Abstract
Hydrolysis of soybean and rapeseed proteins with extract from cod pyloric caeca has been performed. The hydrolysates were analyzed by SDS-PAGE, HPLC, amino acid analysis, and mass spectrometry. The degree of hydrolysis depended on the substrate/enzyme extract ratio, hydrolysis time, temperature, and protein concentration. The hydrolysis of soybean and rapeseed proteins at a substrate/enzyme extract ratio of 1 : 20 and a hydrolysis time of 20 h at 37°C led to complete cleavage of intact proteins. The soluble part of the rapeseed protein hydrolysate consisted of oligopeptides with a molecular weight of less than 14 kDa, as well as free amino acids and short peptides. In sum it was 77% of the original protein material (including 6% of the free amino acids). According to the results of the amino acid analysis, the soluble part of the soybean proteins hydrolysate contained a set of oligopeptides and free amino acids, which comprised 83% of the starting protein material (including 20.16% of the free amino acids).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Plant proteins are widely used as a protein component in a number of food industry products and feed production for animals, poultry, and aquaculture (replacement of fish meal due to the growing expansion of aquaculture throughout the world and the continued depletion of marine bioresources). Soy and rapeseed are the most promising sources of proteins of plant origin. The huge production volumes of these oilseeds provide a wide availability of their processed products in the form of oil cake, meal, flour, and the protein concentrates and isolates obtained from them, which are characterized by a high protein content [1, 2]. According to the literature, the protein content in rapeseed seeds is 30–45% by weight. In the soybean flour, the proteins make up 35–50% on average, depending on the soy variety. The amino acid composition of the soybean and rapeseed proteins is well balanced; they contain all of the essential amino acids,but are characterized by a low content of cysteine and methionine [2]. However, the introduction of protein isolates and concentrates from soybeans and rapeseed into the animal ration is complicated by the presence of antinutrients, which are not digestible and may be even harmful to the health and development of animals; thus, they reduce the digestion and adsorption of the food components: they are inhibitors of digestive enzymes, glucosinolates, phenol derivatives, saponins, phytic acid, and a number of other compounds [3]. This requires the introduction of an additional stage of purification of these vegetable proteins. The second problem is their rather large size, the tendency to aggregate and gel formation (especially in the soybean proteins), and their partial denaturation during heat treatment in the process of pressing and extracting the oil from these oilseeds. The obtainment of protein hydrolysates expands the possibilities for the use of soybean and rapeseed proteins in feed production, which increases the availability of protein material of plant origin for the digestive system of animals [4]. The production of protein hydrolysates for introduction of protein into starter feed for juvenile fish grown in aquaculture is an issue of particular importance. Fish meal hydrolysates are еraditionally used for this purpose. Since the stocks of fish species used for preparation of the fish meal are reduced, the development of alternative feeds is becoming increasingly important. Since the activity of alkaline and acidic intestinal proteinases in fish larvae is not high enough, the feed proteins at the stage of their postembryonic development should be represented by free amino acids, di- and oligopeptides, and low molecular weight soluble proteins in ratios that are close to the composition of plankton organisms—the natural food of most fish species, including their juveniles [5]. However, there are а few studies on the obtainment of plant protein hydrolysates. Several works have been published on the soybean protein hydrolysis with proteolytic enzyme preparations Flavourzyme 1000 L, Novozyme FM 2.0 L, Alcalase 2.4 L [6], a complex of pancreatic enzymes [7], the preparation subtilysin Carlsberg [8], and rapeseed proteins with such proteolytic enzyme preparations like Alcalase, Pronase, and Neutrase [4, 9, 10].
