Abstract
The placement of pea plants (Pisum sativum L.) under flooding conditions led to an increase in lactate dehydrogenase (EC 1.1.1.27) activity in the roots. The enzyme was purified to an electrophoretic homogeneous state by a multistage purification method including ammonium sulfate fractionation, ion exchange chromatography on DEAE-Sephacel, and gel chromatography on Sephadex G-200. The degree of purification was 43.4, the yield was 2.5%, and the specific activity was 80.5 U/mg protein. Its physicochemical properties were studied: the molecular weight of the native lactate dehydrogenase molecule was 138 kDa. The molecular weight of the subunits was determined by PAGE by electrophoresis in the presence of DDS-Na. Its value was 34 kDa, which indicates that the enzyme is a homotetramer. The kinetic and regulatory properties of the enzyme and the values of the Michaelis constants were established. he effect of the concentration of hydrogen ions and temperature on direct and reverse reactions catalyzed by it was obtained. It was determined that lactate dehydrogenase inhibited ATP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Lactate dehydrogenase (LDH, EC 1.1.1.27) catalyzes the conversion of lactate to pyruvate and back, which is followed by NADH and NAD+ turnover. Since pyruvate is a key intermediate of the carbohydrate metabolism, this enzyme is widespread in nature. An important LDH function is regulation of the NAD+/NADH ratio, because its value affects the rate of a number of catalytic reactions [1].
Animal LDH is presently well studied. In most mammals it is represented by five isoforms, each of which is a tetramer consisting of four subunits of two types, muscle (M and A) and heart (H and B), in accordance with their localization. These subunits are encoded by two genes: ldh A and ldh B [2]. The loci of both the ldh-A and ldh-B genes occurred in evolution as a result of the duplication of a common ancestor gene, ldh [3, 4], due to the necessity of animal adaptation to the environmental conditions. Indeed, the LDH-5 homotetramer was found in tissues that underwent oxygen deficiency (i.e., performed their functions under anaerobic conditions) [5].
Electrophoretic analysis of LDH of different plants revealed several protein components that possessed enzymatic activity. These observations were interpreted as the presence of isoenzymes, which were represented by tetramers formed by two different peptide sequences [6]. Analysis of hypoxic roots and sprouts of rice revealed two subunits [7].
There are currently limited data on the physicochemical properties, kinetics, and regulation of LDH activity in the majority of plants. To analyze these properties, it is necessary to obtain electrophoretically pure enzyme preparation, because lactate utilization in some plants is coupled with the glycolate oxidase pathway [8].
The goal of this work was to isolate and purify electrophoretically homogenous LDH from pea roots grown under hypoxic conditions and to study its physicochemical and regulatory proteins.
MATERIALS AND METHODS
Plant material. The objects of study were 20-day-old pea shoots (Pisum sativum L., cultivar Ambrosia) cultivated hydroponically at 25°С. The shoots were placed in water 2–3 cm above the root collar in order to model oxygen deficiency. After 72 h of incubation, plants were used for the experiments [9].
The enzyme was extracted from homogenized plant roots. The extract was separated first by gel filtration on a G-25 sephadex column (1.5 × 20 cm) (Pharmacia, Switzerland) and then by ion-exchange chromatography on a DEAE-cellulose column (1.5 × 15 cm) (GE Healthcare, Switzerland) equilibrated with 50 mM Tris-HCl buffer, pH 7.4. The target protein was eluted by a linear gradient of 0.1–0.35 М NaCl and further purified by gel-filtration chromatography on a G-200 sephadex column (2.0 × 30 cm) (GE Healthcare, Switzerland) as described in [10, 11].
The enzyme activity was assessed spectrophotometrically by the rate of NADH oxidation at 340 nm with a SF-56 spectrophotometer (LOMO, Russia). The reaction mixture contained 2 mL of 50 mM Tris-HCl buffer, pH 7.4, 0.06 mM NADH, and 1 mM sodium pyruvate. The reaction was initiated by the addition of sodium pyruvate [12]. The LDH activity in the direct reaction was measured in 50 mM Tris-HCl buffer, pH 7.4, which contained 0.5 mM NAD+ and 25 mM sodium lactate. The amount of enzyme that either formed (direct reaction) or conversed (reverse reaction) 1 µM NADH in 1 min at 25°С was considered the unit of enzymatic activity.
