was purified by triple precipitation with 50, 40, and 33% ammonium sulfate solutions at 4°C. The volume of the final preparation corresponded to the initial volume of the antiserum. The resulting IgG fraction was dissolved in PBS, mixed with glycerol at 1 : 1 ratio, and stored at –20°C.
Competitive ELISA. Solutions (100 μL) of conjugates (1 mg/mL in PBS) were immobilized in the wells of a 96-well polystyrene plate with a high sorption capacity (XEMA, Russia) overnight at 4°C. Then, the microplate was washed four times with PBS containing 0.05% Triton X-100 (PBST). For assaying, 50 μL of an analyte (from 1 μg/mL to 10 pg/mL), and 50 μL of an antibody solution were added to each well. The microplate was incubated for 1 h at 37°C and washed four times with PBST, and then 100 μL of immunoperoxidase conjugate (1 : 6000 dilution in PBST) were added to the wells. The mixture was incubated for another 30 min at 37°С. After washing (three times with PBST and once with distilled water), the peroxidase activity of the enzyme label bound to the carrier was determined. To do this, 100 μL of the substrate mixture (0.4 mM TMB solution in 40 mM Na-citrate buffer, pH 4.0, with 3 mM Н2О2) were added to all wells. The mixture was incubated for 15 min, and the reaction was stopped by adding 50 μL of 2 M sulfuric acid. The optical density of the reaction product was measured at 450 nm (D450) using an EFOS 9305 microplate photometer (Sapphire, Russia).
Analytical characteristics of ELISA. All approximations of the obtained concentration dependences were performed with the Origin 8.5.1 program (OriginLab, USA), using the four-parameter sigmoid function y = (A – D)/(1 + (x/c)B) + D. As a detection limit, we used IC10, which is the concentration at which the analytical signal (D450) decreased by 10% of the difference between the maximum and minimum signals. The lower and upper limits of the range of detectable concentrations were calculated as IC20 and IC80, which are the concentrations at which the analytical signals decreased by 20 and 80% of the difference between the maximum and minimum signals, respectively. The specificity of the method was assessed as the percentage of cross-reactivity (CR) with analogues of the analyte (X):
where IC50 is the analyte concentration causing a 50% inhibition of antibody binding.
Preparation of extracts from milk samples. Methanol (0.8 mL) and hexane (0.2 mL) were added to 0.2 mL of a milk sample, stirred vigorously for 2 min, and centrifuged at 15 000 g for 10 min. A water-alcohol layer under layers of hexane and fat was collected, and the extract was diluted 6-fold with PBST and used in the assay.
RESULTS AND DISCUSSION
Preparing immunogens. To prepare the polyclonal antibodies, two variants of protein conjugates with derivatives of CIP were used.
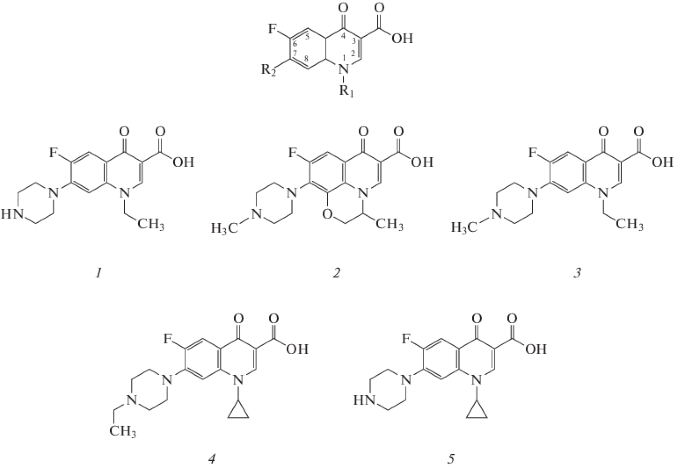
In the first variant, CIP was conjugated to BSA by the carbodiimide method. Conjugation was carried out via the carboxyl group of the constant region of the CIP molecule and with the secondary amino group of the piperazine radical in the seventh position of the quinolone nucleus (Fig. 2). In the second cycle of immunization, carboxylated BSA was used as a carrier, which minimized the production of antibodies against the hapten conjugated via the carboxyl group of the constant region of the molecule. The resulting antisera were designated as AS/CIP-A1 and AS/CIP-A2 (the first and second immunization cycles, respectively).