In previous studies, soybean and rapeseed protein hydrolysates obtained with enzyme preparation from the red king crab hepatopancreas [11, 12], as well as protosubtilin [13] had been obtained. In this study, an extract from the pyloric caece of cod (bag-shaped outgrowths at the beginning of the gastrointestinal tract) was used to hydrolyze soybean and rapeseed proteins. The number of pyloric caece vary in different fish. In salmon, for example, their number is 300—400; in sturgeon they grow together and form one glandular organ. A study of the pyloric caece of cod, salmon, and some perch species confirms that they play an important role in food digestion [14]. It was found in all four listed fish species that the cecum (pyloric caece) is the main location at which sugars, amino acids, and dipeptides are found in much larger quantities than in the main intestines of salmon and cod. The bristle membranes of the pyloric caece contain hydrolytic enzymes that make the fish cecum the result of their adaptation via an increase the intestinal surface area. It was proven that in the pyloric caeca there are enzymes that digests proteins, carbohydrates, and fats [15, 16]. At present, the fish intestines are the objects of intensive study as a potential source of various enzymes [17, 18]. Trypsin and trypsin-like enzymes from the pyloric caece were isolated and characterized for a wide range of both cold-water and warm-water fish species [19, 20]. The pyloric caece of cod used in this work are a cheap source of highly active enzymes, since cod is one of the most important commercial fish species.
The goal of the work is to study the use of an enzyme preparation from pyloric caece of cod for the production of soybean and rapeseed proteins hydrolysates.
MATERIALS AND METHODS
Materials. Shansun-90 soybean isolate (YANTA group of enterprises, Atlant, Russia) with a crude-protein content of 92% (according to the manufacturer) and rapeseed oil cake (Grainlux, Russia, GOST 11048-95, crude protein content of 37.3%) were used in the study. Frozen pyloric caece of cod were obtained from Murmansk Trawl Fleet, Russia. All of the reagents used were manufactured by the Sigma company (USA).
Obtaining the extract from pyloric caece of cod (EPCC). The frozen material was homogenized in 0.1% NaCl at a ratio of 1 : 20 (weight : volume); the homogenate was filtered through a 100 kDa membrane, and the filtrate was freeze-dried. The lyophilized material was homogenized in 0.4 M Tris-HCl buffer (pH 7.5) at a ratio of 1 : 25 (weight : volume), and the insoluble material was separated by centrifugation at 25 000 g for 15 min in a J2-21 centrifuge (Beckman, USA). The supernatant was concentrated to a protein concentration of 2.5 mg/mL by ultrafiltration through a 3 kDa membrane and then used to hydrolyze the proteins.
Extraction of proteins from rapeseed meal [12]. According to the literature, the protein content in the rapeseed meal is 32–40%, depending on the variety. The starting material was ground in a mortar for 15 min to obtain a fine powder. The powder was washed with 60% ethanol for 30 min with stirring and then centrifuged at 25 000 g for 15 min in a J2-21 centrifuge (Beckman, United States). The residue was air-dried. The proteins were then extracted from the dried preparation by a solution of 0.5 M NaCl (pH 10.5) for 2 h at room temperature with vigorous stirring; the powder-to-liquid ratio was 1–3%. Insoluble plant residues were removed by centrifugation at 35000 g for 20 min in a J2-21 centrifuge (Beckman, United States). The supernatant was subjected to ultrafiltration through a membrane with a pore size of 10 kDa to remove low molecular weight compounds, which were extracted with proteins. During the ultrafiltration process, the solution of proteins above the membrane was diluted several times with either water (pH 8.0) or buffer solution (pH 8.0) to maintain a concentration close to the concentration in the original extract. To remove the salts, the proteins were precipitated with 80% ethyl alcohol for 2 h at –4 °C and centrifuged; the precipitated proteins were suspended either in water and freeze-dried or in 0.1 M Tris-HCl buffer (pH 8.0) and then used for enzymatic hydrolysis.
Evaluation of the total protein content in the starting material. The evaluation was performed by the Kjeldahl method [21].
Analysis of protein preparations for the polyphenol content. The analysis was performed by qualitative color reaction with iron (III) chloride [22]. When 2–3 drops of a 5% solution of FeCl3 were added to aqueous solutions of the obtained protein preparations, no change in the color of the solutions occurred, which indicated the absence of phenol compounds.