The molecular weight of the protein was estimated by gel filtration on a G-200 sephadex column calibrated with Dextran blue (2000 kD). The molecular weight was calculated as follows:
where Ve is the protein elution volume and V0 is the interstitial volume.
The molecular weight of the subunits was estimated by electrophoresis in 12% SDS-PAAG. A standard set of proteins, which included β-galactosidase (116.0), bovine serum albumin (BSA) (66.2), ovalbumin (45.0), LDH (35.0), REase Bspl98 (25.0), β-lactoglobulin (18.4), and lysozyme (14.4), was used as the molecular weight standard. The protein bands in the gel were stained with silver nitrate as described in [13].
The electrophoresis of LDH was also carried out in 8% PAAG by the Devis method under non-denaturing conditions [14]. Protein bands were visualized by staining with silver nitrogen [13]. The enzyme was specifically identified by the tetrazole test [15]. The protein concentration was measured by the Lowry method.
The effect of the pH of the rate of the enzymatic reaction was assessed in 50 mM Tris-HCl buffer, рН 5.0–10.0 [6].
The kinetic constants (Kм) of the direct and reverse reactions, which were catalyzed by LDH from the pea leaves, were estimated by the Lineweaver–Burk approach. The catalytic constants (Kм) for pyruvate and L-lactate were estimated in the standard type reaction mixture at рН 7.5 and 25°С. The concentration of the substrates varied from 0 to 60.0 mM, while the concentrations of other components remained constant [8].
The effect of temperature on the LDH-catalyzed reaction rate was assessed in the temperature range of the reaction mixture from 15 to 80°С.
The experiments were carried out in four to six biological and three analytical repeats. The mean values of three assays are shown in Table 1 and the figures. The significance of differences was assessed by methods of variation statistics with the Student’s t-test. Statistically significant differences at р ≤ 0.05 are discussed. Graphics are composed on the basis of data processed with programs for linear and parabolic approximation.
RESULTS AND DISCUSSION
The increase in the LDH activity in the pea roots, which underwent hypoxia, was already observed 3 h after the beginning of the experiment. The maximal activity was registered after 2 days and remained for 4 days thereafter (Fig. 1). The LDH activity increased by 11–12 times as compared with the control plants. This may be due to adaptation to hypoxia by the cell metabolism in the root tissues. A similar effect was observed earlier in sorghum [7, 17] and pea leaves [18].
Pea roots were incubated under the conditions of oxygen deficiency for three days. These roots were then used to obtain a highly purified LDH preparation. Table 1 shows the data on LDA purification from the pea roots.
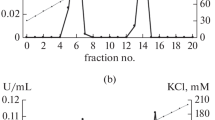
The enzyme obtained by multistep purification with a yield of 2.5% was electrophoretically homogenous and demonstrated high level of specific activity (80.53 U/mg protein). The maximal LDH amount was eluted with 120–150 mM NaCl by ion-exchange chromatography on DEAE-cellulose. PAAG-electrophoresis under non-denatured conditions revealed a single protein component (Rf—0.41), which was stained with silver nitrate (Fig. 2). This component interacted with tetrazolium blue, which is used to reveal the specific LDH activity (Fig. 2). It was determined that the obtained LDH preparation was electrophoretically homogenous and possessed LDH activity. The previously isolated, purified, and electrophoretically homogenous LDH from the pea leaves was characterized by a higher level of purity, higher yield, and higher molecular weight [18].
Analysis of the molecular weight values of the non-denatured LDH, which were obtained by gel-filtration chromatography on a G-200 sephadex column, as well as the data from SDS-PAAG electrophoresis (Fig. 3), suggested that the enzyme molecule is characterized by quaternary structure. Based on the molecular weight (138 kD) and on the molecular weight of the subunit (34 kD), one may conclude that the LDH molecule from pea roots, like the LDH from pea leaves, is represented by a homotetramer [18].
The study of catalytic characteristics of purified LDH showed that the KM value for pyruvate was 35 µM, which indicates a lower affinity of the studied enzyme to the substrate in comparison with LDH from pea leaves [18]. The high affinity of LDH to pyruvate may allow rapid oxidation of glycolytic NADH [19].