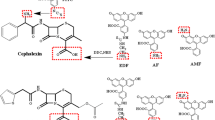
In the second variant, a spacer was introduced into the immunogen between the CIP and the BSA. For this purpose, CIP modified with 6-bromohexanoic acid (Fig. 3) was used, similarly to the previously performed modifications of SAR and NOR [15, 16]. Such a hapten could conjugate to the protein via one of two carboxyl groups, while its secondary amino group would be blocked. Antisera prepared using this immunogen were designated as AS/CIP-B.
ELISA based on AS/CIP-A; a choice of solid-phase antigen and assay conditions. As solid-phase antigens, the conjugates of various fluoroquinolones with an alternative carrier protein (OVA) were tested, which excluded the binding of antibodies contained in antisera to BSA. The following conjugates were compared: CIP-OVA, MOX-OVA, DAN-OVA, GAR-OVA, CLI-OVA, ENO-OVA, NOR-OVA, SAR-OVA, ENR-OVA, PIP-OVA, CIP-N-C5-CO-NH-OVA, and CLI-NH-C5-NH-OVA adsorbed into microplate wells at a concentration of 0.5 µg/mL. For antibodies of two immunization cycles, the binding to the conjugate (optical density D0 registered in ELISA) and its inhibition in the presence of a high concentration of CIP (10 ng/mL, D) were analyzed.
The obtained values of ΔD = (D0– D)/D0 are given in Table 1. The high ΔD values indicate that the antibodies of the first immunization cycle were predominantly associated with free hapten molecules. At the second stage of immunization, ΔD decreased dramatically, but the binding to the conjugates was maintained. An ELISA based on the use of the conjugate CLI-NH-C5-NH-OVA significantly exceeded all others in the value of ΔD; therefore, it was chosen for ELISA implementation.
We determined the optimal parameters of ELISA that ensure a high sensitivity of CIP detection in combination with a high level of the assay signal. The concentration of the conjugate CLI-NH-C5-NH-OVA used for immobilization in the microplate wells was 0.25 μg/mL; the dilution of AS/CIP-A1 antibodies was 1 : 4000, and the dilution of AS/CIP-A2 antibodies was 1 : 6000.
The calibration curve of ELISA for CIP obtained at optimized conditions is shown in Fig. 4. The range of detectable CIP concentrations was 0.3–4.0 ng/mL, and the detection limit was 0.2 ng/mL.
Choice of solid-phase antigen and optimization of ELISA based on antibodies AS/CIP-B. ELISA variants (based on the use of antibodies against the second immunogen) and the same panel of fluoroquinolone conjugates with OVA were compared in relation to the ΔD value determined at a CIP concentration of 100 ng/mL.
The CIP-N-C5-CO-NH-OVA conjugate was characterized by a high ΔD value and was selected for an ELISA. The optimal concentration of the conjugate during immobilization in the microplate wells was shown to comprise 0.05 µg/mL, and the optimal antibody dilution was 1 : 6000. Under the chosen conditions, the range of detectable concentrations of CIP was 0.3–10.0 ng/mL, and the detection limit was 0.2 ng/mL.
Detection of a combination of fluoroquinolones. The two ELISA variants described above were tested. Specificity was assessed as the percentage of detection when assaying samples with fluoroquinolone concentrations of 100 ng/mL. For DAN, FLU, and MAR, samples with concentrations of 30, 50, and 75 ng/mL were tested, which corresponded to the regulations.