Protein concentration. The concentration was determined by the Bradford method [23].
Electrophoresis in PAGE. Electrophoresis of protein preparations and hydrolysates was carried out according to the Lamley method [24] in 15% PAGE in the presence of 10% Na-DDS.
Amino acid analysis. For amino acid analysis, the dried hydrolysates were hydrolyzed with 5.6 M HCl at 110°C for 24 h. The analysis was performed on an SYKAM S430 amino acid analyzer (Sykam, Germany). The total content of free amino acids in the hydrolysates was determined without acid hydrolysis.
Hydrolysis of commercial soybean protein isolate and rapeseed proteins. The hydrolysis was carried out at a protein concentration in a solution of 3 mg/mL, pH 8.0, at room temperature or 37°C for 1, 3, and 20 h. The EPCC/protein ratio was 1 : 20 or 1 : 100 (weight : weight by protein). The reaction was stopped via heating of the reaction mixture at 90°C for 5 min. The hydrolysates were cooled and stored frozen at –20°C.
Mass spectrometric analysis of protein hydrolysates. The analysis was performed after peptide separation on an EASY-nLC 1000 nanoflow chromatograph (Thermo Scientific, USA). A high-resolution OrbiTrap Elite mass spectrometer (Thermo Scientific, United States) was used as the detector. Panoramic spectra were recorded in the range of 500 to 2000 m/z with a resolution of 240 000; the ion fragmentation was performed in a high-pressure HCD dissociation chamber, and the fragmentation spectra were recorded at a resolution of 60 000. The peptides were separated on a capillary column (150 mm × 75 µm, Acuity UHPL BEN300 C4 1.7 µm, USA), balanced with 0.05% formic acid, in a gradient of 0–95% acetonitrile. The detection of peptides was carried out at 220 nm.
Chromatographic analysis of hydrolysates. The analysis was performed on a Phenomenex Luna C18 (2) column (100 Å, 5 μm, 250 × 4.6 mm) equilibrated with 0.1% trifluoroacetic acid in an acetonitrile concentration gradient (2% → 95% within 30 min) at an elution rate of 1 mL/min and a column temperature of 30°C using a BREEZE high pressure chromatographic system (Waters, USA). Twenty microliters of hydrolysate was applied to the column. The detection of peptides was carried out at 215 nm.
RESULTS AND DISCUSSION
Oilseed by-products attract increasing interest in the world as a source of available proteins. Currently, soy is a world-leading species used as a global source of plant-based protein for the food industry and feed production. This is due to the availability of its processing products and its relatively high protein content. Rapeseed is one of the five most cultivated oilseeds in the world, ranking fifth after soy, cotton, peanuts, and sunflower. Since rape is a more cold-resistant culture as compared with the others listed, it acquires special significance under the climatic conditions of Russia as a source of vegetable protein. The main proteins of rapeseed, globulins (molecular weight ~300 kDa), consist of six subunits and have a quaternary structure. The polypeptide chains of the subunits are partially interconnected by disulfide bridges. Albumin has a low molecular weight (~12–14 kDa) and is highly soluble in water. According to the balance of amino acids, rapeseed proteins correspond to a full-fledged diet of fish with fish meal–based food [2]. The amino acid composition of rapeseed [25] and soybean proteins [2] is well balanced and contains enough sulfur-containing amino acids and lysine.
The main proteins of soybeans are globulins, glycinin, and β-conglycinin in a ratio of 75/115, constituting 80% of the soybean proteins [26, 27]. The proteins have a complex quaternary structure. Glycinin consists of five subunits and has a sedimentation coefficient of 11S, and β-conglycinin (sedimentation coefficient of 7S) consists of three subunits (76, 72, and 53 kDa) [28]. In addition to globulins, which are insoluble in water but soluble in solutions of neutral salts, soybean contains water-soluble proteins characterized by a lower molecular weight. These include a minor γ-conglycinin and a relatively small amount of other proteins, including “whey proteins,” which together comprise from 9 to 15% of the total mass of soybean proteins. Having a complex quaternary structure formed by large polypeptides, soybean globulins quickly denature upon the unfolding of polypeptide chains under certain conditions. Based on the results of a number of studies on the enzymatic hydrolysis of soybean proteins, it is concluded that, due to their compact quaternary and tertiary structures, soybean proteins are not readily available for enzymatic hydrolysis, since many of their sections are closed and are difficult for enzymes to access [29–31].