It was shown that the KM of LDH-5 from muscles of the thorax of benthic isopod (Saduria entomon) with respect to pyruvate as a substrate was 180 µM [20], whereas it was 20 µM for the enzyme isolated from skeletal muscles of lizard (Agama stellio) [21]. The KM of LDH from pea roots with respect to NADH was 63 µM (Fig. 4), which indicates a quite high affinity of LDH to this coenzyme. The KM of LDH-5 from the pig brain and lizard muscles varied from 15.6 µM to 40 µM respectively [21, 22].
Assessment of kinetic parameters of the isolated LDH with respect to the reverse reaction substrates revealed much higher KM values in comparison with the enzyme isolated from pea leaves [18]. For example, the KM values of LDH isolated from roots was 33 mM and 5.1mM with respect to lactate and NAD+, respectively (Fig. 4). The study of the affinity of LDH isolated from other sources to the reverse reaction substrates revealed similar results. Indeed, the KM of LDH obtained from goanna liver and benthic isopod with respect to lactate were 12.4 mM [23] and 90.04 mM [20], respectively, whereas the KM values of LDHs obtained from lizard muscles and rat liver were 20 µM and 3.2 mM, respectively [21, 24].
It is noteworthy that the 4A isoform of LDH is inhibited by ATP. It was shown that LDH-5 from soy sprouts was inhibited by 30 µM of ATP [25]. The LDH activity of the pea roots was partially inhibited by 30–60 µM of ATP and totally inhibited by 240 µM ATP. The obtained data may be considered evidence that the studied enzyme is a homotetramer consisting of four A-type subunits.
The effect of temperature on the activity of isolated LDH was studied in the temperature range of 15–70°C. The optimal temperature was 37°C for the direct reaction and 42°C for the reverse reaction (Fig. 5). The data correlated with optimal temperatures for LDHs obtained from different organisms, which typically vary from 30°C to 60°C [9, 21]. It was also shown that the working temperature range of LDH-5 was lower than that of LDH-1 [22].
The study of the effect of hydrogen ions on the activity of isolated LDH showed that the optimal pH was 7.2 for the direct reaction and 8.3 for the reverse reaction (Fig. 6). It was shown that the pH values 7.5 and 9.5 were optimal for direct and reverse reactions, respectively, which were catalyzed by LDH-5 from the goanna liver, whereas the pH values 6.8 and 9.5 were optimal for direct and reverse reactions catalyzed by the enzyme isolated from soy sprouts [23, 25].
It was determined that purified LDH remained stable for 6 months if stored at –74°С. It was also stable at –20°С if supplemented with a mixture of 5 mM EDTA and 5mM MgCl2.
CONCLUSIONS
Hence, electrophoretically homogenous LDH was isolated and purified from pea roots incubated under hypoxic conditions. The study of the physicochemical and regulatory properties of the obtained enzyme revealed it to be different from enzymes obtained from other sources. In particular, the LDH obtained from pea roots was characterized by a higher affinity to pyruvate as compared with lactate, which enabled its participation with reoxidation of glycolytic NADH. The optimal pH value was 7.2 for pyruvate reduction and 8.3 for lactate oxidation. This may be considered indirect evidence of the involvement of this enzymatic system in the adaptation metabolism of the root [23, 25]. Analysis of the efficacy of ATP enzyme inhibition allowed us to assign the purified LDH to the A-type of these enzymes [25]. The data are considered to be promising for research on the role of LDH (together with LDH isolated from pea leaves) in the development of adaptive reactions of the cellular metabolism to conditions of hypoxia that occur during flooding [17].
REFERENCES
Rider, C. and Taylor, C., Izoenzymes, London: Chapman and Hall, 1980.
Rossignol, F., Solares, M., Balanza, E., Coudert, J., and Clottes, E., J. Cell Biochem. Physiol., 2003, vol. 89, no. 1, pp. 67–79.
Korochkin, L.I., Serov, O.L., Pudovkin, A.I., Aronshtam, A.A., Borkin, L.Ya., Maletskii, S.I., Polyakova, Ya.V., and Manchenko, G.P., Genetika izofermentov (Genetics of Isozymes), Moscow: Nauka, 1977.
Tsuji, S., Qureshi, M.A., Hou, E.W., Fitch, W.M., and Li, S.S., Proc. Natl. Acad. Sci. U. S. A., 1994, vol. 91, no. 20, pp. 9392–9396.