The ELISA results (Table 2) were interpreted on the basis of the ΔD assessment. The choice of the minimum and maximum ΔD values enabled assessments of the ΔD levels of each fluoroquinolone. For the AS/CIP-A/CLI-NH-C5-NH-OVA test system, the minimum threshold value of ΔD was 0.45 (ΔD for CIP according to Table 2), and the maximum value was 0.76 (ΔD for NOR, PEF, and MOX). The ΔD values of fluoroquinolones, the content of which was impossible to control by this method, are indicated in Table 2 in italics, and the values of detectable amounts of fluoroquinolones are in bold. According to the characteristics for the second configuration of the test system provided in Table 2, the corresponding threshold values of ΔD were 0.58 and 0.68.
As follows from the presented data, the variants under consideration allowed us to control the total content of a variety of fluoroquinolones, with the exception of three compounds (danofloxacin, flumequin and marbofloxacin) that have individual MPLs and require additional highly selective analysis. The broadest specificity for the compounds of the fluoroquinolone group (16 compounds) had the test system implementing antibodies AS/CIP-A and the conjugate CLI-NH-C5-NH-OVA.
During testing of the samples, we did not know which fluoroquinolone (or a combination of several fluoroquinolones) was present in them, so the choice of criteria for interpreting ELISA results that would allow making the most reliable conclusions despite the scarcity of the available information was of great importance (Table 3). It was established that if 0.45 were taken as the threshold value of ΔD for this system, then the conclusion about the presence of fluoroquinolone would correspond to the situation when the individual concentrations of 16 fluoroquinolones studied (ciprofloxacin, enrofloxacin, ofloxacin, norfloxacin, pefloxacin, clinafloxacin, garenoxacin, nadifloxacon, pazufloxacin, orbifloxacin, enoxacin, gatifloxacin, lomefloxacin, sparfloxacin, moxifloxacin and nalidixic acid) would be significantly higher than the MPL (100 ng/mL). At high ΔD levels up to the value of 0.76, it is necessary to conduct a verification, since the total content of fluoroquinolones may be either above or below the MPL. If ΔD is above 0.76, then the total content of the 16 fluoroquinolones under study was reliably below 100 ng/mL. For the second configuration of the test system, the decision-making criteria (Table 3) were based on the ΔD threshold levels comprising 0.58 and 0.68, which were selected using the data from Table 2.
It should be noted that the comparison of ΔD with two threshold values allowed us to draw evidential conclusions about exceeding the MPL, regardless of which of the 16 fluoroquinolones and in what proportion were present in the sample. This opportunity represents a significant advantage of the proposed method for practice, since the ELISA methods for fluoroquinolone control described earlier are characterized by a narrower specificity and allow the researcher to control no more than 11–12 compounds.
Testing milk samples. ELISA methods were approbated on 83 samples of milk and milk mixes from different manufacturers. As the extractant for sample preparation, we chose methanol, since it provides the most complete extraction of various fluoroquinolones. All the tested samples did not contain fluoroquinolones in the amount exceeding the regulatory requirements of the Customs Union.
REFERENCES
Ezelarab, H.A.A., Abbas, S.H., Hassan, H.A., and Abuo-Rahma, G.E.A., Arch. Pharm. (Weinheim), 2018, vol. 351, no. 9. e1800141.
Blondeau, J.M., Surv. Ophthalmol., 2004, vol. 49, no. 2, pp. 73–S78.
Gouvea, R., Dos, S.F.F., Aquino, M.H.C.D., and Pereira, V.L.D., Bras. J. Poultry Sci., 2015, vol. 17, no. 1, pp. 1–10.
Brown, S.A., J. Vet. Pharm. Therapeutics, 1996, vol. 19, no. 1, pp. 1–14.
Stahlmann, R. and Lode, H.M., Expert Opin. Drug Saf., 2013, vol. 12, no. 4, pp. 497–505.
Council Regulation (EEC) No 2377/90 of 26 June 1990 laying down a Community procedure for the establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin.
Uniform sanitary-epidemiological and hygienic requirements for goods subject to sanitary-epidemiological supervision (control). Approved by the Decision of the Commission of the Customs Union dated May 28, 2010, no. 299.