The rapeseed proteins were obtained from fat-free oil cake, according to the method described previously [12], which was provided to obtain protein preparations that did not contain antinutritional substances present in the rape cake. The rapeseed meal crushed to fine powder was washed with 60% ethyl alcohol to remove colored compounds and some impurities soluble in alcohol. This removes 75% of phenolic compounds [33], as well as about 50% of mono- and oligosaccharides [2]. The proteins were then extracted with 0.5 M NaCl at a pH of 10.5 and a flour concentration of not more than 3% (wt/vol). The antinutrient low-molecular compounds extracted together with proteins were separated by ultrafiltration of the extracts through membranes with pores that accomodated molecules with a molecular mass of not more than 10 kDa. To remove NaCl, the proteins were precipitated with ethyl alcohol at a ratio of 1 : 9 (wt/wt) and 4°C; they were then suspended in water and freeze-dried. According to amino acid analysis, the protein content in the resulting protein preparations was about 92%. When the reaction with iron(III) chloride was conducted, no change in the color of protein solutions was found, which indicated the absence of phenol compounds in them [22]. The amino acid composition of protein preparations was comparable to the amino acid composition of fish meal, but it differed somewhat in the content of a number of amino acids [11].
The extract from the pyloric caece of cod (EPCC) in 0.1% NaCl solution was used to hydrolyze rapeseed proteins and commercial soybean protein isolate. The protein concentration in the extract was used to calculate the ratio of the enzyme preparation and the hydrolysable protein. Since rapeseed proteins contain albumin with a pI of about 10 [32] and globulins (~ 70% of the total protein), their pI values range from 4 to 7 [33], and the pI values of soybean proteins range from 4.5 to 6.4; the hydrolysis was carried out at a pH of 8.0.
Commercial soybean protein isolate and rape proteins (3 mg/mL) were hydrolyzed with EPCC at an EPCC/protein ratio of 1 : 20 or 1 : 100 (EPCC weight : protein weight) for 1, 3, and 20 h either at room temperature or at 37°C. The pre-denaturation of proteins did not affect the hydrolysis depth. The resulting hydrolysates were analyzed by PAGE-electrophoresis in the presence of Na-DDS, HPLC, and mass spectrometry; the content of free amino acids was also determined.
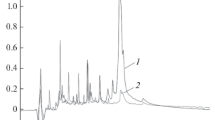
Figure 1 presents the electrophoregrams of rapeseed protein hydrolysates obtained at room temperature (lanes nos. 9–14). When the EPCC/protein ratio was 1 : 20, almost all high-molecular proteins hydrolyzed completely within 3 h. While some part of the hydrolysate consisted of oligopeptides with a molecular mass of less than 14 kDa. The rest of the protein material, which constituted the majority of the total protein, was hydrolyzed to the free amino acids and short peptides, which passed the gel at electrophoresis. When the EPCC/protein ratio was 1 : 100, this result was achieved only after 20 h of hydrolysis, but some amount of the original protein material was present at the same time in the hydrolysate. However, insoluble fragments were present among the hydrolysis products. Figure 2 presents the HPLC chromatograms of rapeseed protein hydrolysates obtained after EPCC hydrolysis for 20 h at various EPCC/protein ratios. When the peak areas of the starting protein material and hydrolysates are compared, it is obvious that, during the indicated time, when the EPCC/protein ratio was 1 : 20 (Fig. 2a) and 1 : 100 (Fig. 2b), the original protein material was almost completely hydrolyzed to oligopeptides with a low molecular weight. It can be seen that the hydrolysis of the original protein material for the same hydrolysis time at a ratio of 1 : 20 occurred more completely than at a ratio of 1 : 100. According to the results of amino acid analysis, the soluble part of the hydrolysate was only 18.7% of the original protein by weight at a EPCC/protein ratio of 1 : 100 and 36.4% at a ratio of 1 : 20. As shown by mass spectrometric analysis (Fig. 3a–3d), very short peptides of less than 1 kDa and oligopeptides of 1–10 kDa were present in soluble hydrolysis products.