Unzhakov, A.R., Ilyukha, V.A., Matsuk, N.V., and Belkin, V.V., Trudy Karel. Nauchn. Tsentra Ross. Akad. Nauk, 2007, no. 11, pp. 118–126.
Mulcahy, P. and O’Carra, P., Phytochemistry, 1997, vol. 45, no. 5, pp. 889–896.
Jain, V., Singla, N.K., Jain, S., and Gupta, K., Physiol. Mol. Biol. Plants, 2010, vol. 16, no. 3, pp. 241–247.
Engqvist, M.K.M., Schmitz, J., Gertzmann, A., Florian, A., Jaspert, N., Arif, M., Balazadeh, S., Mueller-Roeber, B., Fernie, A.R., and Maurino, V.G., Plant Physiol., 2015, vol. 169, no. 2, pp. 1042–1061.
Rivoal, J., Richard, B., and Pradet, A., Plant Physiol., 1991, vol. 95, no. 3, pp. 682–686.
Hoffman, N.E. and Hanson, A.D., Plant Physiol., 1986, vol. 82, no. 3, pp. 664–670.
Eprintsev, A.T., Fedorin, D.N., Nikitina, M.V., and Igamberdiev, A.U., Plant Physiol., 2015, vol. 181, pp. 14–19. https://doi.org/10.1016/j.jplph.2015.03.012
Setsuko, K., Takahiro, M., and Hiroshi, Y., PLoS One, 2013, vol. 8, no. 6. www.ncbi.nlm.nih.gov/pmc/articles/ PMC3683008. Accessed October 15, 2018.
Shevchenko, A., Wilm, M., and Vorm, O., Anal. Chem., 1996, vol. 68, no. 5, pp. 850–858.
Davis, B.J. and Ornstein, L., Ann. N.Y. Acad. Sci., 1964, vol. 121, no. 2, pp. 404–427.
Fieldes, M.A., Electrophoresis, 1991, vol. 13, nos. 1–2, pp. 82–86.
Lakin, G.F., Biometriya (Biometry), Moscow: Vysshaya Shkola, 1990.
Narsai, R. and Whelan, J., Front. Plant Sci., 2013, vol. 4, pp. 349–363.
Eprintsev, A.T., Komarova, N.R., and Falaleeva, M.I., Appl. Biochem. Microbiol., 2019, vol. 55, no. 2, pp. 159–164.
Zheng, Y., Si, X., He, Q., Jin, S., and Hong, J., Essays Biochem., 2015, vol. 59, pp. 1–4. https://doi.org/10.1042/bse0590001
Mulkiewicz, E., Zietara, M.S., Stachowiak, K., and Skorkowski, E.F., Comp. Biochem. Physiol. B Biochem. Mol. Biol., 2000, vol. 126, no. 3, pp. 337–346.
S. Al-Dzhasabi, Biokhimiya, 2002, vol. 67, no. 7, pp. 786–789.
Goto, T., Sugawara, K., Nakamura, S., Kidokoro, S., Wakui, H., and Nunomura, W., Biochem. Biophys. Res. Commun., 2016, vol. 479, no. 4, pp. 860–867.
Massod, J.H., Syed, M.I.A., Abida, N.H., Asifa, A., and Ishaq, M., Exp. Mol. Med., 1997, vol. 29, no. 1, pp. 25–30.
Fregoso-Peñuñuri, A.A., Valenzuela-Soto, E.M., Figueroa-Soto, C.G., Peregrino-Uriarte, A.B., Ochoa- Valdéz, M., Leyva-Carrillo, L., and Yepiz-Plascencia, G., Protein Expr. Purif., 2017, vol. 137, pp. 20–25. www.ncbi. nlm.nih.gov/pubmed/28625911.
Tihanyi, K., Fontanell, A., Talbot, B., and Thiron, J.P., Arch. Biochem. Biophys., 1989, vol. 274, no. 2, pp. 626–632.
Funding
This work was supported by the government order of the Ministry for Science and Higher Education of Russia, project no. 6.6927.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by M. Bibov
Rights and permissions
About this article
Cite this article
Eprintsev, A.T., Komarova, N.R., Falaleeva, M.I. et al. Isolation and Cleaning of Lactate Dehydrogenase from Pea (Pisum sativum L.) Roots by Hypoxia and the Study of Its Regulatory Properties. Appl Biochem Microbiol 55, 544–548 (2019). https://doi.org/10.1134/S000368381905003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000368381905003X