De, A.K., Bera, A.K., and Pal, B., Int. J. Pharm. Sci. Res., vol. 7, no. 2, pp. 531–542.
Sousa, J., Alves, G., Fortuna, A., and Falcao, A., Anal. Bioanal. Chem., 2012, vol. 403, no. 1, pp. 93–129.
Lehotay, S.J. and Chen, Y.B., Anal. Bioanal. Chem., 2018, vol. 410, no. 22, pp. 5331–5351.
Xu, F., Ren, K., Yang, Y.Z., Guo, J.P., Ma, G.P., Liu, Y.M., Lu, Y.Q., and Li, X.B., J. Integr. Agricult., 2015, vol. 14, no. 11, pp. 2282–2295.
Duffy, G.F. and Moore, E.J., Anal. Lett., 2017, vol. 50, no. 1, pp. 1–32.
Gaudin, V., Biosens. Bioelectron., 2017, vol. 90, pp. 363–377.
Dzantiev, B.B., Byzova, N.A., Urusov, A.E., and Zherdev, A.V., TrAC, Trends Anal. Chem., 2014, vol. 55, pp. 81–93.
Tittlemier, S., Gelinas, J.-M., Dufresne, G., Haria, M., Querry, J., Cleroux, C., and Godefroy, S.B., Food Anal. Methods, 2008, vol. 1, no. 1, pp. 28–35.
Huet, A.-C., Charelier, C., Tittlemier, S.A., Singh, G., Benrejeb, S., and Delahaut, P., J. Agric. Food Chem., 2006, vol. 54, no. 8, pp. 2822–2827.
Liu, Y.Z., Zhao, G.X., Wang, P., Liu, J., Zhang, H.C., and Wang, J.P., J. Environ. Sci. Health, 2013, vol. 48, no. 2, pp. 139–146.
Li, Y., Ji, B., Chen, W., Liu, L., Xu, C., Peng, C., and Wang, L., Food Agric. Immunol., 2008, vol. 19, no. 4, pp. 251–264.
Wang, Z., Zhu, Y., Ding, S., He, F., Beier, R.C., Li, J., and Shen, J., Anal. Chem., 2007, vol. 79, no. 12, pp. 4471–4483.
Bucknall, S., Silverlight, J., Coldham, N., Thorne, L., and Jackman, R., Food Additives Contam., 2003, vol. 3, no. 3, pp. 221–228.
Burkin, M.A., Food Agric. Immunol., 2008, vol. 19, no. 2, pp. 131–140.
Wen, K., Nolke, G., Schillberg, S., Wang, Z., Zhang, S., Wu, C., and Shen, J., Anal. Bioanal. Chem., 2012, vol. 403, no. 9, pp. 2771–2783.
Lu, S., Zhang, Y., Liu, J., Zhao, C., Liu, W., and Xi, R., J. Agric. Food Chem., 2006, vol. 54, no. 19, pp. 6995–7000.
Chu, F.S., Lau, H.P., Fan, T.S., and Zhang, G.S., J. Immunol. Methods, 1982, vol. 55, no. 3, pp. 73–78.
Liu, Z.Q., Lu, S.X., Zhao, C.H., Ding, K., Cao, Z.Z., Zhan, J.H., Ma, C., Liu, J.T., and Xi, R.M., J. Sci. Food Agric., 2009, vol. 89, no. 7, pp. 1115–1121.
Funding
The work was performed with partial financial support from the Russian Foundation for Basic Research (Grant no. 18-58-00038) and the Belarusian Republican Foundation for Fundamental Research (Grant no. H18P-060).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shanin, I.A., Zvereva, E.A., Eremin, S.A. et al. Development of an Immunoenzyme Assay to Control the Total Content of Antibiotics of the Fluoroquinolone Group in Milk. Appl Biochem Microbiol 55, 563–569 (2019). https://doi.org/10.1134/S0003683819050132
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683819050132