SDS-PAGE pattern of the commercial soybean protein isolate (1) and rapeseed proteins (8) and hydrolysates of soybean protein isolate (lanes 2–7) and rapeseed proteins hydrolysates (lanes 9–14) by EPCC at room temperature at EPCC/protein ratio of 1 : 20 (lanes nos. 2–4 for soybean and 9–11 for rapeseed) and 1 : 100 (lanes 5–7 for soybean and 12–14 for rapeseed); hydrolysis time: 1 h (lanes 2, 5, 9, 12), 3 h (lanes 3, 6, 10, 13) and 20 h (lanes 4, 7, 11, 14).
Mass spectrometric analysis of the products of the hydrolysis of rapeseed proteins extracted at EPCC/protein ratio of 1 : 20 (a, b) and 1 : 100 (c, d) for 20 h; a, c—chromatograms of hydrolysates (see Materials and Methods), b, d—mass chromatograms of the chromatographic eluate; the ranges of the molecular masses of the hydrolysis products are indicated on the top (a) and (c).
The hydrolysis of commercial soybean protein isolate was performed with EPCC preparation under the same conditions as the rapeseed protein hydrolysis. The hydrolysis of soybean proteins under the same conditions was much slower than the hydrolysis of rapeseed proteins. In the course of hydrolysis, turbidity of the reaction mixture was observed. It did not disappear by its end, which was apparently associated with the formation of a certain amount of insoluble protein fragments. Figure 1 presents the electrophoregrams of soybean hydrolysate (lanes 2–7). It is clear that the hydrolysis of both soybean and rapeseed proteins proceeded faster with a higher enzyme load (an EPCC/protein ratio of 1 : 20). During 1 h of hydrolysis with an EPCC/protein ratio of 1 : 20, all soybean proteins were split, and the hydrolysate contained protein fragments with a molecular weight below 30 kDa, mostly below 20 kDa. Over the next 20 h, further hydrolysis of the newly formed protein fragments occurred, and the final hydrolysate contained mainly peptide fragments with a molecular weight below 16 kDa. The hydrolysis of soybean proteins at an EPCC/protein ratio of 1 : 100 was much slower. Thus, 3 h after the start of a hydrolysis, a significant amount of high molecular weight protein fragments was still present in the hydrolysate. However, after 20 h, the degree of hydrolysis of soybean proteins was approximately the same as when the EPCC/protein ratio was 1 : 20, but some high-molecular protein fragments with a molecular mass of about 50–30 kDa were still present in the hydrolysate. The soluble part of the hydrolyzed soybean protein isolate was analyzed by HPLC (Fig. 4). It is clear that in both hydrolysates obtained at different EPCC/protein ratios, even after 20 h of hydrolysis, a number of large protein molecules were present. Mass spectrometry analysis of the soluble part of hydrolyzed soybean protein (Fig. 5) showed that, after hydrolysis for 20 h and an EPCC/protein ratio of 1 : 20 and 1 : 100, the soluble part of the hydrolysate contained very short peptides of less than 1 kDa and a set of oligopeptides of 1–10 kDa. However, according to the results of amino acid analysis of the soluble part of the hydrolysate, it was found that the soluble part of the hydrolysate obtained at EPCC/protein ratio of 1 : 20 after hydrolysis at room temperature contained only 10.16% (by weight) of the initial protein amount; with a ratio of 1 : 100, it was 22%.
Mass spectrometric analysis of soluble part of the soybean protein hydrolysates at EPCC/protein ratio of 1 : 20 (a, b) and 1 : 100 (c, d) for 20 h; a, b—chromatograms of hydrolysates (see Materials and Methods); b, d—mass chromatograms of the components of the chromatographic eluate (the ranges of the molecular masses of the hydrolysis products are indicated on the top of (a) and (c)).
Therefore, at room temperature, which is more economically advantageous in the preparation of protein hydrolysates for feed, the hydrolysis of soybean and rapeseed proteins with EPCC was not sufficiently because the insoluble fragments were produced. In this work, it was shown that soybean and rapeseed proteins hydrolyzed more deeply at 37°C with the same other conditions. In this case аmino acid analysis of hydrolysates showed that the soluble part of soybean protein hydrolysate (at EPCC/protein ratio of 1 : 20) contaned 82.7% by weight of the amount of the original protein, including 20.16% of the free amino acids. At a ratio of 1 : 100, the soluble part of the hydrolysate was 88.4%. This analysis also showed that the yield of soluble products of rapeseed protein hydrolysate at 37°С, EPCC/protein ratio of 1 : 20 was 72% of the amount of the original protein and 5.7% of free amino acids (both values by weight) when the hydrolysis was performed.
CONCLUSIONS
The results showed that the enzyme complex from the pyloric caece of cod contained enzymes exhibiting high proteolytic activity that resulted in a high hydrolysis depth, even for such complex proteins as soybean proteins. This result is of particular importance at the production of starter feeds for aquaculture, which are used to rear larvae until they start active protein digestion; this is an important technological stage in artificial fish cultivation. During this period, a high mortality (over 50%) of young fish is noted, because the activity of intestinal alkaline and acid proteinases is not suffiecient. In this regard, it is considered necessary to introduce into the composition of larval (starter) feeds a significant amount of low molecular weight protein substances that can be digested and absorbed in the process of membrane and intracellular digestion [5].
ACKNOWLEDGMENTS
The work was supported by the Russian Science Foundation (project no. 16-14-00133).
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
REFERENCES
Salunkhe, D.K., Adsule, R.N., Chavan, J.K., and Kadam, S.S., World Oilseeds. Chemistry, Technology and Utilization, New York: Springer, 1992.
Shahidi, F., in Canola and Rapeseed. Production, Chemistry, Nutrition and Processing Technology, Shahadi, F., Ed., New York: Springer Science and Business Media, 1990.
Francis, G., Makkar, H., and Bekker, K., Aquaculture, 2001, vol. 199, no. 3, pp. 197–227.
Vioque, J., Sanchez-Vioque, R., Clemente, A., Pedroche, J., Bautista, J., and Millan, F., JAOCS, vol. 76, no. 7, pp. 819–823.
Grozesku, Yu.N., Bakhareva, A.A., and Shul’gina, E.A., Rybovod. Rybn. Kh-vo, 2011, no. 4, pp. 49–52.
Hrĉková, M., Rusňáková, M., and Zemanoviĉ, J., Czech. J. Food Sci., 2002, vol. 20, no. 1, pp. 7–14.
Caldéron De La Barca, A.M., Ruiz-Salazar, R.A., and Jara-Marini, M.E., J. Food Sci., 2000, vol. 65, no. 2, pp. 246–253.
Kuipers, B.J., Gruppen, H., and Voragen, A.G., J. Agric. Food Chem., 2005, vol. 53, no. 4, pp. 1031–1038.
Rodrigues, I.M., Coelho, J.F.J., and Carvalho, M.G., J. Food Eng., 2012, vol. 109, no. 3, pp. 337–346.
Kim, C.H., Kim, H.S., Lee, J.S., and Kang, Y.J., J. Food Eng., 1992, vol. 21, no. 4, pp. 519–524.
Muranova, T.A., Zinchenko, D.V., Kononova, S.V., Belova, N.A., and Miroshnikov, A.I., Appl. Biochem. Microbiol., 2017, vol. 53, no. 6, pp. 680–687.
Muranova, T.A., Zinchenko, D.V., Melanina, L.A., and Miroshnikov, A.I., Appl. Biochem. Microbiol., 2018, vol. 54, no. 1, pp. 76–82.
Zinchenko, D.V., Muranova, T.A., Melanina, L.A., Belova, N.A., and Miroshnikov, A.I., Appl. Biochem. Microbiol., 2018, vol. 54, no. 3, pp. 294–300.
Buddington, R.K. and Diamond, J.M., Proc. Natl. Acad. Sci. U. S. A., vol. 83, no. 3, pp. 8012–8014.
Mankura, M., Kayama, M., and Saito, S., Bull. JSSF, 1984, vol. 50, no. 12, pp. 2127–2131.
Khantaphant, S. and Benjakue, S., Food Chem., 2010, vol. 120, no. 3, pp. 658–664.
Hau, P.V. and Benjakul, S., J. Food Biochem., 2006, vol. 30, no. 3, pp. 478–495.
Li, B.J., Zhou, L.G., Cai, Q.F., Hara, K., Maeda, A., Su, W.J., and Cao, M.J., Food Chem., 2008, vol. 110, no. 2, pp. 352–360.
Simpson, B.K. and Haard, N.F., Comp. Biochem. Phys., vol. 79, no. 4, pp. 613–622.
Klomklao, S., Benjakul, S., Visessanguan, W., Kishimura, H., and Simpson, B.K., J. Agric. Food Chem., 2006, vol. 54, no. 15, pp. 5617–5622.
Busev, A.I., Kolorimetricheskie (fotometricheskie) metody opredeleniya nemetallov (Colorimetric (Photometric) Methods for Determination of Non-Metals), Moscow: Izd. Inostr. Literatury, 1963.
Zaprometov, M.N., Biokhimiya katekhinov (Biochemistry of Catechins), Moscow: Nauka, 1964.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, no. 7, pp. 248–254.
Laemmly, U.K., Nature, 1970, vol. 227, no. 8, pp. 680–685.
Cheftel, J.C., Cuq, J.L., and Lorient, D., Protéines Alimentaires. Biochimie—Propriétés Fonctionnelles—Valeur Nutritionnelle—Modifications Chimiques, Paris: Technique et Documentation—Lavoisier, 1985.
Nielsen, N.S., in Structure of Soy Protein, Altschul, A.M. and Wilcke, H.L., Eds., New York: Academic Press, 1985, pp. 26–66.
Nishinary, K.Y., Fang, S.Guo., and Philips, G.O., Food Hydrocolloid, 2014, vol. 39, no. 3, pp. 301–318.
Sexton, P.J., Paek, N.C., and Shibles, R.M., Field Crops Res., 1998, vol. 59, no. 1, pp. 1–8.
Tsumura, K., Saito, T., Kugimiya, W., and Inouye, K., J. Food Sci., 2004, vol. 69, no. 5, pp. 363–367.
Chen Lin, Jianshe Chen, Jianyan Ren, and Mouming Zhao, Food Hydrocolloid., 2011, vol. 25, no. 5, pp. 887–897.
Xiang Dong Sun., Int. J. Food Sci. Technol., 2011, vol. 46, no. 12, pp. 2447–2459.
Krause, J.P. and Schweke, K.D., Colloids Surf., 2001, vol. 21, no. 1, pp. 29–36.
Chabanon, G., Chevalot, I., Framboisier, X., Chenu, S., and Marc, I., Proc. Biochem., 2007, vol. 42, no. 10, pp. 1419–1428.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Martynova
Rights and permissions
About this article
Cite this article
Zinchenko, D.V., Muranova, T.A., Melanyina, L.A. et al. Hydrolysis of Soybean and Rapeseed Proteins with Enzyme Complex Extracted from the Pyloric Caeca of the Cod. Appl Biochem Microbiol 55, 165–172 (2019). https://doi.org/10.1134/S0003683819020182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683819020182